Review Article - (2018) Volume 8, Issue 6
The Role of Connexin 43 in Vascular Cognitive Diseases
- Corresponding Author:
- Xia Zhang
Department of Neurology, Second Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China
Tel: 86-512-67783663
Fax: 86-512-68284303
Abstract
Cerebrovascular disease is the most important modern disease affecting human health, subsequently the incidence of vascular dementia increases year by year. However, a significant percentage of patients were not diagnosed and didn't receive standard treatment, which is mostly related to the insufficient understanding of vascular dementia. Cerebral blood vessels play crucial roles in the pathogenesis of vascular dementia, not only for the delivery of oxygen and nutrients, but also for the trophic signaling that links the well-being of neurons and glia to that of cerebrovascular cells. In recent years, more and more evidence has shown that Cx43 and its gap junction channels are the potential therapeutic targets for neurodegenerative and vascular diseases. This review sums up the related researches of Cx43 and its gap junction channels in recent years about neurovascular unit(NVU) and vascular cognitive impairment (VCI), puts forward a new prospect in the field of vascular cognitive impairment and provide theoretical basis for more in-depth researches and clinical transformation in the future.
Introduction
In the early 1990s, vascular dementia (VD) was classified as an independent form of dementia in the Alzheimer’s disease Diagnostic and Treatment Centers (ADDTC) criteria, further extending the concept of dementia. Where after, the broader term ‘‘vascular cognitive impairment’’ (VCI).
Vascular cognitive impairment’’ (VCI) was introduced to recognize the heterogeneous nature of the contribution of vascular pathology to dementia, including many different subtypes and more subtle deficits that would not fulfill dementia criteria [1,2]. As the two commonest forms of cognitive impairment in the elderly [3], Alzheimer’s disease (AD) and VD bring more attention to the conception of the neurovascular unit (NVU) and more curiosities about the overlap between neurodegenerative and vascular factors in the pathogenesis of dementia.
The concept of the NVU was proposed as a functional entity with both neurons and microvessels together, including neurons, glia, perivascular and vascular cells. They are structurally, functionally, and developmentally interrelated to maintain the homeostasis of the cerebral microenvironment. As is well-known, the NVU plays a fundamental role in the broad spectrum of pathologies underlying VCI. It regulates blood flow, controls the exchange across the blood–brain barrier (BBB), contributes to immune surveillance in the brain, and provides trophic support to brain cells [4]. In normal brain, cellular types composing the NVU, include neurons, astrocytes and endothelial cells which all express pannexins and connexins. Pannexins and connexins are protein subunits of two families that form plasma membrane channels [5]. In central nervous system (CNS), gap junction (GJ) coupling occurs among both neurons and glial cells to form a highly coupled intercellular network. Each GJ is composed of two opposing hemichannels and each hemichannel is made of six connexin subunits arranged around a central pore. And connexin43 (Cx43) is one of the most abundant GJ proteins and the predominant connexins in astrocytes, which is expressed ubiquitously in astrocytes and microglia throughout the CNS [6].
There is increasing evidence outlining changes in Cx43 expression following CNS ischemic injury. Previous studies have shown the upregulation of an unlocked hemichannel (CxHc) in human postmortem tissue following ischemia [7], suggesting the involvement of CxHc in the pathophysiological process of ischemic injury. More evidences demonstrated that the opening of unlocked CxHc led to consequent cell death after ischemic insults in different cell models including myocytes [8], astrocytes [9], and renal tubule cells [10]. In our previous in-vivo study, we proved that targeting Cx43 hemichannels is a therapeutic strategy for cerebral ischemic injury. Gap26 regulated the expression and distribution of Cx43 via Akt activation-dependent ubiquitinproteasome pathway and showed a remarkable protection for neurological functions [11].
In recent years, more and more evidence has shown that hemichannels, the sub-component of GJs, play a role in neurodegenerative diseases. Similar high expression of Cx43 is reported in models of AD [12], amyotrophic lateral sclerosis (ALS) [13], as well as Parkinson’s disease (PD) [14]. Changes of gap junction intercellular communication (GJIC) reflected by alterations in connexin expression and dye coupling have been associated with numerous CNS diseases [15,19]. Treatment with channel inhibitors can significantly improve the clinical symptoms of experimental animals [20]. Therefore we speculate that Cx43 may play a vital role in the pathogenesis of dementia between neurodegenerative and vascular factors.
Cx43 and Dementia
AD is currently the leading cause of dementia in elder population [21] featuring gradually progressive cognitive and functional deficits as well as behavioral changes, eventually with social or occupational disability [22]. Studies have shown the correlation between AD and expression of Cx43. The expression of Cx43 is increased in the reactive astrocytes surrounding plaques in both human AD brain and murine models of familial AD (FAD) [23,24]. And Cx43 immunoreactivity co-localizes with astrocytes within 80% of amyloid plaques of human post-mortem tissues. Aβ has also been reported to increase CxHc activity in neurons, astrocytes, and microglia [25]. The opening of CxHc among neighbor neurons causes the spread of inflammatory injury, neurotoxic glutamate release and propagates the neurodegenerative process [26]. Meanwhile, Takeuchi et al reported that CxHC inhibitor improved cognitive function and reduced cytotoxic damage both in vitro and in vivo of AD [27].
In tissue from AD individuals, activated astrocytes were closely associated with amyloid plaques in the molecular layer of the cerebral cortex. Astrocytes might be activated by human amyloid- β (Aβ) [28]. All evidences show that there is a close relationship between Cx43/ astrocyte and dementia. In our previous behavioral tests, the spatial memory and learning abilities as well as the muscle strength and balance abilities of rats significantly decreased after ischemic injury, which have been reversed by Gap26, the inhibitor of Cx43 hemichannel. Gap26 significantly inhibited the excessive activation of astrocytes and improve astrocytic functions to maintain homeostasis in CNS [11].
VD and NVU
More and more evidences showed cerebrovascular dysfunction associated with Cx43/astrocytes in both ischemia and neurodegenerative impairment, therefore we speculate that Cx43 plays an important role in the pathophysiology of VD. Abnormal expression of Cx43 protein or channel dysfunction may impair cerebral blood vessels and composing cells of NVU, thus affecting the occurrence, progression and outcome of VCI.
VD is a syndrome with heterogeneous pathophysiological mechanisms, exposed to some common vascular risk factors [29]. The incidence of post-stroke or stroke-associated cognitive dysfunction is extremely high. In 2011, a scientific consensus statement about VCI and dementia from the American Heart Association(AHA) and American Stroke Association(ASA), pointed out that the NVU dysfunction is likely to be an important component of pathophysiological processes underlying VCI, and might be the common target of cerebrovascular and neurodegenerative disease [30]. Neurovascular dysfunction leads to brain tissue vulnerability, including the cerebrovascular regulation dysfunction, the BBB destruction, and reduced nutrition supply and impaired repairing potential of damaged brain tissue. Chronic, longterm low perfusion damage, oxidative stress, inflammatory response, and vascular barrier permeability change are all underlying mechanisms for VCI and VD [4]. Glial cells, including astrocytes and microglial cells, play an important neuromodulatory role in the development of dementia. Astrocytes were proved to be capable of cell-to-cell communication through gap junctions, suggesting an important role for modulation of neuronal and vascular function [31]. At the same time, Cx43, expressed ubiquitously in astrocytes and microglia throughout the CNS, appears to be the major connexin to do this job.
The NVU and Cx43
▪ Regulation of cerebral blood flow
The brain vasculature has an intimate developmental, structural, and functional relationship with the brain tissue; their cellular elements forming a functional domain termed the neurovascular unit [32]. Astrocytes comprise a part of celluar elements of the NVU. How astrocytes participate in cerebral blood flow regulation focused on their foot processes. Zonta et al. found that electrical stimulation of astrocytes resulted in an increase of intracellular Ca2+ followed by vasodilation of arterioles contacted with astrocytic foot processes [33]. It was inferred that astrocytic foot processes sensed the synaptic activity and then responded by inducing vasodilation of the appropriate blood vessels [34].
On the other hand, emerging evidence implicates the microvascular bed as one of the key players in the overall regulation of cerebral blood flow. Rosenblum et al. first presented evidence for the presence of coordinated vasomotor responses (CVR) in mouse pial arterioles [35]. Dietrich et al. [36] suggested that endothelial cells appeared to have a key role in the conducted responses of intracerebral arterioles to potassium. Cx43 has been identified in the smooth muscle and endothelial cells of blood vessels [37,38] and abundant at end-foot processes along blood vessels, as well as within astrocytic processes that surround chemical synapses[39]. An endothelial cell-specific Cx43 knock-out mouse shows hypotension and bradycardia correlating with elevated plasma levels of nitric oxide and angiotensins I and II, suggesting the importance of Cx43 gap junctions for maintenance of vascular tone [40]. Hemichannels formed by Cx43 are mostly active in pathological conditions, which have recently been shown to regulate cognitive function. The activity of astroglial Cx43 hemichannels in resting states regulates basal excitatory synaptic transmission [41,42]. After ischemic insult and other possible harm on cerebral blood flow, the opening of Cx43 channel regulates electrolyte balance inside and outside cells while connexin expression changes to shape the vasomotor conductivity.
▪ Blood–brain barrier(BBB) dysfunction
The BBB is a highly selective semipermeable membrane barrier separating the central nervous system (CNS) from peripheral circulation, permitting essential nutrients to reach the brain while restricting the passage of harmful substances [43]. As a complex celluar gate for CNS, BBB is one of the underexplored brain structures in ageing and dementia. Within the NVU, the endothelial cells forming the BBB restrict the entry of potentially neurotoxic plasma components, blood cells, and pathogens into the brain [44]. White matter BBB alterations are early findings in VCI [45,46]. BBB hyper permeability further contributes to cerebral microvascular diseases such as lacunar strokes and leukoaraiosis, which are the typical imaging manifestations of VCI [47].
Connexin 43 hemichannels, the most ubiquitously expressed gap junction proteins in the choroid plexus (CP), were found to be the important par cellular route for solute transport. The opening of Cx43 hemichannels destroys BBB and has been found to be associated with neurodegenerative disorders, such as PD and AD [48,49]. On the other hand, there are some other proteins involved in maintaining the BBB integrity, including zonula occluden (ZO)-1. Evidences showed ZO-1 deficiency disrupts tight junction (TJ), associated with BBB breakdown in many neurological disorders [50]. Recent studies demonstrated that Cx43 could interact with ZO-1, and the inhibition of the interaction would decrease hemichannelmediated membrane permeability [51,52]. Our previous study proved after cerebral ischemic injury, the opening of CxHc broke the BBB and caused the cytotoxic release of glutamate, ATP and other harmful elements, and then cell death followed [11]. But studies of Cx43 on BBB destruction in vascular dementia lack.
Cx43 activities might be regulated through Wnt/ β-catenin signaling pathway for the BBB formation. Dickkopf-1(Dkk-1) as a classic Wnt/ β-catenin pathway inhibitor, has been confirmed to be related to a variety of pathophysiological processes including AD, temporal lobe epilepsy, ischemic brain damage and so on, with downregulation of Cx43. Ana Martin et al. have shown that lack of DKK-1 can improve spatial memory function, memory consolidation and emotional behavior in adult mice. In AD, dkk-1 was involved in the pathological process of β-amyloid deposition and Tau hyperphosphorylation. In the transient ischemic and permanent cerebral artery ischemia model, the secretion of Dkk-1 was found to inhibit the occurrence of nerve cell damage induced by Wnt/ β-catenin pathway. Thus, Dkk 1 and Wnt signaling pathway associated with Cx43 is also the common target for neurodegenerative and vascular disease [53,54].Whether Cx43 participate in the development of VD through Dkk-1 and Wnt signaling pathway? We don’t yet know.
It was suggested that BBB disruption in cerebrovascular disease is due to the function alteration of adherens and TJs through modulation of protein expression, which are calcium dependent [55]. Cx43 homozygous null mice revealed an important role for Cx43 in the regulation of intercellular calcium [Ca2+] signaling and functional dye coupling [56]. Astrocytic Cx43 hemichannels play a key role in maintaining the balance of intracellular and extracellular calcium [57]. Astrocytes also influence the formation and the maintenance of the BBB [58,59]. However, it remains unclear whether astrocytes are essential for it. Future studies should provide more definitive answers to whether astrocytes play a key role in BBB maintenance in the mature and aging brain, especially with vascular cognitive impairment that we’re talking about.
▪ Role of oxidative stress and inflammation
The NVU is an important checkpoint regulating the afferent and efferent arms of the immune system and shaping the immune responses of the brain. The cells of the NVU are involved in the initiation and expression of adaptive and innate immune responses of the brain.
Vascular oxidative stress and inflammation are key pathogenic factors for neurovascular dysfunction [60-63]. Park et al. proved that radicals produced by superoxide-producing enzyme nicotinamide adenine dinucleotide phosphate(NADPH) oxidase are responsible for the deleterious cerebrovascular effects associated with various VCI risk factors [64-67].Vascular oxidative stress and inflammation impede the proliferation, migration, and differentiation of oligodendrocyte progenitor cells and compromise repairing of the damaged white matter [68-70]. On the other hand, astrocytes contribute to a variety of dynamic regulations in the neural system and emerge as pivotal regulators of CNS inflammation responses, with important implication for diverse CNS disorders [71,72]. Changes of GJIC reflected by dye coupling and connexin expression have been associated with numerous CNS inflammatory diseases. A general consequence of inflammation responses is reactive gliosis distinguished by astrocyte hypertrophy and proliferation of astrocytes and microglia. In our study, accompanied by the excessive expression of Cx43 after ischemia hypoxia, we observed the excessive activation of astrocytes, and Cx43 inhibitors can obviously restrain this phenomenon and restore the normal function of astrocytes. Chanson et al. also proved that inhibition of Cx43 hemichannels could restrict the intercellular diffusion of inflammatory molecules and effectively limiting the spread of inflammatory responses [73]. Indeed, gap junctional communication and Cx43 expression among astrocytes are affected during cerebral ischemia, with a large number of activated macrophages/microglia [24,74,75].
As another important cellular component of the NVU, pericytes have a vital role in many neurological diseases, such as AD [76-79], mild dementia [80], amyotrophic lateral sclerosis (ALS) [81], and stroke [82], because of the physiological roles in maintaining BBB integrity, aiding in angiogenesis and microvascular stability [44,83], regulating capillary diameter and CBF [82,84], and phagocytosing toxic metabolites and exotic pathogens [85]. Connexins as the important connection between pericytes and endothelial cells, contribute to maintaining pericytic function.
▪ Trophic coupling in the neurovascular unit
Cellular components in the NVU such as neurons, astrocytes, oligodendrocytes, vascular and perivascular cells are in a state of close trophic and metabolic codependence, playing a defining role in the physiological functions of brain. In the adult nervous system, neuroblasts migrate along blood vessels, a process dependent on brain derived neurotrophic factor (BDNF) secretion by endothelial cells [86]. Endothelial cells can also promote the proliferation and survival of oligodendrocytes (oligovascular niche) by activating the Akt/PI3 kinase pathway through BDNF and fibroblast growth factor (FGF) [87].
After brain injury, growth factors released from endothelial cells like BDNF, vascular endothelial derived growth factor (VEGF), stromal-derived factor 1, and angiopoietin-1 orchestrate the migration and differentiation of neuroblasts [88-90]. Moreover, astrocytes also release neurotrophic factors, contributing to neurotransmitter metabolism, in addition to their well-established interactions with neurons [91].
Loss of trophic support may impede the proliferation, migration, and differentiation of oligodendrocyte progenitor cells, and compromise the repair of the damaged white matter in VCI [68-70,92]. Dysfunction of neurons and glia is associated with endothelial cell atrophy and microvascular rarefaction for their trophic support to vascular cells [93]. Decreased capillary density not only occurs at lesioned sites, but also in normal appearing white matter in VCI patients, possibly due to loss of neuron and/or glial-derived growth factors [94]. Trophic interactions are also critically involved in the demyelination and remyelination associated with leukoaraiosis.
In our previous study, Cx43 inhibitor Gap26 protected brain through the Akt activation dependent pathway after hypoxic-ischemic damage [11].Our unpublished pre-experiments showed that the overexpression of Cx43 after ischemic hypoxic brain injury was accompanied by the reduction of BDNF. Therefore, whether the trophic coupling in the NVU is related to Cx43 function requires further proofs.
Conclusion
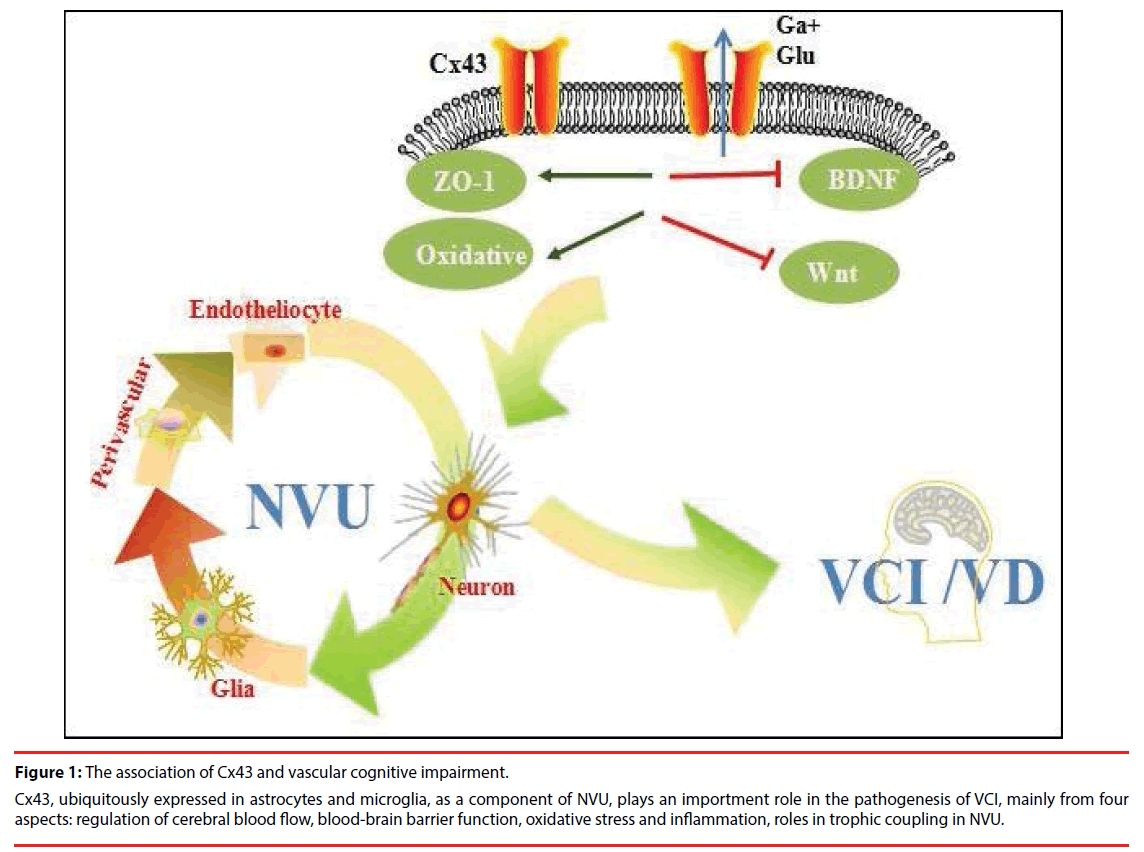
Vascular pathologies contribute to neurodegenerative changes and vice versa. Although there is evidence that ischemia promotes Aβ accumulation by enhancing its production and reducing its elimination, the impact that ischemia exerts on tau pathology is still less well understood. The vascular pathologies could synergistically contribute to the neurodegenerative changes resulting in cognitive decline more than one by one pathology do alone (synergistic effects) (Figure 1). Synergistic pathogenic interaction between vascular and neurodegenerative pathologies is biologically plausible because in animal models ischemia promotes Aβ accumulation and, in turn, Aβ aggravates ischemic injury. In conclusion, vascular lesions are detrimental to cognitive function either by directly damaging neural pathways involved in higher integrated functions or by aggravating AD pathological process.
Figure 1: The association of Cx43 and vascular cognitive impairment.
Cx43, ubiquitously expressed in astrocytes and microglia, as a component of NVU, plays an importment role in the pathogenesis of VCI, mainly from four aspects: regulation of cerebral blood flow, blood-brain barrier function, oxidative stress and inflammation, roles in trophic coupling in NVU.
Due to a blowout of cerebrovascular diseases and the unhealthy lifestyle in modern society, the incidence of VD has increased. In recent years, cerebral small vessel disease (CSVD) has been studied a lot by researchers, and VD is one of the most important clinical manifestations of CSVD. Considering that some vascular risk factors are modifiable, approaches to treat dementia should rely heavily on strategies to regulate cerebrovascular risk factors. The coexistence of ischemic and neurodegenerative pathologies raises a number of questions related to their impacts on cognition, and has vital implications for the prevention, diagnosis, and treatment of VCI and VD.
Cx43 assumptions in the future, which is clinically meaningful for early screening of high-risk population of VD and providing a new target for VD intervention. Cx43 might become an important target for prognosis and treatment of cognitive dysfunction. Researches on cerebrovascular diseases and neurodegenerative diseases have been relatively comprehensive; however, systematic researches on Cx43 roles in the NVU, especially vascular dementia, are rare. Based on the previous fragmented researches and our results on Cx43 in ischemic stroke, we look forward to providing a new perspective on VD and more related researches to verify the theoretical.
References
- Hachinski VC, Bowler JV. Vascular dementia. Neurol 43(10), 2159-2160 (1993).
- Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta. Neuropathol 120(3), 287-296 (2010).
- Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nat. Rev. Neurol 5(12), 649-658 (2009).
- Iadecola C. The pathobiology of vascular dementia. Neuron 80(4), 844-866 (2013).
- Nagy JI, Rash JE. Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain. Res. Brain. Res. Rev 32(1), 29-44 (2000).
- Chew SS, Johnson CS, Green CR, et al. Role of connexin43 in central nervous system injury. Exp. Neurol 225(2), 250-261 (2010).
- Nakase T, Yoshida Y, Nagata K. Enhanced connexin 43 immunoreactivity in penumbral areas in the human brain following ischemia. Glia 54(5), 369-375 (2006).
- Kondo RP, Wang SY, John SA, et al. Metabolic inhibition activates a non-selective current through connexin hemichannels in isolated ventricular myocytes. J. Mol. Cell. Cardiol 32(10), 1859-1872 (2000).
- John SA, Kondo R, Wang SY, et al. Connexin-43 hemichannels opened by metabolic inhibition. J. Biol. Chem 274(1), 236-240 (1999).
- Vergara L, Bao X, Bello-Reuss E, et al. Do connexin 43 gap-junctional hemichannels activate and cause cell damage during ATP depletion of renal-tubule cells? Acta. Physiol. Scand 179(1), 33-38 (2003).
- Li X, Zhao H, Tan X, et al. Inhibition of connexin43 improves functional recovery after ischemic brain injury in neonatal rats. Glia 63(9), 1553-1567 (2015).
- Wisniewski HM, Wegiel J. Spatial relationships between astrocytes and classical plaque components. Neurobiol. Aging 12(5), 593-600 (1991).
- Patel SA, Maragakis NJ. Amyotrophic lateral sclerosis: pathogenesis, differential diagnoses, and potential interventions. J. Spinal. Cord. Med 25(4), 262-273 (2002).
- Nutt JG, Wooten GF. Clinical practice. Diagnosis and initial management of Parkinson's disease. N. Engl. J. Med 353(10), 1021-1027 (2005).
- Haughey NJ, Mattson MP. Alzheimer's amyloid beta-peptide enhances ATP/gap junction-mediated calcium-wave propagation in astrocytes. Neuromolecular. Med 3(3), 173-180 (2003).
- Nakase T, Fushiki S, Naus CC. Astrocytic gap junctions composed of connexin 43 reduce apoptotic neuronal damage in cerebral ischemia. Stroke 34(8), 1987-1993(2003).
- Naus CC, Ozog MA, Bechberger JF, et al. A neuroprotective role for gap junctions. Cell. Commun. Adhes 8(4-6), 325-328 (2001).
- Rouach N, Avignone E, Meme W, et al. Gap junctions and connexin expression in the normal and pathological central nervous system. Biol. Cell 94(7-8), 457-475 (2002).
- Rozental R, Giaume C, Spray DC. Gap junctions in the nervous system. Brain. Res. Rev 32(1), 11-15 (2000).
- Takeuchi H, Suzumura A. Gap junctions and hemichannels composed of connexins: Potential therapeutic targets for neurodegenerative diseases. Front. Cell. Neurosci 8(1), 189 (2014).
- Tiiman A, Palumaa P, Tougu V. The missing link in the amyloid cascade of Alzheimer's disease - metal ions. Neurochem. Int. 62(4), 367-378 (2013).
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev 81(2), 741-766 (2001).
- Mei X, Ezan P, Giaume C, et al. Astroglial connexin immunoreactivity is specifically altered at beta-amyloid plaques in beta-amyloid precursor protein/presenilin1 mice. Neurosci 171(1), 92-105 (2010).
- Nagy JI, Li W, Hertzberg EL, et al. Elevated connexin43 immunoreactivity at sites of amyloid plaques in Alzheimer's disease. Brain. Res 717(1-2), 173-178 (1996).
- Orellana JA, Shoji KF, Abudara V, et al. Amyloid beta-induced death in neurons involves glial and neuronal hemichannels. J. Neurosci 31(13), 4962-4977 (2011).
- Perez Velazquez JL, Frantseva MV, Naus CC. Gap junctions and neuronal injury: Protectants or executioners? Neuroscientist 9(1), 5-9 (2003).
- Takeuchi H, Mizoguchi H, Doi Y, et al. Blockade of gap junction hemichannel suppresses disease progression in mouse models of amyotrophic lateral sclerosis and Alzheimer's disease. PLoS. One 6(6), e21108 (2011).
- DeWitt DA, Perry G, Cohen M, et al. Astrocytes regulate microglial phagocytosis of senile plaque cores of Alzheimer's disease. Exp. Neurol 149(2), 329-340 (1998).
- KorczynAD, Vakhapova V. The prevention of the dementia epidemic. J. Neurol. Sci 257(1-2), 2-4 (2007).
- Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 42(9), 2672-2713 (2011).
- Simard M, Arcuino G, Takano T, et al. Signaling at the gliovascular interface. J. Neurosci 23(27), 9254-9262 (2003).
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci 5(5), 347-360 (2004).
- Zonta M, Sebelin A, Gobbo S, et al. Glutamate-mediated cytosolic calcium oscillations regulate a pulsatile prostaglandin release from cultured rat astrocytes. J. Physiol 553(Pt 2), 407-414 (2003).
- Iliff JJ, D'Ambrosio R, Ngai AC, et al. Adenosine receptors mediate glutamate-evoked arteriolar dilation in the rat cerebral cortex. Am. J. Physiol. Heart. Circ. Physiol 284(5), H1631-1637 (2003).
- Rosenblum WI, Weinbrecht P, Nelson GH. Propagated constriction in mouse pial arterioles: Possible role of endothelium in transmitting the propagated response. Microcirc. Endothelium. Lymphatics 6(4-5), 369-387 (1990).
- Horiuchi T, Dietrich HH, Hongo K, et al. Mechanism of extracellular K+-induced local and conducted responses in cerebral penetrating arterioles. Stroke 33(11), 2692-2699 (2002).
- Saez JC, Berthoud VM, Branes MC, et al. Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev 83(4), 1359-1400 (2003).
- De Wit C, Hoepfl B, Wolfle SE. Endothelial mediators and communication through vascular gap junctions. Biol. Chem 387(1), 3-9 (2006).
- Danesh-Meyer HV, Huang R, Nicholson LF, et al. Connexin43 antisense oligodeoxynucleotide treatment down-regulates the inflammatory response in an in vitro interphase organotypic culture model of optic nerve ischaemia. J. Clin. Neurosci 15(11),1253-1263 (2008).
- Liao Y, Day KH, Damon DN, et al. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc. Natl. Acad. Sci 98(17), 9989-9994 (2001).
- Chever O, Lee CY, Rouach N. Astroglial connexin43 hemichannels tune basal excitatory synaptic transmission. J. Neurosci 34(34), 11228-11232 (2014).
- Stehberg J, Moraga-Amaro R, Salazar C, et al. Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. FASEB. J. 26(9), 3649-3657 (2012).
- Popescu BO, Toescu EC, Popescu LM, et al. Blood-brain barrier alterations in ageing and dementia. J. Neurol. Sci 283(1-2), 99-106 (2009).
- Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat. Neurosci 14(11), 1398-1405 (2011).
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57(2), 178-201 (2008).
- Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiol. Aging 30(3), 337-352 (2009).
- Wardlaw JM, Sandercock PA, Dennis MS, et al. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 34(3), 806-812 (2003).
- Alimonti A, Bocca B, Pino A, et al. Elemental profile of cerebrospinal fluid in patients with Parkinson's disease. J. Trace. Elem. Med. Biol 21(4), 234-241 (2007).
- Bakulski KM, Rozek LS, Dolinoy DC, et al. Alzheimer's disease and environmental exposure to lead: the epidemiologic evidence and potential role of epigenetics. Curr. Alzheimer. Res 9(5), 563-573 (2012).
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat. Rev. Neurosci 12(12), 723-738 (2011).
- O'Quinn MP, Palatinus JA, Harris BS, et al. A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia following ventricular injury. Circ. Res 108(6), 704-715 (2011).
- Rhett JM, Jourdan J, Gourdie RG,. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol. Biol. Cell 22(9), 1516-1528 (2011).
- Daneman R, Agalliu D, Zhou L, et al. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci 106(2), 641-646 (2009).
- Liebner, S., M. Corada, T. Bangsow, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J. Cell. Biol 183(3), 409-417 (2008).
- Brown RC, Davis TP. Calcium modulation of adherens and tight junction function: a potential mechanism for blood-brain barrier disruption after stroke. Stroke 33(6), 1706-1711 (2002).
- Naus CC, Bechberger JF, Zhang Y, et al. Altered gap junctional communication, intercellular signaling, and growth in cultured astrocytes deficient in connexin43. J. Neurosci. Res 49(5), 528-540 (1997).
- Contreras JE, Sanchez HA, Eugenin EA, et al. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc. Natl. Acad. Sci 99(1), 495-500 (2002).
- Duvdevani R, Roof RL, Fulop Z, et al. Blood-brain barrier breakdown and edema formation following frontal cortical contusion: does hormonal status play a role? J. Neurotrauma 12(1), 65-75 (1995).
- Abbott, NJ, Revest PA, Romero IA. Astrocyte-endothelial interaction: physiology and pathology. Neuropathol. Appl. Neurobiol 18(5), 424-433 (1992).
- Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell. Metab 7(6), 476-484 (2008).
- Faraci FM. Reactive oxygen species: Influence on cerebral vascular tone. J. Appl. Physiol (1985). 100(2), 739-743 (2006).
- Simpson JE, Hosny O, Wharton SB, et al. Microarray RNA expression analysis of cerebral white matter lesions reveals changes in multiple functional pathways. Stroke 40(2), 369-375 (2009).
- Simpson JE, Ince PG, Haynes LJ, et al. Population variation in oxidative stress and astrocyte DNA damage in relation to Alzheimer-type pathology in the ageing brain. Neuropathol. Appl. Neurobiol 36(1), 25-40 (2010).
- Park L, Anrather J, Girouard H, et al. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J. Cereb. Blood. Flow. Metab 27(12), 1908-1918 (2007).
- Kazama K, Anrather J, Zhou P, et al. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ. Res 95(10), 1019-1026 (2004).
- Kitayama J, Faraci FM, Lentz SR, et al. Cerebral vascular dysfunction during hypercholesterolemia. Stroke 38(7), 2136-2141 (2007).
- Park L, Zhou P, Pitstick R, et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci 105(4), 1347-1352 (2008).
- Simpson JE, Fernando MS, Clark L, et al. White matter lesions in an unselected cohort of the elderly: astrocytic, microglial and oligodendrocyte precursor cell responses. Neuropathol. Appl. Neurobiol 33(4), 410-419 (2007).
- Arai K, Lo EH, Astrocytes protect oligodendrocyte precursor cells via MEK/ERK and PI3K/Akt signaling. J. Neurosci. Res 88(4), 758-763 (2010).
- Sim FJ, Zhao C, Penderis J, et al. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J. Neurosci 22(7), 2451-2459 (2002).
- Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat. Rev. Neurosci 14(5), 311-321 (2013).
- Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci 16(5), 249-263 (2015).
- Chanson M, Berclaz PY, Scerri I, et al. Regulation of gap junctional communication by a pro-inflammatory cytokine in cystic fibrosis transmembrane conductance regulator-expressing but not cystic fibrosis airway cells. Am. J. Pathol 158(5), 1775-1784 (2001).
- Cotrina ML, Kang J, Lin JH, et al. Astrocytic gap junctions remain open during ischemic conditions. J. Neurosci 18(7), 2520-2537 (1998).
- Lin JH, Weigel H, Cotrina ML, et al. Gap-junction-mediated propagation and amplification of cell injury. Nat. Neurosci 1(6), 494-500(1998).
- Baloyannis SJ, Baloyannis IS. The vascular factor in Alzheimer's disease: a study in Golgi technique and electron microscopy. J. Neurol. Sci 322(1-2), 117-121 (2012).
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog. Neurobiol 64(6), 575-611 (2001).
- Hagan NBen-Zvi A. The molecular, cellular, and morphological components of blood-brain barrier development during embryogenesis. Semin. Cell. Dev. Biol 38(1), 7-15 (2015).
- Sengillo JD, Winkler EA, Walker CT, et al. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer's disease. Brain. Pathol 23(3), 303-310 (2013).
- Montagne A, Barnes SR, Sweeney MD, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85(2), 296-302 (2015).
- Winkler EA, Sengillo JD, Sullivan JS, et al. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta. Neuropathol 125(1), 111-120 (2013).
- Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508(7494), 55-60 (2014).
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21(2), 193-215 (2011).
- Peppiatt CM, Howarth C, Mobbs P, et al. Bidirectional control of CNS capillary diameter by pericytes. Nature 443(7112), 700-704(2006).
- Sagare AP, Bell RD, Zhao Z, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat. Commun 4(1), 2932(2013).
- Schneider JA, Arvanitakis Z, Leurgans SE, et al. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann. Neurol. 66(2), 200-208(2009).
- Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J. Neurosci 29(14), 4351-4355(2009).
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends. Neurosci 30(9), 464-472(2007).
- Ohab JJ, Fleming S, Blesch A, et al. A neurovascular niche for neurogenesis after stroke. J. Neurosci 26(50), 13007-13016 (2006).
- Snapyan M, Lemasson M, Brill MS, et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J. Neurosci 29(13), 4172-4188(2009).
- Wolburg H, Noell S, Mack A, et al. Brain endothelial cells and the glio-vascular complex. Cell. Tissue. Res 335(1), 75-96(2009).
- Pang Y, Campbell L, Zheng B, et al. Lipopolysaccharide-activated microglia induce death of oligodendrocyte progenitor cells and impede their development. Neurosci 166(2), 464-475 (2010).
- Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol. Appl. Neurobiol 37(1), 56-74(2011).
- Brown WR, Moody DM, Thore CR, et al. Vascular dementia in leukoaraiosis may be a consequence of capillary loss not only in the lesions, but in normal-appearing white matter and cortex as well. J. Neurol. Sci 257(1-2), 62-66 (2007).