Research Article - (2019) Volume 9, Issue 2
The Event-Based Prospective Memory Impairment as an Early Indicator of Amnestic Mild Cognitive Impairment
- *Corresponding Author:
- Huaidong Cheng
Department of Oncology, the Second Affiliated Hospital of Anhui Medical University
Hefei, Anhui 230601, China
Abstract
Background
Amnestic Mild Cognitive Impairment (aMCI) is an early stage of Alzheimer’ disease (AD).
Memory loss is the hallmark of aMCI. In comparison to a vast literature on Retrospective
memory (RM) disorders in aMCI, relatively little is known about Prospective memory (PM) in
aMCI.
Methods
aMCI patients (n=52) and normal controls (n=52) were administered with a battery of
neuropsychological tests including prospective memory(PM) (event and time-based
prospective memory; EBPM and TBPM) and retrospective memory(RM) (Item memory and
Source memory) tasks.
Results
Compared with normal controls, aMCI group were impaired in EBPM as well as in TBPM and
RM (P<0.01; P<0.05; P<0.05) furthermore the EBPM were impaired more significantly (P<0.01).
The results show that there are significant PM and RM impairment in aMCI patients, especially
on EBPM.
Conclusion
The present study show that aMCI patients manifest PM and RM impairment, but EBPM
impairment is worse than TBPM and RM. It indicated that the EBPM impairment may be an
early behavioral marker in aMCI patients.
Keywords
Prospective memory, Item memory, Source memory, Mild cognitive impairment
Introduction
Cognitive impairment in aging remains a challenging problem [1]. A great deal of research in aging and dementia is focused on identifying subjects at the earliest stage of cognitive impairment. Memory loss as the predominant complaint strongly predicts early dementia due to probable AD (Alzheimer’s disease) [2], in those individuals the term aMCI (amnestic mild cognitive impairment) is used. Recently, the concept of MCI (mild cognitive impairment) has come to represent an intermediate stage between healthy aging and early stage of dementia, and MCI is becoming an increasingly recognized clinical entity. MCI is considered a heterogeneous syndrome with several different clinical neuropsychological profiles (e.g. amnestic single domain, amnestic multi domain, nonamnestic single domain, and non-amnestic multi domain). aMCI individuals are known to be an early stage for clinically probable Alzheimer’s disease, and most clinicians are aware of that aMCI is the threshold for the clinical diagnosis of early AD [3]. It has been well proved that PM loss is considered one of the earliest signs of AD [4], and memory impairment is one of the frequently symptoms of aMCI which is often impaired on RM (retrospective memory) examined by recall and recognition test [5,6]. In addition to memory complaints by patients with aMCI, some evidence found that among a battery of neuropsychological tests administered to persons with memory difficulty, indicators of learning over repeated trials were the best predictors of conversion to AD. Much research on aMCI has focused on RM, or recollection of past events (source and item). It has also been reported that memory impairment may be associated with normal aging [7].
In comparison to a vast literature on retrospective memory in aMCI, relatively little is known about event-based prospective memory (EBPM) and time-based prospective memory (TBPM) in aMCI. So interest has been shifted to investigating PM, which is a type of memory for intentions.
PM is a kind of memory for intention and considered to be relatively independent of retrospective memory [8]., which can be divided into EBPM and TBPM [9]. EBPM involves remembering to perform an intended action when a specific event occurs (e.g, remembering to mail a letter when you pass the post office). TBPM involves remembering to perform an intended action at a specified time (e.g., remembering to watch TV at 8 pm tonight). Previous neuropsychological research has provided the direct evidence of PM impairment in older adults [10], however there is little evidence to distinguish between PM and explicit episodic memory [11]. Recently, there are a lot of evidence supporting a distinction between PM and RM [12]. Simons et al. [13] have suggested that PM is supported by multiple component processes, which can be divided into prospective component and a retrospective component.
Although many studies have found evidence of age related decline on tests of PM [14,15], it is not sufficient to infer the presence of a differential deficit in patients with aMCI. Yet, the feature of the memory deficits found in aMCI is of both clinical and theoretical importance. With the aim of creating a test that was sensitive to different areas of memory impairment in aMCI, we designed a kind of EBPM and TBPM task at present study.
The objective of this work is to investigate different kinds of memory impairment, especially PM impairment in aMCI patients. Fifty-two aMCI patients and 52 healthy controls were administered with a battery of neuropsychological tests including PM (EBPM, TBPM) and RM (Item memory, Source memory) tasks. We aim to examine which memory problems are due to early aMCI.
Material and Methods
▪ Participants
There are two older group adults were recruited for this study, one is healthy controls, the other group is aMCI patients. General cognitive function was assessed by the Montreal Cognitive Assessment (MoCA) and Mini- Mental Status Examination (MMSE). The two groups subjects were age, and education level matched.
▪ Normal control group
Fifty-two normal controls included 36 men and 16 women (age 56–84 years) were recruited from research volunteers. Their education years was all over five years, The average level was 11.6 years, ranging from 8 to 15 years. They all have no history of psychiatric disorder, substance abuse, and other affecting cognition factors. Normal performance on the follow tests: (a) general cognitive status (MoCA and MMSE); (b) Auditory Verbal Learning Test Delayed Recall (AVLT); (c)self-reported mood (Self-Rating Depression Scale, SDS) ; (d) clinical interview (Clinical Dementia Rating, CDR).
▪ aMCI group
Fifty-two adults with a diagnosis of aMCI included 37 men and 15 women (age 56–84 years) were recruited in this study, referred to the outpatient memory clinic of the second hospital of Anhui Medical University. aMCI diagnosis according to Petersen’s criteria [15]. The assessment was performed by an experienced neuropsychiatrist who administered a structured interview to aMCI patients.
Participants were given informed consent and were excluded from the study if they had a past history of known concurrent cerebrovascular disease, stroke, hydrocephalus, alcohol abuse, head injury, Parkinson’s disease, epilepsy, major depression (excluded by Self-Rating Depression Scale) or other neurological or psychiatric illness (excluded by clinical assessment and case history), major medical illness (e.g., cancer, anemia, thyroid dysfunction), or severe visual or hearing loss.
All patients and controls underwent a standardized dementia screening, including general medical, brain MRI (magnetic resonance imaging) and neuropsychological examination. The study was approved by the Research Ethics Committee of The Second Affiliated Hospital AnHui Medical University, and all participants were asked to write the informed consent.
▪ Experimental procedure
Neuropsychological assessment: A battery of neuropsychological tests was administered to assess general cognition and memory: the MMSE and MoCA to measure general cognition, the Verbal Fluency Test (VFT) is to assess the frontal lobe functions. The VFT was to name animals or vegetables within 1 min. The total score the subject could receive on the VFT was the number of animals named and the number of vegetables named. Digit Span (DS) to measure the working memory. The Digit Span Test included the Forwards and the Backwards Digit Span Test. In the Digit Span Test, a series of lists of numbers is presented verbally to the subject. The subject is asked to recall the numbers in ascending numerical order (forward) or reverse numerical order (backward). The total performance is the numbers of lists that are correctly remembered in ascending numerical order and reverse numerical order.
Event based prospective memory task: The participant was initially instructed that they should tap the desk whenever the two words which belong to animals (target events) appeared during the subsequent tasks, and also that they should tell their telephone number after the test finished without any mention. Next the participant was given a word selection task in which the target events for the prospective task were embedded. The task consisted of 32 question cards. On each card, 12 Chinese words were printed. Ten of the 12 words belonged to one category, and the remaining two words belonged to another category. The participant was told to select the two words that belonged to a category that differed from the other 10 words. The experimenter presented each card to the participants, who were then instructed to answer verbally at their own pace. The target events for the prospective memory task occurred on the sixth, 11th, 16th, 21st, 25th, and 31st card of the word selection task. After the word selection task, the participant should remember to speak out their telephone number, and the participant was also asked to recall the animal words.
A prospective memory (EBPM) score was recorded respectively for correct response during the test. The maximum score of EBPM is 8. 1 was given for each correct response to a target event (totally 6 target events), while 0 was given for an incorrect response 2 was given for remembering to tell their telephone number after the test.
Time based prospective memory task: The participant was instructed that they should tap the desk after each 5 minutes had elapsed during the upcoming test (i.e. to remember to tap the desk exactly at the time points of 5, 10 and 15 minute respectively after the test). Participants were allowed to use a digital clock to check the time. To exclude any visible external cue, the digital clock (the clock was set to display 0 hour 0 min 0 s at the beginning) was located one meter away behind the participant’s right shoulder, and the participants might turn their head to the clock when they manage to check the time during the test. After the clock was started, the participant was shown 100 cards one by one, on each of which 12 Arabic numerals were printed. The participant was required to select the biggest and the smallest number for each card and the test is not stop until the digital clock indicated 17 minutes. The participants were expected to recall check the time and tap the desk at each time points.
A prospective memory (TBPM) score was recorded respectively for correct response. The maximum score of TBPM is 6. Each exactly punctual response at time point (from 10 seconds before to 10 seconds after the target time) was given a score of 2, and nearly punctual response (from 30 seconds before to 30 seconds after the target time) were given a score of 1. A score of 2 was given for correct recalling each time point when he tap the desk (maximum score 6).
For both groups, 15 subjects performed the event based prospective memory task first and the other 15 subjects performed the time based prospective memory task first. The stimuli were presented using a computer display, with subjects seated 100 cm away from the screen.
Item memory task: Following a practice block, participants studied two lists of words in each of sixteen study-test blocks,
A total of 32 nouns was divided into two pools which served as either target or foil items, counterbalanced across participants. Participants were instructed to memorize the nouns and the list in which they occurred for a subsequent memory test. In the item memory test phase, Participants were asked to make speeded and accurate old/new judgments to each 32 nouns. Proportions of correct recognitions of study list nouns and of false alarms to foils were used to compute item scores, reflecting the discriminability of old from new items.
Source memory task: During the study, there were eight sorts of objects including fruits or tools or furniture or animals or clothes or vehicles or writing tools and household electric appliances, two objects were selected from each sort. One was presented with word, the other was presented with picture, Participants were instructed to provide the third object which belong to the same sort, and memorize the objects names which would occur for a subsequent memory test after five minutes. In the source memory test phase, a trial consisted of the sequential presentation of 48 objects name, each displayed for 200 ms, Participants were asked to make speeded and accurate old/new judgments to each noun, if either noun had been judged old, they also had to point out which they belong to from the words or pictures or their own supply. The strength of source memory in the task was measured as the proportion of all target nouns whose source was correctly identified. The use of total old items in the denominator of this measure proportion of correct source identifications.
▪ Statistical analysis
Comparisons among aMCI and NC diagnostic groups on sociodemographic characteristics (i.e., age and educational level), global cognitive level (i.e., MMSE scores), was performed by using analysis of T-test. Comparisons among MCI and NC diagnostic groups on performance of EBPM and TBPM or Item memory and Source memory tasks were performed by using analysis of T-test, the ratio of impairment among the EBPM and TBPM or Item and Source memory on aMCI groups were performed by using Nonparametric Mann-Whitney U-test. The level of statistical significance was defined as p<0.05.
Results
▪ Background neuropsychological evaluation
In Table 1, scores on the neuropsychological tests administered to aMCI groups and healthy normal controls are presented. There is no significant difference between aMCI patients and controls on CDT or DS (p>0.05). The performance of patients with aMCI was significantly poorer than that of the control group on the MMSE or MoCA or AVLT and VFT (p<0.01).
| Items | aMCI (n=52) |
NC (n=52) |
P-values | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 73.0 | 5.8 | 71.0 | 5.3 | ns |
| Education (years) | 9.0 | 3.6 | 9.9 | 3.1 | ns |
| CDR | 0.5 | - | 0 | - | |
| MMSE | 26.5* | 1.3 | 29.2 | 0.9 | 0.000 |
| MoCA | 26 .3* | 1.4 | 28.1 | 1.2 | 0.000 |
| AVLT | 3.2* | 1.5 | 8.5 | 1.2 | 0.000 |
| VFT | 16.3* | 3.3 | 20.9 | 1.7 | 0.000 |
| CDT | 3.4 | 0.7 | 3.5 | 0.7 | ns |
| DS | 11.2 | 1.1 | 11.6 | 1.5 | ns |
| *P<0.01 ABB: aMCI: amnestic Mild Cognitive Impairment; NC: normal comparison subjects; CDR: Clinical Dementia Rating; MMSE: Mini-Mental Status Examination; MoCA: Montreal Cognitive Assessment; AVLT: Auditory Verbal Learning Test; VFT: Verbal Fluency Test; CDT: Clock Drawing Test; DS: Digit Span | |||||
Table 1: Demographic and neuropsychological data between groups.
▪ Performance of EBPM and TBPM task in aMCI and NC
In Table 2, the means and standard deviations for the performance of EBPM and TBPM are presented for the two groups. The result shows the significant difference between the performance of EBPM and TBPM in patients with aMCI and normal controls (p<0.01, p<0.05). The EBPM score of the patients with aMCI was significantly lower than that of the normal control group (t=-6.143, p<0.01). There was also a significant difference between the normal control and patients with aMCI in TBPM score (t=-2.383, p<0.05).
| PM | N | aMCI | NC | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| EBPM | 52 | 1.08** | 1.12 | 3.26 | 0.86 |
| TBPM | 52 | 4.21* | 1.24 | 5.12 | 1.06 |
| ABB: aMCI: Amnestic Mild Cognitive Impairment; NC: Normal Comparison Subjects; *P<0.05, **P<0.01 EBPM: Event-Based Prospective Memory; TBPM: Time-Based Prospective Memory |
|||||
Table 2: Performance of EBPM and TBPM task in aMCI and HC.
▪ Performance of Item and Source memory task in aMCI and NC
In Table 3, the means and standard deviations for the performance of retrospective memory task are presented for the two groups. The result shows the significant difference between the performance of item and source memory patients with aMCI and normal controls. The item and source memory score of the patients with aMCI was significantly lower than that of the normal control group (t=- 2.345, p<0.05; t=-2.419, p<0.05).
| RM | N | aMCI | NC | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| IM | 52 | 0.56* | 0.14 | 0.68 | 0.14 |
| SM | 52 | 0.53* | 0.12 | 0.66 | 0.16 |
| ABB: aMCI: Amnestic Mild Cognitive Impairment; NC: Normal Comparison Subjects; *P<0.05IM: Item Memory; SM: Source Memory | |||||
Table 3: The performance of Item and Source memory in aMCI and NC.
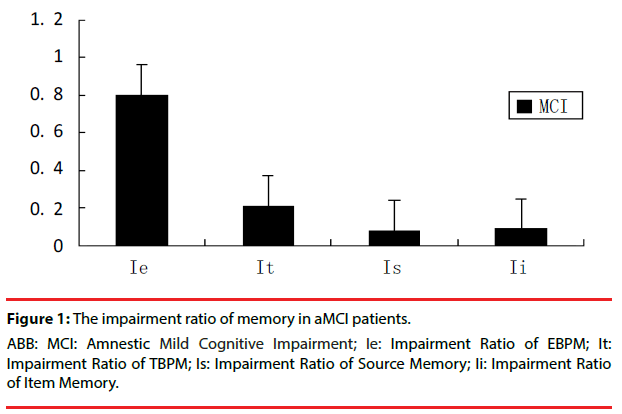
▪ Different memory impairment ratio in patients with aMCI
In order to find out the level of different memory impairment in patients with aMCI, the impairment ratio of EBPM and TBPM or Item and Source memory were compared. The impairment ratio of EBPM(Ie)=performance in EBPM of each patients with aMCI–mean performance in EBPM of NC/mean performance in EBPM of NC, The other memory impairment ratio was made out similar to EBPM. Compared with impairment ratio of TBPM (It) or Item and Source memory (Ii, Is), there was a significant (P<0.05) deference between impairment ratio of EBPM and the others by using Nonparametric Mann-Whitney U-test (Figure 1).
Discussion
The results of our study suggest that patients with aMCI (the pre-dementia stage of AD) have deficits in EBPM and TBPM, as well as in retrospective memory including source memory and item memory. The main findings are noted in present study is that the impairment ratio of EBPM in aMCI was more severe than TBPM and RM. Because evidence has suggested that there is dysfunction of entorhinal cortex in aMCI patients [16], one clear prediction was that subjects with aMCI would show a selective, or at least a disproportionate, impairment in memory, especially for episodic memory. The fact that even patients with early aMCI show substantial deficits in episodic memory is now well established3, but rare is known about PM impairment in aMCI. The result of our study suggested that patients with aMCI did indeed demonstrate a highly significant impairment in the retrospective memory including item and source memory but they also showed severe impairment in the event and time based prospective memory, especially reduced in EBPM. This indicates that EBPM impairment may be an early behavioral marker for aMCI patients.
The main advantage of early detection or prediction of progression in aMCI is the opening of a therapeutic window of opportunity to AD. Early detection of aMCI, and prediction of future AD among aMCI subjects are critical issues facing clinicians. It is likely that a combination of clinically practical memory disorders and neuroimaging approaches is required to enhance the accuracy of the prediction of progression or detection of early signs of probable AD by specialists [17]. The definition of aMCI could thus potentially be improved by adding criteria concerning the different memory impairment, a point relatively easy to assess during neuropsychological assessment using adequate memory tests including EBPM and TBPM or item and source memory test.
Although the aMCI often can progress to AD, there is heterogeneity on conversion rates to AD. One clear prediction was that subjects with aMCI would show a selective, or at least a disproportionate impairment in different kinds of memory. To find out the sensitive behavioral marker of conversion, a larger sample memory test to aging and aMCI is required, which may include a higher proportion of old adults whose memory impairment is due to early aMCI and AD. There are a lot of evidence suggested that PM disorder can occur in this group, and some previous evidence indicated that PM disorder may be an early behavioral marker of dementia [18,19]. however there was rarely little study on aMCI about EBPM and TBPM impairment. The results of present study suggest that patients with aMCI have deficits in EBPM and TBPM, especially for EBPM, suggesting that EBPM are particularly vulnerable to deterioration in early aMCI.
The advent of functional neuroimaging techniques such as fMRI has also allowed investigators to examine the functional neuroanatomy of EBPM [20]. These studies have examined changes in regional cerebral blood flow (rCBF) during EBPM test and shown obvious rCBF changes in the rostral prefrontal cortex, situated in the most anterior part of the frontal lobes (consisting of the superior frontal gyrus, part of the middle frontal gyrus and the medial frontal lobe). Okuda et al. [21] reported activation in the left frontal pole, as well as right dorsolateral and ventrolateral prefrontal cortex, when participants remembered and acted upon a list of target words relative to performing an ongoing routine activity (word repetition). Activation in the Rostral prefrontal cortex was also found by Benoit et al. [22] across several EBPM tasks. There were widely EBPM impairment in all a lot of patients such as Parkinson’s disease [23], Korsakoffs [19] and schizophrenia [24], who are all with prefrontal lobe dysfunction. All of this evidence has shown repeatedly that PFC is likely to be of central importance to EBPM.
The neural basis of the different memory disorders in aMCI and early AD remains uncertain In the resent fMRI study by Poettrich et al. patients with MCI would exhibit alterations in the neural network supporting memory as compared to elderly control subjects. Previous functional studies with fMRI have focused on the modulation of hippocampus activity in aMCI patients during experimental memory tasks. Using a memory task during fMRI scanning, Dickerson et al. showed a correlation between the extent of activation within the hippocampal formation and parahippocampal gyrus with better memory performance in aMCI patients.
There were retrospective and prospective component included in EBPM task [15], and the retrospective component of EBPM was identical to the ability that is evaluated by RM tasks. So, the EBPM impairment in aMCI patients may be related to impairment of the retrospective and prospective component. In present study, the RM impairment of aMCI has been found on item and source memory impairment, but EBPM impairment was more severe than RM impairment. So, it may be sure that the prospective component of EBPM was impaired in aMCI patients.
Conclusion
All in all, the present results suggest that PM and RM are all differentially impaired in aMCI patients. Compared with TBPM and RM, EBPM impairment was worse. According to recently study [25-27], we speculate that the brain area neuropathology of aMCI may be evolved in memory systems of the brain. EBPM disorder in aMCI patients implicates that dysfunction of a neural network in aMCI might be centered on the prefrontal cortex, as prefrontal cortex involvement in EBPM processing which had been confirmed from evidence of previous patients with lesions in the prefrontal cortex and neuroimaging studies. It indicated that memory disorders in aMCI may be associated with dysfunction of a widely network of neocortical structures, beyond medial temporal lobe and hippocampus dysfunction.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 81872504; 81372487).
References
- Pei X, Lai S, He X, et al. Mild cognitive impairment in maintenance hemodialysis patients: a cross-sectional survey and cohort study. Clin. Interv. Aging 14(1), 27-32 (2018).
- Hampstead BM, Sathian K, Bikson M, et al. Combined mnemonic strategy training and high-definition transcranial direct current stimulation for memory deficits in mild cognitive impairment. Alzheimers. Dement (NY) 3(3), 459-470 (2017).
- Kontaxopoulou D, Beratis IN, Fragkiadaki S, et al. Exploring the profile of incidental memory in patients with amnestic mild cognitive impairment and mild Alzheimer's disease. J. Alzheimers. Dis 65(2), 617-627 (2018).
- Schultz SA, Oh JM, Koscik RL, et al. Subjective memory complaints, cortical thinning, and cognitive dysfunction in middle-aged adults at risk for AD. Alzheimers. Dement (Amst) 1(1), 33-40 (2015).
- Elizabeth L, Susan RR, Davidson PSR. Source memory in older adults. J. Exp. Psychology 27(5), 1131-1146 (2001).
- Cycowicz YM, Friedman D, Snodgrass JG, et al. Recognition and source memory for pictures in children and adults. Neurosychologia 39(3), 255-267 (2001).
- Maylor EA, Smith G, Della SS, et al. Prospective and retrospective memory in normal aging and dementia: an experimental study. Mem. Cognit 30(6), 871-884 (2002).
- Graf P, Uttl B. Prospective memory: a new focus for research. Conscious. Cogn 10(4), 437-450 (2001).
- Okuda J, Fujii T, Ohtake H, et al. Differential involvement of regions of rostral prefrontal cortex (Brodmann area 10) in time-and event-based prospective memory. Int. J. Psychophysiology 64(3), 233-246 (2007).
- Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia 39(6), 545-555 (2001).
- Burgess PW, Scott SK, Frith CD. The role of the rostral frontal cortex (area 10) in prospective memory: A lateral versus medial dissociation. Neuropsychologia 41(8), 439-453(2003).
- den Ouden HEM, Frith U, Frith C, et al. Thinking about intentions. NeuroImage 28(4), 787-796 (2005).
- Simons JS, Scho¨ lvinck M, Gilbert SJ, et al. Differential components of prospective memory? Evidence from fMRI. Neuropsychologia 44(8), 1388-1397 (2006).
- Henry JD, MacLeod MS, Phillips LH, et al. A Meta-analytic review of prospective memory and aging. Psychol. Aging 19(1), 27-29 (2004).
- Salthouse TA, Berish DE, Siedlecki KL. Construct validity and age sensitivity of prospective memory. Memory. Cognit 32(7), 1133–1148 (2004).
- .Barbeau EJ, Ranjeva JP, Didic M, et al. Profile of memory impairment and gray matter loss in amnestic mild cognitive impairment. Neuropsychologia 46(4), 1009-1019 (2008).
- Duchesne S, Bocti C, De Sousa K, et al. Amnestic MCI future clinical status prediction using baseline MRI features. Neurobiol. Aging 31(9), 1606-1617 (2010).
- Huppert FA, Beardsall L. prospective memory impairment may be an early indicator of dementia.
J. Clin. Exp. Neuropsychol 15(5), 805-821 (1993). - Brunfaut E, Vanoverberghe V, d‘Ydewalle G. Prospective remembering of korsakoffs and alcoholics as a function of the prospective-memory and on-going tasks. Neuropsychologia 38(7), 975-984 (2000).
- McCauley SR, Wilde EA, Merkley TL et al. Patterns of cortical thinning in relation to event-based prospective memory performance three months after moderate to severe traumatic brain injury in children. Dev. Neuropsychol 35(3), 318-832 (2010).
- Okuda J, Fujii T, Ohtake H, et al. Differential involvement of regions of rostral prefrontal cortex (Brodmann area 10) in time- and event-based prospective memory. Int. J. Psychophysiol 64(3), 233-246 (2007).
- Benoit RG, Gilbert SJ, Frith CD, et al. Rostral prefrontal cortex and the focus of attention in prospective memory. Cereb. Cortex 22(8), 1876-1886 (2012).
- Katai S, Maruyama T, Hashimoto T, et al. Event based and time based prospective memory in Parkinson’s disease. J. Neurol. Neurosur. Psychiatry 74(6), 704-709 (2003).
- Shum D, Ungvari GS, Tang WK, et al. Performance of schizophrenia patients on time-, event-, and activity-based prospective memory tasks. Schizophr Bull 30(4), 693-701 (2004).
- Hojjati SH, Ebrahimzadeh A, Khazaee A, et al. Predicting conversion from MCI to AD by integrating rs-fMRI and structural MRI. Comput. Biol. Med 282(1), 69-80 (2018).
- Papma JM, Smits M, de Groot M, et al. The effect of hippocampal function, volume and connectivity on posterior cingulate cortex functioning during episodic memory fMRI in mild cognitive impairment. Eur. Radiol 27(9), 3716-3724 (2017).
- Cai S, Chong T, Peng Y, et al Altered functional brain networks in amnestic mild cognitive impairment: a resting-state fMRI study. Brain. Imaging. Behav 11(3), 619-631 (2017).