Review Article - (2018) Volume 8, Issue 6
The Effects of Emotional Expressions on Attention-Inhibition Processes of Depressed Patients: An ERP Systematic Review
- Corresponding Author:
- Hector WH Tsang
Department of Rehabilitation Sciences, The Hong Kong Polytechnic University, Hong Kong
Tel: +852 2766 6750
Abstract
Major Depressive Disorder (MDD) affects millions of people across the lifespan and causes tremendous burden across the globe. Abnormalities in cognitive functions, including decreased cognitive processing speed, trouble concentrating, and biases towards emotional expressions are major reasons of the debilitating effects of MDD. This systematic review aims to examine the attention-inhibition processes of MDD patients (without comorbidity) in comparison to healthy controls (HCs) based on emotional expressions, using a multistaged Information Processing Model. An online search was conducted in EBSCO, PubMed, ScienceDirect, and Wiley from inception to September 2017. Ten studies were extracted for a detailed review. Behavioural and electrophysiological results reveal that depressed patients are hypersensitive towards negative or sad emotional stimuli but hyposensitive towards positive or happy emotional stimuli. The bias is stronger in MDD when the patients are symptomatic but there is a ‘transition’ from negative emotional bias to positive emotional bias during remission. This bias is also observed at the early/perceptual processing stage, mid/ post-perceptual processing stage, and late/central processing stage. Based on the results, it is recommended that, first, future studies should examine the number of stages involved in information processing in addition to examining which stage remains unaffected by MDD. Second, studies should examine the reasons behind this negativity bias as well as the association between ERP components and daily functions among MDD patients so as to get a holistic view of their condition. Third, ERP studies can investigate in what ways antidepressants improve the information processing and negative bias in MDD patients.
Keywords
Event-Related Potential; Major Depressive Disorder (MDD); Information Processing Model; Emotional Expression; Attention-Inhibition
Introduction
Major Depressive Disorder (MDD) is one of the most prevalent mental disorders which is of public health concern [1] and it is the leading cause of disability [2] worldwide. Its symptoms cause significant clinical distress, impairment in social [3,4], occupational [5,6], or psychological functioning [7,8], leading to a loss of over $36.6 billion per year in the United States of America alone through absenteeism and presenteeism [9].
The advent of state-of-the-art technologies such as Electroencephalography (EEG) and its derivative, Event-Related Potential (ERP), has recently deepened our understanding of cognitive processes of MDD patients. ERP technology nowadays enables us to understand invisible cognitive processes in forms of different chronometric (millisecond timing) and energetically (mental effort) indices [10,11]. Based on these indices, this review tries to model ERP studies (component-based e.g. P1 and N1), following a multi-staged Information Processing Model and using Latency (ERP’s time cursor) as its marker. This will result in a better understanding of the association between behavioural and ERP perspectives and thus the stage-related cognitive processes of MDD patients.
Several studies have examined cognitive processes behaviourally [12,13] and reported that depression affects motor adjustment and response selection stages which ultimately determines reaction time but not the pre-processing stage [12,13]. Electrophysiological results on emotion perception, attention, executive function, and memory suggest that depression affects specific stages of the Information Processing Model [12,14-17]. Previous reviews among patients with depressive disorders (DD), mostly with comorbidities, provided interesting insight as to the effects of depression on patients’ cognitive processes [18-22]. However, there has not been any ERP review to date among MDD patients without comorbidities [18,19,21,22] on attention-inhibition processes. This paper systematically reviews ERP studies in order to fill the knowledge gap by specifically:
1. examining differences in attentioninhibition processes between MDD patients (without comorbidity) and matched healthy controls (HCs) based on emotional expressions, using a multi-staged Information Processing Model; and
2. Suggesting directions for future research.
Methodology
▪ Literature Search
This paper followed the guidelines of Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA; Moher, Liberati [23]. Online databases, specifically EBSCO, PubMed, ScienceDirect, and Wiley- Blackwell, were searched sequentially using the combination of the following keywords: “EEG/ ERP”, electroencephalography, “event-related potential”, “cognitive dysfunction”, cogniti*, “major depressive disorder”, and depress*. The reference lists of the selected articles were also reviewed for relevant studies. The search was limited to English studies published until September 2017.
▪ Selection Criteria
The title and abstract of the generated results were first screened using the following inclusion criteria: (i) focusing on cognitive processes of clinical MDD (i.e., all forms of MDD diagnosed by a mental health professional using DSM-IV/5, ICD-10 or accepted classification standard) patients using ERP; (ii) having at least a comparable or matched healthy control group; (iii) having a set of behavioural data; (iv) using emotional stimuli; and (v) articles published until September 2017. The exclusion criteria for this systematic review included (i) MDD patients with co-morbidities; (ii) animal studies; (iii) a non-case-controlled experimental study; and (iv) articles published in languages other than English.
▪ Quality Appraisal, Data Extraction and Synthesis
Quality appraisals were conducted by two independent reviewers (DKA, PL), using The Joanna Briggs Institute’s MAStARI critical appraisal tools for Comparable Cohort / Case- Control Studies [24]. Comparison of each of the studies was made and reconciliation held for inconsistent results between the two reviewers. Only studies with a quality score of 7 (out of 9) or above were included in this review (Table 1). A specifically designed data extraction form was used in extracting salient information from each study by two independent authors. This included but was not limited to authors’ details, aim, sample size, gender, task, diagnosis type, and results. Relevant information from the data extraction is presented in Tables 2 and 3. Quantitative analysis was not performed due to the heterogeneity of study population, and experimental tasks. Hence, the narrative summary format was used to present and summarise the data.
| Authors | Points |
|---|---|
| Bistricky, Atchley, Ingram, and O'Hare (2014) | 8 points |
| Dai and Feng (2011) | 8 points |
| Dai, Feng, and Koster (2011) | 9 points |
| Liu, Yin, Wu, and Xu (2014) | 8 points |
| Tang, Li, Wang, Li, Li, and Wang (2011) | 7 points |
| Vanderhasselt et al. (2012) | 8 points |
| Xue, Wang, Kong, and Qiu (2017) | 8 points |
| Yang, Zhu, Wang, Wu, and Yao (2011) | 9 points |
| Yao, Liu, Liu, Hu, Yi, and Huang (2010) | 8 points |
| Yu, Zhou [30] | 8 points |
Table 1: Quality Appraisal.
| Author(s) | Aim | N (MA±SD; M/F) | Task | Diagnosis/Type | Results |
|---|---|---|---|---|---|
| Bistricky, Atchley [25] | To examine whether groups with risk factors for depression would show attentional biases or inhibitory deficits related to viewing facial expressions | DfD = 14 (19.29±1.20; 7M/7F) DnD = 13 (19.62±2.47; 5M/8F) NfD = 14(21.79±7.74; 4M/10F) NnD = 14(18.86±0.86; 6M/8F) |
VOT | DSM-IV/ | Past depression was exclusively associated with greater P3 ERP amplitude following sad targets, reflecting a selective attention bias. Dysphoric individuals less effectively inhibited responses to sad distracters than non-dysphoric individuals according to behavioural data, but not psychophysiological data |
| Dai, Feng [27] | To investigate distracter inhibition ability for emotional faces in depression | MDD =17(28.24±5.29; 9M/8F) rMDD=17(27.35±6.48; 8M/9F) HC = 17(26.29±3.80; 7M/10F) |
NAP | DSM-IV/ Remitted MDD & MDD |
MDD patients have deficient distracter inhibition and excessive facilitation for negative stimuli. The RMD partients show a mixed pattern of deficient distracter inhibition and excessive facilitation for both positive and negative stimuli. |
| Tang, Li [26] | To examine specific topographic patterns of altered cortical directed connectivity | MDD = 12 (31.67±13.55; 8M/4F) HC = 13(39.54±9.65; 7M/6F) |
FITCT | ICD-10/ First & Recurrent Episode |
Behavioural responses and ERPs results showed cognitive deficits during attention modulation in depression |
| Vanderhasselt, De Raedt [29] | To investigate cognitive control over emotional stimuli | rMDD = 15 (27.87±7.91; 7M/8F) HC = 18 (27.17±10.88; 9M/9F) |
cECT | DSM-IV/ remitted MDD (rMDD) |
ERP showed no group differences in response to the cues. Remitted MDD patients had selective deficit in cognitive control over sad but not happy stimuli |
| Xue, Wang [28] | To confirm that processing of emotional conflict is impaired in treatment-resistant depression (TRD). | TRD = 17 (41.42±14.74; 5M/12F) HC = 17 (43.06±16.07; 6M/11F) |
FWST | DSM-IV | Results revealed that TRD individuals pay more attention to emotional information (larger frontal region N2 amplitude) and had higher interference as compared to HC |
| Yu, Zhou [30] | To investigate neural substrates of response inhibition to sad faces across explicit and implicit tasks in depressed female patients | MDD = 20 (30.6±9.4; 20F) HC = 21 (25.9±6.2; 21F) |
eGNGT | DSM-IV | MDD patients had decreased discrimination accuracy and amplitudes of the task indicating a selective impairment in response inhibition to sad faces compared to HCs. |
Table 2: Emotional Facial Expression.
| References | Aim | N (MA±SD; M/F) | Task | Diagnosis/Type | Results |
|---|---|---|---|---|---|
| Dai and Feng [33] | To investigate the interference inhibition for emotional words |
MDD = 17 (27.59±3.74; 8M/9F) RMDD = 17 (27.53±6.36; 6M/11F) HC = 17 (25.71±3.72; 7M/10F) |
EST | DSM-IV/ Remitted MDD & MDD |
MDD patients had higher interference effects for negative words with deficient behavioural and neurophysiological indices of attentional inhibition for negative material. Remitted MDD patients’ had no special attentional bias for emotional words in the behavioural data but they had enhanced negativity for negative words in the ERP data. |
| Liu, Yin [34] | To examine how Chinese emotional words are processed among Chinese MDD individuals | MDD = 25 (20.60±2.46; 13M/12F) HC = 25 (20.32±1.95; 14M/11F) |
CEWHT | DSM-IV | MDD patients showed lateralization of brain activity in response to emotional words, whereas healthy individuals did not show this lateralization |
| Yang, Zhu [31] | To investigate the time course of the affective processing bias | MDD = 16 (8M/8F) HC = 20 (10M/10F) |
VTSSOT | DSM-IV/ First episode |
Affective processing bias in MDD begins in the early stages of perceptual processing and continues at later cognitive stages |
| Yao, Liu [32] | To determine whether early or late stages of information processing were impaired | MDD = 18(32.5±6.4; 7M/11F) HC = 18 (32.7±6.6; 7M/11F) |
NAP | DSM-IV/ Unipolar |
MDD is associated with less effective inhibition towards negative information. MDD had an abnormally reduced P2 for negative trials compared with the controls, but not for positive trials. No difference on LPC (evaluative meaning) |
Table 3: Emotional Words Expression.
Results
▪ Description of Results
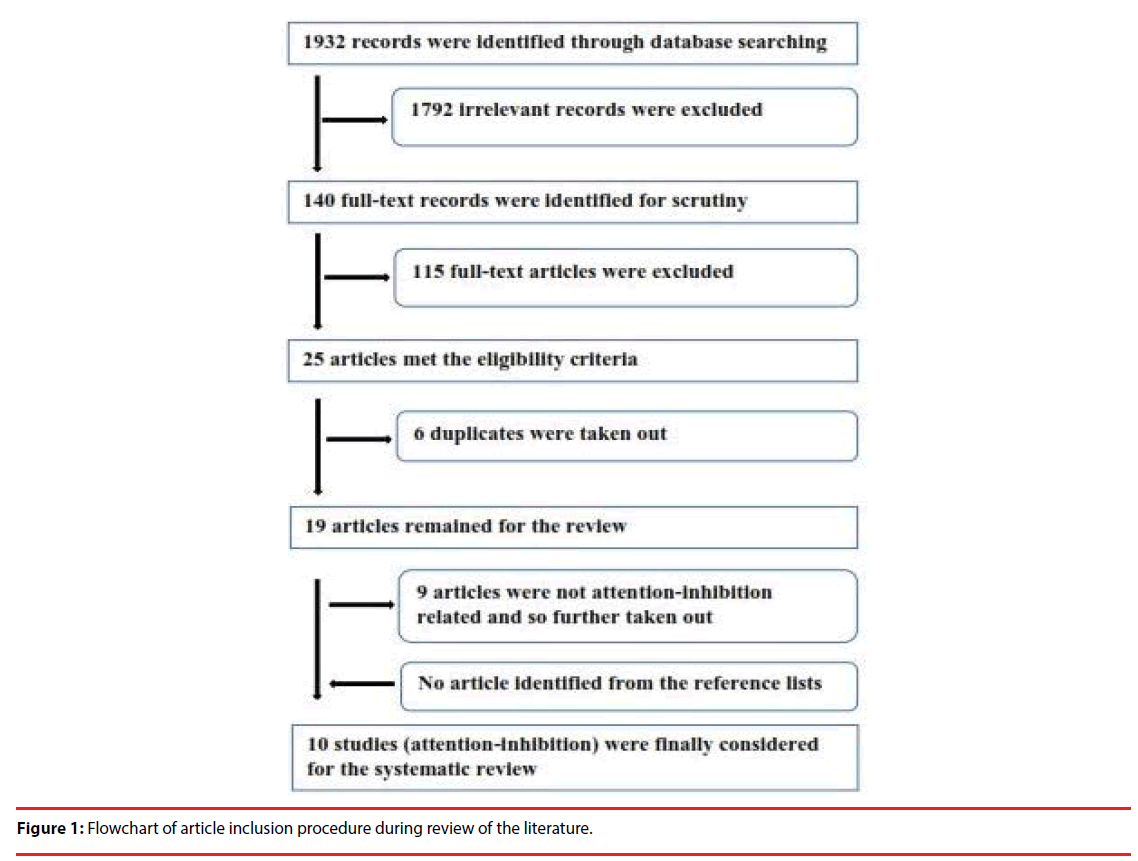
Ten studies, out of 2 674 pre-selected articles, met the inclusion-exclusion criteria. The methodological processes that led to the selection of these 10 studies are shown in Figure 1. Details of each study and its quality scores are shown in Tables 1-3. The results are broadly categorised into two groups based on whether the stimuli used were (A) Emotional Faces or (B) Emotional Words but all presented visually. For Emotional Facial Expression (A), six studies examined Attention and Inhibition (Table 2) whilst four studies examined attention and inhibition for Emotional Words Expression (B) (Table 3). The results reported in this systematic review are based on behavioural (error/ accuracy scores and reaction times—RT) and electrophysiological (amplitudes and latencies) perspectives. For the purpose of this systematic review, electrophysiological perspectives were stratified into early/perceptual (P1, N1, and N170), mid/post-perceptual (P2 and N2), and late/central (P3, Late Positive Component, and Slow wave) processing stages.
▪ Major Depressive Disorder and multistaged information processing model
▪ Emotional Facial Expression
Face-in-the-Crowd, Cued Emotional Conflict, Affective Priming, Oddball, Go/No-go, and Stroop were the tasks used for the attention and inhibition studies.
▪ Behavioural perspective
Although different tasks were used by the authors, the results between depressed patients and HCs were mostly consistent. In an oddball task by Bistricky, Atchley [25], depressed patients (dysphoric formerly depressed and dysphoric never depressed) were found to have lower accuracies as compared to HCs, although no significant difference for reaction time (RT) was found. Li [26], similarly, reported lower accuracy scores for emotional expression among MDD patients but longer RT for detecting negative face as compared to HCs. Other studies on inhibition using negative affective priming [27] and face-word Stroop task [28] have also reported interesting findings. Feng [27] reported that MDD patients had reduced inhibitory control but enhanced priming for sad faces as compared to HCs whilst remitted MDD (r-MDD) patients had inhibitory impairments for all emotional faces as compared to HCs. In the face-word Stroop task, MDD patients had lower accuracy scores but longer RTs as compared to HCs [28]. De Raedt [29] reported that r-MDD patients had weaker cognitive control for sad faces (relative to happy) as compared to HCs. In an emotional go/no-go study by Zhou [30], MDD patients were reported to have smaller effect when inhibiting inappropriate responses as well as shorter response time as compared to HCs.
▪ Electrophysiological perspective
At the early/perceptual processing stage, Feng [27], using negative affective priming, reported that MDD patients had higher P1 amplitude for sad faces in the positive priming condition as compared to r-MDD and HCs but not with N1 amplitude (at occipital area) [27]. MDD patients had lower N1 amplitude than HCs, especially at the FCz electrode site in an emotional go/no-go task [30].
The mid/post-perceptual processing stage results revealed that, in general, MDD patients had higher N2 amplitude compared to HCs [28] whilst Tang’s group observed lower N2 amplitude among MDD patients as compared to HCs when responding to neutral and happy faces but not for sad face at electrode Fz in their Face-in-the-Crowd task. MDD patients were found to have longer N2 latency in response to neutral [26] but not for happy or sad faces [26]. No significant group-emotion expression interaction effect was noticed for N2 and P2 amplitudes and N2 latency [25,29]. N2 amplitude (difference wave) was lower among MDD patients as compared to HCs, especially at FC4 and C4 electrode sites in an emotional go/no-go task [30].
At the late/central processing stage, a study which used affective priming-related task found that, comparatively, MDD patients had significantly higher and lower P3 amplitudes for sad faces in the positive and negative priming condition respectively whilst r-MDD patients had higher P3 amplitude for sad faces but lower P3 amplitudes for both happy and sad faces in the positive and negative priming condition respectively [27]. Further, MDD patients had the lowest amplitude for happy faces in the control condition as compared to HCs and r-MDD patients [27]. Similarly, MDD patients were reported to have higher P3 amplitude compared to HCs in the face-word Stroop task [28]. MDD patients were reported to have lower P3 amplitude than HCs in an emotional go/nogo task [30]. In an oddball task that examined whether abnormal selective attention and/or inhibition for emotional facial expressions exists, patients with past depression were reported to have higher P3 amplitude for sad faces as compared to HCs [25]. A study which used cued emotional conflict task reported higher N450 amplitude (increased recruitment of cognitive control) among HCs as compared to r-MDD patients [29].
▪ Emotional Words Expression
Visual Three-Stimulus Semantic Oddball, Negative Affective Priming, Chinese Emotional Word Hemifield, and Emotional Stroop were the tasks used for these attention and inhibition studies.
▪ Behavioural perspective
A study which used visual three-stimulus semantic oddball task revealed that MDD patients had significantly fewer hits, in general, as compared to HCs due to fewer hits in positive relative to negative stimuli but no betweengroup difference for RT [31]. Liu [32], using negative affective priming, reported that MDD patients, in general, had longer RT as compared to HCs but not for accuracy. MDD patients had longer RTs for both positive and negative targets [32]. In an emotional Stroop task, MDD patients were reported to have the biggest interference effects for negative words (deficient interference inhibition) as compared to HCs and r-MDD patients [33]. In a study which used emotional word hemifield task, MDD patients were reported to have longer RT than HCs [34]. Further, among MDD patients, there was shorter RT to negative words presented on the left hemisphere (LH) than positive words and even to negative words presented on the right hemisphere (RH) [34].
▪ Electrophysiological perspective
At the early/perceptual processing stage, MDD patients were reported to have significantly lower P1 (at O1 and O2 electrode sites for positive words) and N1 (at O1 and O2 electrode sites for negative words) amplitudes as compared to r-MDD patients and HCs [33]. In general, N1 latency was shortest for r-MDD as compared to MDD patients and HCs but no betweengroup difference for P1 latency [33]. Another study reported no significant between-group difference for posterior (O1/O2, P3/P4 and P7/ P8) P1 amplitude [31] but MDD patients were reported to have shorter P1 latency for negative relative to positive target stimuli over the right hemisphere as compared to HCs in their visual three-stimulus semantic oddball task [31].
For the mid/post-perceptual processing stage, a study that focused on action monitoring revealed that MDD patients had higher anterior (FZ, FCZ, CZ) N2 amplitude for negative target stimuli (but not positive target stimuli) as compared to HCs but no between-group difference in anterior (FZ, FCZ, CZ) and posterior (O1/O2, P3/P4 and P7/P8) P2 amplitudes [31]. There was no significant between-group difference for anterior and posterior P2 and anterior N2 latencies [31]. A study on affective priming reported that, in general, MDD patients had significantly lower P2 amplitude, especially for negative targets [32].
At the late/central processing stage, Zhu [31] reported that MDD patients had lower P3 amplitude (FZ, CZ, PZ, OZ) to negative relative to positive target stimuli but no significant between-group difference on P3 latency. Dai and Feng [33] also reported that MDD patients had lower P3 amplitude and shorter latency as compared to r-MDD patients and HCs. Analysis of N450 component revealed that MDD and r-MDD patients had higher amplitude for negative words at P3 and P4 electrode sites compared to HCs [33]. Liu [32] reported significantly lower Late Positive Components (LPC) amplitude in MDD patients than in HCs, although there was no significant betweengroup difference on LPC latency. Another study also confirmed the lower LPC amplitude at the frontal compared to posterior region among MDD patients whilst HCs had higher LPC amplitude in posterior regions in response to negative words [34].
Discussion
This systematic review has examined differences in the attention-inhibition processes between MDD patients (without comorbidity) and matched healthy controls (HCs) based on a multi-staged Information Processing Model using ERP with visual emotional stimuli. Emerging and interesting trends of acquisition, processing, and interpretation following the Information Processing Model are discussed in this section based on the type of emotional stimuli: (A) Emotional Facial Expression and (B) Emotional Words Expression.
▪ Emotional Facial Expression
Converging results from behavioural and electrophysiological perspectives point to general impairment in processing emotional stimuli across different tasks. A study by Bistricky, Atchley [25], for instance, used different types of depressed patients to examine selective attention as well as its trait or state effects on correlates of attention. Bistricky, Atchley [25] revealed that dysphoric never depressed patients failed to inhibit sad distracters as compared to nondysphoric never depressed patients but only at the behavioural and not electrophysiological level [35]. Nonetheless, greater attentional allocation (P3 amplitude) to sad faces (relative to happy faces) which further indicates attention bias towards sad faces was noticed among previously depressed patients (trait) [25]. This, possibly, implies a creation of neural pathway [36,37] as P3 amplitude has been known to be a marker differentiating MDDs and HCs [21]. This postulation has received preliminary support from studies that have affirmed the dysfunction of circuitry essential for mood regulation and cognitive function due to abnormal disruption of neuronal function and morphology [38,39]. This differentiates between state and trait depression and, possibly, indicates the hope of full treatment for state depression since neural pathways might not have been created or entrenched. More so, the authors hypothesized that MDD patients will have response disinhibit ion (N2 amplitude) for sad stimuli, but this was not supported [25]. The non-significant result is thought to be as a result of, comparatively, fewer trials used [25,40].
The orientation of visual attention is vital to attention bias towards emotional information. The face-in-the-crowd study by Li [26] explored this assertion and reported that MDD patients had a cognitive deficit during attention modulation as compared to HCs. Specifically, MDD patients had attenuated orientation of visual attention and cognitive control (anterior N2 amplitude) for happy faces but no betweengroup difference for the sad faces. This explains their bias towards sad facial stimuli as they have defective ability to adjust or accommodate happy facial stimuli, leading to the supposed bias against happy compared to sad facial stimuli. In addition, partial directed coherence (PDC) revealed that there was a frontal hemisphere asymmetry among MDD patients with leftfrontal hypoactivity in response to happy faces but a right-frontal hyperactivity in response to sad faces [26]. This confirms the earlier point that there is impaired attention modulation to happy facial stimuli. It can, therefore, be deduced that MDD patients develop an anomaly which makes them either hypersensitive to sad faces [25] or hyposensitive to happy faces [26].
Deficient cognitive inhibition and excessive facilitation of emotional information have also been implicated in depression. MDD patients reportedly had deficient distracter inhibition and excessive facilitation (higher P1 and P3 amplitudes) for sad facial stimuli whilst r-MDD patients showed a mixed pattern of deficient distracter inhibition and excessive facilitation for both happy and sad facial stimuli (higher N1 amplitude for happy faces and a higher P1 and P3 amplitude for sad faces) as compared to HCs [27]. This confirms the results of other studies [17,33] and indicates that r-MDD patients still have some effects of depression as well as qualities of HCs whilst MDD patients have impaired inhibition of sad information. This result is also supported in a study by Zhou [30] who reported that both behavioural and electrophysiological perspectives indicated an impaired response inhibition (lower frontal N1, N2, and P3 amplitudes) to sad faces among MDD patients as compared to HCs.
Treatment-resistant depression (TRD) patients were also found to spend longer time (longer RT), more cognitive resources and have higher interference effects (higher N2 and P3 amplitudes at the frontal region respectively) in processing the emotional information as compared to HCs [28]. This provides additional support to the result of earlier studies that there may be a disruption of neuronal function and morphology [38,39] which sometimes affect treatment. Similarly, r-MDD patients had decreased cognitive control to overcome interference (N450 amplitude) from sad but not happy faces [29]. This confirms the earlier assertion that there is impaired inhibition and facilitation among MDD patients and it is even worse for treatment-resistant patients. Nonetheless, response monitoring (conflict slow potential) was not impaired among TRD patients similar to HCs [28] as well as during effortful anticipatory processing among r-MDD (frontocentral [FCz] N2 amplitude) [29] which remain controversial even among past studies [41-44]. It should also be noted that progress on newer pharmacological agents has been shown to reverse deficits at the synapses [38,45]. More studies need to be done, especially with similar task and participants.
Evidence from both behavioural and electrophysiological perspectives of the various tasks reveals that there are two ways which depression affects patients. First, MDD patients are inhibited from attending to happy faces, making them biased towards sad faces. Second, MDD patients have enhanced facilitation for sad faces compared to happy faces, making them biased towards sad faces. Depression (currently or remitted) has debilitating effects on information processing and not merely its symptoms (state) which has inclusive results on attention bias towards sad emotion. More so, the review found that bias towards sad facial stimuli starts at the early/perceptual processing stage through mid/ post-perceptual processing stage to late/central processing stage but not during the anticipatory and response monitoring phases.
▪ Emotional Words Expression
The time course of emotional information processing between F-MDD patients and HCs using a visual three-stimulus semantic oddball indicated that processing bias begins at the early stages of perceptual processing. Although no between-group differences were noted for RT, there was, comparatively, shorter P1 latency for negative target stimuli at the right posterior regions, indicating earliest stages of preconscious processing in the right posterior region among F-MDD patients [31]. The negative bias among F-MDD patients was also evident at strategic evaluation stages of processing (anterior N2 amplitude), although there was less attribution of resources to category processing at later stages (P3 amplitude) [31]. Similarly, the study by Yao, Liu [32] revealed that MDD patients had a comparatively decreased attention allotted to negative target stimuli but increased attention for positive stimuli, indicating that negative targets stimuli occupied less attention resources due to an inhibition deficit [32] even though they were slower in processing the emotional information (longer RT). Further, MDD patients had decreased LPC amplitude for both positive and negative trials as compared to HCs [32], which further strengthens the point that inhibition deficit that starts at the early phase of information processing mostly continue through to later stages. These studies [31,32] corroborate each other in revealing the attentional biases for negative and positive emotional target stimuli among depressed patients and HCs respectively [46].
Studies which examined inhibitory functioning tasks among MDD and r-MDD patients in comparison to HCs suggested that the effect of depression on inhibition is inherent in its symptoms. MDD patients had impaired inhibition for negative words in both behavioural and electrophysiological perspectives whilst r-MDD patients had no impairment in terms of behavioural and electrophysiological perspectives except at N450 component where they had enhanced negativity for negative words at the parietal sites [33]. This implies that impairment among r-MDD patients seems to have qualities of both depressed patients and HCs, thereby confirming results of other studies that r-MDD patients are in a “transition” from depression to normalcy reflected in biases towards emotional stimuli. The Left Hemisphere preferentially processes negative emotional words with MDD patients having lower levels of activation in the central and left frontal brain regions compared to HC in response to emotional words [34]. The right posterior region was noted to play a role in emotional stimuli processing [34,46] whilst the left and right prefrontal cortex areas are specialized for negative and positive emotions respectively [34] contrary to previously reported finding [46].
Negativity bias in emotional perception starts earlier among MDD patients [31] and, depending on the task, it continues to the mid/ post-perceptual processing stage and then to late/ central processing stage [31-34]. More so, the inhibitory functioning towards emotional stimuli among r-MDD patients seems to be in a “transition” from negative to positive bias [33] with the former more prevalent during “symptomatic” clinical depression. The left and right prefrontal cortex areas have been confirmed to be specialised for negative and positive emotions respectively with impaired activation at the central and left frontal brain regions among MDD patients [34].
▪ Implications
Evidence from both emotionally laden facial and word expressions reveal that MDD patients are biased towards negative emotional stimuli. This is likely due to some kind of inhibition in the brain which does not allow the patient to attend to positive emotional stimuli. Meanwhile, we hypothesize that there will be a simultaneous effect of enhancing the attention to negative emotional stimuli. This implies that there may be some bio-psychological anomalies as revealed by MRI-related studies [38,39,47] in the information processing stream leading to the above bias. Although the study by Kennedy [48] tried to investigate neuron firing rates and emotional states in the subjects, their results raised more questions than answers. Further research needs to look at the inhibition and facilitation mechanisms within the Information Processing Framework so as to help us track and identify the exact anatomical sites of anomaly. This will also aid pharmacologists to work on new medications targeting for the exact anatomical sites. Clinicians and therapists can at the same time explore the use of innovative psychosocial therapeutic procedures that shift, alternate, and focus attention from negative to positive emotional stimuli like meditation and qigong. Lastly, community and family members should learn to use positive-related words in their communication among themselves and especially with people living with depressive disorders. This is not only for facilitating the recovery process and well-being of people living with depressive disorders but also to serve as a protective mechanism as well as promoting good living relationship among themselves.
Conclusion and Future Suggestions
This systematic review provides the most updated ERP results on the effects of emotional expressions on attention-inhibition processes of MDD (without comorbidity) patients. It was noted that negativity bias is stronger during clinical MDD when the patient is symptomatic but there seems to be a transition from negative emotional bias to positive emotional bias during remission. It was also observed that depressed patients are biased towards negative/sad emotional stimuli by being either hypersensitive towards negative/sad emotional stimuli or hyposensitive to positive/happy emotional stimuli. This bias starts at the early/perceptual processing stage, through the mid/post-perceptual processing stage to late/central processing stage. Anatomical reasons why MDD patients have negative emotional bias have been suggested by neuroimaging (e.g., fMRI) studies [38] but more ERP studies are needed to understand the reasons behind the negativity bias and why this is so. Based on the results, it is recommended that, first, future studies should examine the number of stages involved in information processing in addition to examining which stage remains unaffected by MDD without comorbidity. Second, future studies should also examine the reasons behind this negativity bias as well as the association between ERP components and daily functions among MDD patients to get a holistic view of their condition. Third, as fMRI studies have demonstrated the effects of antidepressants in many ways [38], ERP studies can investigate in what ways antidepressants improve the information processing and negative bias in MDD patients.
References
- Monroe SM, Anderson SF. Depression: The Shroud of Heterogeneity. Current. Directions. Psychological. Science 24(3), 227-231 (2015).
- World Health Organisation. Depression. (2017).
- Weightman MJ, Air TM, Baune BT. A review of the role of social cognition in major depressive disorder. Frontiers. psychiatry 5(1), 179 (2014).
- Sewall KB. Social Complexity as a Driver of Communication and Cognition. Integr. Comp. Biol 55(3), 384-395 (2015).
- Then FS1, Luck T, Luppa M, et al. Association between mental demands at work and cognitive functioning in the general population - results of the health study of the Leipzig research center for civilization diseases. J. Occup. Med. Toxicol 9, 23 (2014).
- Hunt E, Madhyastha TM. Cognitive Demands of the Workplace. J. Neuroscience. Psychology. Economics, 2012. 5(1), 18-37 (2012).
- American Psychiatric Association, D.S.M.T.F, Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. DSM-5. 2013, Arlington, VA: Arlington, VA, American Psychiatric Association, (2013).
- Schulz EP, Arora EG. Continuum: Lifelong Learning in Neurology. Depression 21(3), 756-771 (2012).
- Lépine JP, Briley M. The increasing burden of depression. Neuropsychiatr. Dis. Treat 7(1), 3-7 (2011).
- Light GA, Williams LE, Minow F, et al. Electroencephalography (EEG) and Event-Related Potentials (ERP’s) with Human Participants. Curr. Protoc. Neurosci 6(25),1-24 (2010)
- Pierson A, Ragot R, Van Hooff J, et al. Heterogeneity of information-processing alterations according to dimensions of depression: An event-related potentials study. Biological. Psychiatry 40(2), 98-115 (1996).
- Bonin-Guillaume S, Blin O, Hasbroucq T. An additive factor analysis of the effect of depression on the reaction time of old patients. Acta. Psychologica 117(1), 1-11 (2004).
- Azorin, J.M., et al. Stimulus preprocessing and response selection in depression: A reaction time study. Acta. Psychologica 89(2), 95-100 (1995).
- Mathews A, MacLeod C. Cognitive Vulnerability to Emotional Disorders. Annu. Rev. Clin. Psychol 1(1), 167-195 (2005).
- Chen J, Ma W, Zhang Y, et al. Distinct facial processing related negative cognitive bias in first-episode and recurrent major depression: evidence from the N170 ERP component. PloS. One 9(10), e109176 (2014).
- Dai Q, Feng Z, More excited for negative facial expressions in depression: evidence from an event-related potential study. Clin. Neurophysiol 123(11), 2172-2179 (2012).
- Pišljar M, Repovš G, Pirtošek Z. Cognition in late onset depression. Psychiatry. Res 210(1), 89-94 (2013).
- Delle-Vigne D, Wang W, Kornreich C, et al. Emotional facial expression processing in depression: Data from behavioral and event-related potential studies. Neurophysiol. Clin 44(2), 169-187 (2014).
- Leppänen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr. Opin. Psychiatry 19(1), 34-39 (2006).
- Samamé C. Social cognition throughout the three phases of bipolar disorder: A state-of-the-art overview. Psychiatry. Res 210(3), 1275-1286 (2013).
- Bruder GE, Kayser J, Tenke CE. Event-related brain potentials in depression: clinical, cognitive and neurophysiologic implications. Oxford. University. Press 563-592 (2012).
- Olofsson JK, Nordin S, Sequeira H, et al. Affective picture processing: an integrative review of ERP findings. Biol. Psychol 77(3), 247-265 (2008).
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med 151(4), 264 (2009).
- The Joanna Briggs Institute, Joanna Briggs Institute Reviewers’ Manual: 2014 edition. Australia: The Joanna Briggs Institute (2014).
- Bistricky SL, Atchley RA, Ingram R, et al. Biased processing of sad faces: an ERP marker candidate for depression susceptibility. Cogn. Emot 28(3), 470-492 (2014).
- Tang Y, Li Y, Wang N, et al. The altered cortical connectivity during spatial search for facial expressions in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 35(8), 1891-1900 (2011).
- Dai Q, Feng Z, Koster EH. Deficient distracter inhibition and enhanced facilitation for emotional stimuli in depression: An ERP study. Int. J. Psychophysiol 79(2), 249-258 (2011).
- Xue S. Abnormal Neural Basis of Emotional Conflict Control in Treatment-Resistant Depression: An Event-Related Potential Study. Clinical. EEG. Neurosci 48(2), 103-110 (2017).
- Vanderhasselt MA, De Raedt R, Dillon DG, et al. Decreased cognitive control in response to negative information in patients with remitted depression: an event-related potential study. J. Psychiatry. Neurosci 37(4), 250-258 (2012).
- Yu F, Zhou X, Qing W, et al. Decreased response inhibition to sad faces during explicit and implicit tasks in females with depression: Evidence from an event-related potential study. Psychiatry. Res. Neuroimaging 259(1), 42-53 (2017).
- Yang W, zhaoZhu X, WangX, et al. Time course of affective processing bias in major depression: ERP. study. Neurosci 487(3), 372-377 (2011).
- Yao S. Inhibition dysfunction in depression: Event-related potentials during negative affective priming. Psychiatry. Res. Neuroimaging 182(2), 172-179 (2010).
- Dai Q, Feng Z. Deficient interference inhibition for negative stimuli in depression: An event-related potential study. Clinic. Neurophysiol 122(1), 52-61 (2011).
- Liu H=, Yin HF, Wu DX, et al. Event-Related Potentials in Response to Emotional Words in Patients with Major Depressive Disorder and Healthy Controls. Neuropsychobiology 70(1), 36-43 (2014).
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. J. Abnorm. Psychol 116(1), 135 (2007).
- Dai Q, Feng Z. Deficient inhibition of return for emotional faces in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 33(6), 921-932 (2009).
- Steidtmann D, Ingram RE, Siegle GJ. Pupil response to negative emotional information in individuals at risk for depression. Cognition. Emotion 24(3), 480-496 (2010).
- Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med 22(3), 238-249 (2016).
- Liu W, Ge T, Leng Y, et al. The role of neural plasticity in depression: from hippocampus to prefrontal cortex. Neural. Plast 2017(1): 6871089 (2017).
- Krompinger JW, Simons RF. Electrophysiological indicators of emotion processing biases in depressed undergraduates. Biol. Psychol 81(3), 153-163 (2009).
- Olvet DM, Klein DN, Hajcak G. Depression symptom severity and error-related brain activity. Psychiatry. Res 179(1), 30-37 (2010).
- Ruchsow M, Herrnberger B, Wiesend C, et al. The effect of erroneous responses on response monitoring in patients with major depressive disorder: A study with event-related potentials. Psychophysiology 41(6), 833-840 (2004).
- Hartlage S, Alloy LB, Vázquez C, et al. Automatic and effortful processing in depression. Psychol. Bull 113(2), 247 (1993).
- Hammar Å. Automatic and effortful information processing in unipolar major depression. Scand. J. Psychol 44(5), 409-413 (2003).
- Li XL. Changed Synaptic Plasticity in Neural Circuits of Depressive-Like and Escitalopram-Treated Rats. Int. J. Neuropsychopharmacol 18(10), pyv046 (2015).
- Atchley RA. The right hemisphere's contribution to emotional word processing in currently depressed, remitted depressed, and never-depressed individuals. J. Neurolinguistics 20(2), 145-160 (2007).
- Karki P, Smith K, Johnson JJr, et al. Astrocyte-derived growth factors and estrogen neuroprotection: Role of transforming growth factor-α in estrogen-induced upregulation of glutamate transporters in astrocytes. Mol. Cell. Endocrinol 389(1), 58-64 (2014).
- Kennedy P. Changes in emotional state modulate neuronal firing rates of human speech motor cortex: A case study in long-term recording. Neurocase 17(5), 381-393 (2011).