Research Article - Neuropsychiatry (2017) Volume 7, Issue 4
The effect of rehabilitation on the development of dementia in Parkinsons disease
- Corresponding Author:
- Ching-Ju Chiu, PhD
Institute of Gerontology, National Cheng Kung University, No. 1, University Road, Tainan, Taiwan.
Tel: +886-6-2353535 ext.5739
Fax: +886-6-3028175
Abstract
Objective:
This study investigate the influence of rehabilitation on prevent dementia in Parkinson’s disease patients.
Methods:
This was a retrospective cohort study analyzing data from the National Health Insurance Research Database in Taiwan between January 1, 1997, and December 31, 2007. Patients with newly onset Parkinson’s disease initially receiving any anti-parkinsonism drug were followed up to evaluate the effect of rehabilitation on Parkinson disease dementia. Cox proportional hazard models were employed to evaluate the relationship between cumulative times of rehabilitation and the incidence of subsequent dementia in adult with Parkinson’s disease, adjusting for age, sex, cerebral vascular accident, hypertension, diabetes, dyslipidemia, mood disorders, drug use and comorbidity.
Results:
Each rehabilitation program is able to significantly decrease the incidence of dementia 0.8% in Parkinson’s disease patients (p<0.0001). Other risk factors of dementia in Parkinson’s disease include age, hypertension, stroke, mood disorder and diabetes.
Conclusion:
Rehabilitation may be able to prevent the subsequent dementia in Parkinson’s disease patients. And the effect of rehabilitation in dementia prevention among Parkinson’s disease patients is dose-dependent.
Keywords
Parkinson’s disease, Dementia, Therapeutic exercise, Rehabilitation
Introduction
Parkinson’s disease is the second most common neurodegenerative disorder, after Alzheimer’s disease. The prevalence of Parkinson’s disease is approximately 0.5 to 1% among persons 65 to 69 years of age, rising to 1 to 3% among persons 80 years of age and older [1]. As worldwide life expectancy has increased, the prevalence of Parkinson’s disease will increase rapidly [2]. Taiwan is one of the fastest aging countries in the world [3]. So the burden of Parkinson’s disease in Taiwan will likely continue to grow in further.
Cognitive dysfunction is common in Parkinson’s disease. Aarsland et al. reported that 24 to 31% of PD patients have dementia [4]. Dementia in Parkinson disease is associated with reduced patient and caregiver quality of life [5], reduced survival [6], and increased risks of institutionalization [7]. Unfortunately, the current treatment of Parkinson’s disease and dementia in those with Parkinson’s disease is mainly to treat motor symptom, such as tremor or bradykinesia. No drug has been shown to modify the course of the Parkinson’s disease or slow the progression of Parkinson’s disease unequivocally.
Recently, several prospective studies focused on the therapeutic effects of exercise and/or rehabilitation programs on cognitive functions or Parkinson’s disease. First, a recent metaanalysis of prospective studies confirmed the association of diminished Parkinson’s disease risk with moderate to vigorous physical activities in the preceding years [8]. The reason that exercise can reduce the risk of Parkinson disease may be due to the neuroprotective effect in the brain pathology before Parkinson’s disease clinically manifests. If the neuroprotective effect continues after the onset of Parkinson’s disease, it might also slow the progression of Parkinson’s disease. Secondarily, Tabak, et al. reported that an 8-weeks physical therapy program was associated with short-term cognitive benefits among patients with Parkinson’s disease with dementia [9]. If the rehabilitation program is also able to improve the cognitive function among Parkinson’s disease before the onset of dementia, it may delay the onset of dementia among Parkinson’s disease. These studies imply that exercise and/or the rehabilitation programs may be an opportunity to delay the onset of dementia among the Parkinson’s disease community. But there are limitations in these studies. For example, these studies followed individuals over a short-term period. They didn’t evaluate the effect of long-term rehabilitation programs on dementia in Parkinson’s disease. Nor did these studies identified the intensity of exercises and rehabilitations to gain the effects. Importantly, these findings do not provide any data on answering if physical therapy and rehabilitation program slow Parkinson’s disease progression or delay the onset of dementia among Parkinson’s disease.
To further explore this issue, we investigated the association between rehabilitation treatment and risk of dementia occurrence among Parkinson’s disease patients in Taiwan by reviewing the medical data of a sample from the National Health Insurance database, a claims database that is representative of the general population in Taiwan.
Methods
▪ Study population and design
This retrospective cohort study used the National Health Insurance Research Database (NHIRD). The National Health Insurance program covers more than 99% of the population and contracts with 97% of the hospitals and clinics in Taiwan. The National Health Research Institutes has released a sub-dataset composed of claims data for one million randomly selected insurance enrollees (approximately 5% of the entire insured population in The National Health Insurance program) to the public for research and administrative purposes. The database contains medical claims information regarding such things as ambulatory care, inpatient care, and dental services.
Patients with Parkinson’s disease were included in this study if they met fulfilled the following three criteria: (i) diagnosis of new onset of Parkinson’s disease from the year 1997 to 2005; (ii) older than 50 years of age at the onset of Parkinson’s disease; and (iii) lack of diagnosis of dementia before the onset of Parkinson’s disease. The follow-up period was from January 1, 1997 until December 31, 2007. Parkinson’s disease was defined according to ICD-9-CM code 332.0. To enhance diagnostic validity, we selected only patients who had at least three or more consistent diagnoses of Parkinson’s disease in outpatient settings or one discharge diagnosis of Parkinson’s disease in an inpatient setting, and also received single or multiple anti-parkinsonian medication (levodopa, amantadine, bromocriptine, pergolide, and selegiline). The date of the first prescription of an anti-parkinsonian medication was set as the cohort entry date.
Rehabilitations were identified according to NHI medical records. Rehabilitative programs comprised therapeutic exercise, posture, muscle strengthening, endurance, ambulation and balance training to achieve functional improvement and ambulation balance. The times of rehabilitation that each patient received are calculated and are treated as time-dependent variables.
ICD-9-CM codes 290.0, 290.1, 290.2, 290.3 and 331.0 were used to search for outpatients and inpatients with a new diagnosis of dementia during the follow-up period ending on December 31, 2007. Person-years of followup were determined by calculating the interval between the date on which Parkinson’s disease was diagnosed and whichever of the following dates was first: date of dementia diagnosis; date of withdrawal from the NHI program; date of death, or the end of 2007.
▪ Statistical analysis
Chi-square test or Student’s t test was used to compare differences in age, sex, urbanization levels of residential area and comorbidity, including diabetes, hypertension, stroke, hyperlipidemia, and incidence of dementia, between Parkinson’s disease patients with and without rehabilitation. The dementia-free survival rates were estimated using the Kaplan–Meier method between the patients with and without rehabilitation.
Time-dependent analysis was performed using the Cox proportional hazard model to investigate the time-effect of rehabilitation on reduction in dementia risk. All analyses were performed using SAS statistical software. The results were considered statistically significant when two-tailed p-values were less than 0.05.
Result
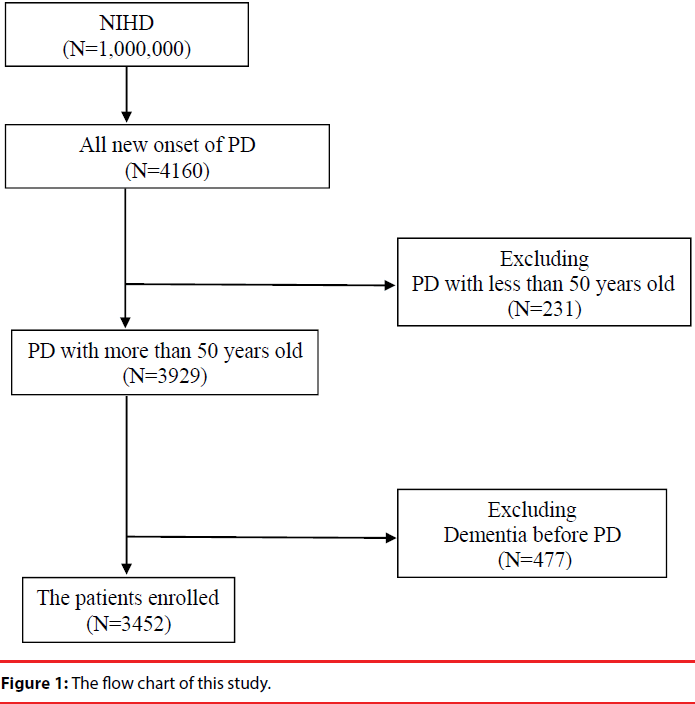
We identified 4160 patients with new onset Parkinson’s disease during the period from 1997 to 2007. A total of 3452 patients were rerolled in this study after excluding those who were less than 50 years old and those who already had dementia at the baseline (Figure 1). The mean follow up period of patients was 4.9 years. Of the 3452 patients, 1069 subjects received rehabilitation care during the follow up period and 2383 did not. Compared with patients who did not receive rehabilitations, patients who received rehabilitation services had significantly higher prevalence of hypertension (Table 1).
| Parameters | Rehabilitation | |||
|---|---|---|---|---|
| all | with | without | P value | |
| Age | 71.3 ± 8.27 | 70.9±7.93 | 71.4 ± 8.42 | 0.0919 |
| Sex | 0.2958 | |||
| Male | 1767 (51.2%) | 533 (49.9%) | 1234 (51.8%) | |
| Female | 1685 (48.8%) | 536 (50.1%) | 1149 (48.2%) | |
| HTN | 1669 (48.4%) | 485 (45.4%) | 1184 (49.7%) | 0.0190* |
| DM | 741 (21.5%) | 226 (21.1%) | 515 (21.6%) | 0.7557 |
| Mood disorder | 152 (4.4%) | 39 (3.7%) | 113 (4.7%) | 0.1476 |
| Stroke | 983 (28.5%) | 328 (30.7%) | 655 (27.5%) | 0.0543 |
| Dyslipidemia | 643 (18.6%) | 183 (17.1%) | 460 (19.3%) | 0.1274 |
| Drug type | 1.6 ± 0.87 | 1.5 ± 0.87 | 1.6 ± 0.86 | 0.2911 |
Table 1: Demographic characteristics and comorbidity medical disorders of Parkinson’s disease with and without rehabilitation.
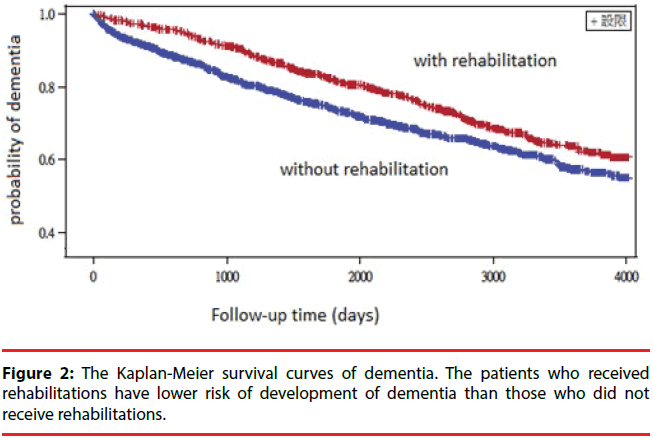
A total of 914 patients developed dementia within the follow-up period. The Kaplan–Meier log-rank test showed a significantly lower rate of development of dementia in patients with Parkinson’s dementia with rehabilitation treatment than those without rehabilitation treatment (p<0.0001) (Figure 2). We constructed a Cox proportional hazards model to adjust for possible confounding variables (Table 2). After controlling for demographic characteristics and comorbidities, Parkinson’s disease subjects who received rehabilitation treatment had a decreased risk of dementia compared to those who did not receive rehabilitation treatment (HR=0.992, 95% CI 0.996-0.988) (Table 2). In addition, age, mood disorder, stroke and diabetes were also independent predictors of dementia.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | Hazard ratio | P value | Hazard ratio | P value |
| Sex | 0.4892 | 0.7335 | ||
| Male | 1 | 1 | ||
| Female | 0.955 | 1.023 | ||
| Age (1 year) | 1.052 | <0.0001 | 1.047 | <0.0001 |
| Rehabilitation time | 0.993 | 0.0002 | 0.992 | <0.0001 |
| Hypertension | 1.484 | <0.0001 | 1.093 | 0.2634 |
| Mood disorder | 1.486 | 0.0001 | 1.641 | <0.0001 |
| Stroke | 2.003 | <0.0001 | 1.764 | <0.0001 |
| DM | 1.268 | 0.0009 | 1.178 | 0.0296 |
| Dyslipidemia | 1.034 | 0.6616 | 0.892 | 0.1612 |
| Drug type | 0.982 | 0.6229 | 1.041 | 0.2945 |
Table 2: Cox proportional hazard models measured crude and adjusted hazard ratios (HRs) of dementia by associated factors.
Discussion
Our study used a large population cohort data to investigate the association between rehabilitation treatment and risk of dementia occurrence in Parkinson’s disease patients in Taiwan. The mean follow-up period is 4.9 years. The results suggested that rehabilitation program decreased the incidence of dementia in patients with Parkinson’s disease. There is dose-dependent impact of reducing incidence of dementia in Parkinson’s disease patients who received rehabilitation, and the effect persists in the follow up period. The more rehabilitation times the patient receives, the less risk the patient develops the dementia. The other risk factor of dementia in Parkinson’s disease included age, stroke, mood disorder and diabetes.
During the following period, there were only 31% Parkinson’s disease patients receiving the rehabilitation program during the following period. Many studies demonstrate the effect of physical therapy in the motor function, ambulation balance or falling down prevention in Parkinson disease patients [10-12]. Several clinical guidelines, such as those from the United Kingdom National Institute for Health and Clinical Excellence (NICE) [13], European Federation of Neurological Societies/Movement Disorder Society–European Section (EFNS/ MDS–ES) [14] and the Royal Dutch Society of Physical Therapy [10] suggest that Parkinson disease patient should receive rehabilitation program for enhancement of aerobic capacity and improvement of functional independence. Our data showed that still more than half of Parkinson’s disease patients in Taiwan did not receive any physical therapy or evaluation of physiotherapist. The utility ratio of rehabilitation is less than other developed country [13]. Future, we should improve the availability to rehabilitation within Parkinson’s disease patients in Taiwan.
These results support previous studies of exercise-induced cognitive function improvement in people with Parkinson’s disease. Several studies demonstrate that the shortterm cognitive benefits among patients with Parkinson’s disease that had already suffered from dementia [9,15,16]. Other studies also suggested that rehabilitation programs are able to improve cognitive function in several different neurological disorder, such as stroke [17,18], Alzheimer disease [19] and traumatic brain injury [20]. Parkinson’s disease is a common neurodegenerative disease. Many animal models have revealed that exercise is able to enhance brain neuroplasticity [21] and slow the progression of Parkinson’s disease. This may be the mechanism of the effect of rehabilitation in Parkinson’s disease dementia.
This study has several limitations. First, we did not consider the severity of Parkinson’s disease due to data limitation. Previous study showed that the severity and type of motor dysfunction wound affect the incidence of dementia. But patients with severe motor dysfunction have more prevalent in the rehabilitation cohort. These patients had higher risk to develop dementia. Thus, the effect of rehabilitation may be underestimated. Secondly, Parkinson’s disease and dementia may have genetic factors. However, confounding of genetic factors was not likely to occur because there is no evidence that the genetic factors ought to differ between patients being selected to receive or not to receive rehabilitation therapy. In addition, we did not consider the difference between physiotherapists and the rehabilitation program, and it is possible that different rehabilitation programs might have different effects on patients. Future research should focus on the different effect between different rehabilitation programs. Finally, we did not evaluate the effect of drugs that may affect the cognitive function, such as benzodiazepines and antidepressants, though we did adjust for comorbid mood disorder. These patients had more prevalent to use these drugs.
In summary, the present study used a large population-based cohort data to investigate the risk of dementia for patients with Parkinson’s disease. The result indicates that rehabilitation is able to prevent development of dementia in Parkinson’s disease patients. Future study should consider the interaction between drug and rehabilitation program and the mechanism of Parkinson’s disease dementia.
Conflicts of Interest
The authors declared no conflict of interest.
References
- Nussbaum RL, Ellis CE. Alzheimer's disease and Parkinson's disease. New Eng. J. Med 348(14), 1356-1364 (2003).
- Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68(5), 384-386 (2007).
- Lin MH, Chou MY, Liang CK, et al. Population aging and its impacts: Strategies of the health-care system in Taipei. Ageing Res. Rev 9(1), S23-S27 (2010).
- Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson's disease. Mov. Disord 20(10), 1255-1263 (2005).
- Weintraub D, Moberg PJ, Duda JE, et al. Effect of Psychiatric and Other Nonmotor Symptoms on Disability in Parkinson's Disease. J. Am. Geriatr. Soc 52(5), 784-788 (2004).
- Levy G, Tang MX, Louis ED, et al. The association of incident dementia with mortality in PD. Neurology 59(11), 1708-1713 (2002).
- Aarsland D, Larsen JP, Tandberg E. Predictors of nursing home placement in Parkinson's disease: a population-based, prospective study. J. Am. Geriatr. Soc 48(8), 938-942 (2000).
- Xu Q, Park Y, Huang X, et al. Physical activities and future risk of Parkinson disease. Neurology 75(4), 341-348 (2010).
- Tabak R, Aquije g, Fisher BE. Aerobic Exercise to Improve Executive Function in Parkinson Disease: A Case Series. J. Neurol. Phys. Ther 37(2), 58-64 (2013).
- Keus SHJ, Bloem BR, Hendriks EJ, et al. Evidence-based analysis of physical therapy in Parkinson's disease with recommendations for practice and research. Mov. Disord 22(4), 451-460 (2007).
- de Goede CJ, Keus SH, Kwakkel G, et al. The effects of physical therapy in Parkinson's Disease: A research synthesis. Arch. Phys. Med. Rehabil 82(4), 509-515 (2001).
- Tomlinson CL, Herd CP, Clarke CE, et al. Physiotherapy for Parkinson's disease: a comparison of techniques. Cochrane. Database. Syst. Rev (6), CD002815 (2001).
- Tomlinson CL, Patel S, Meek C, et al. Physiotherapy intervention in Parkinson’s disease: systematic review and meta-analysis. BMJ 345:e5004 (2012).
- Ferreira JJ, Katzenschlager R, Bloem BR, et al. Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson's disease. Eur. J. Neurol 20(1), 05-15 (2013).
- Tanaka K, Quadros AC Jr, Santos RF, et al. Benefits of physical exercise on executive functions in older people with Parkinson’s disease. Brain. Cogn 69(2), 435-441 (2009).
- Cruise KE, Bucks RS, Loftus AM, et al. Exercise and Parkinson’s: benefits for cognition and quality of life. Acta. Neurol. Scand 123(1), 13-19 (2011).
- Pyöriä O, Talvitie U, Nyrkkö H, et al. The effect of two physiotherapy approaches on physical and cognitive functions and independent coping at home in stroke rehabilitation. A preliminary follow-up study. Disabil. Rehabil 29(6), 503-511 (2007).
- Quaney BM, Boyd LA, McDowd JM, et al. Aerobic Exercise Improves Cognition and Motor Function Poststroke. Neurorehabil. Neural. Repair 23(9), 879-885 (2009).
- Farina N, Tabet N, Rusted J. Habitual physical activity (HPA) as a factor in sustained executive function in Alzheimer-type dementia: A cohort study. Arch. Gerontol. Geriatr 59(1), 91-97.
- Grealy MA, Johnson DA, Rushton SK. Improving cognitive function after brain injury: The use of exercise and virtual reality. Arch. Phys. Med. Rehabil 80(6), 661-667 (1999).
- van Praag H, Christie BR, Sejnowski TJ, et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. USA 96(23), 13427-13431 (1999).