Research Article - (2018) Volume 8, Issue 6
Significance of the Ventral Striatal-Network in Dialysis Patients: Structural Covariance Analysis and Relationship with Neurobehavioral Outcomes
- Corresponding Author:
- Chiung-Chih Chang, MD, PhD
Department of Neurology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, #123, Ta-Pei Road, Niaosung, Kaohsiung County 833, Taiwan
Tel: 886-7-7317123 Ext. 3399
Abstract
Background
Patients undergoing dialysis often experience executive dysfunctions, depressive mood and memory deficits. As the cerebral striatal network may modulate these functions, no systemic approaches have been performed.
Methods
In this study, we investigated the striatal structural covariance networks (SCNs) in 27 patients undergoing dialysis and compared with 27 age-matched controls. The SCNs were constructed using 3D T1 magnetic resonance imaging and seed-based analysis, focusing on six striatal networks. Detailed neuropsychiatric assessments and the geriatric depression scale served as the major outcome factors. The role of seed or peak cluster volumes, or a covariance strength showing controls > patients were tested for the network effects.
Result
Our results revealed a lower verbal and visual memory, slower executive speed and higher geriatric depression scale scores in the patients. The clinical correlations suggested the clinical significance of ventral inferior striatum volume in predicting depression, execution and memory domains, and ventral superior striatum volume in execution and memory domains.
Conclusion
ventral striatum-anchored SCN mediates the cortical degenerative process and modulates the emotional-executive profiles in patients undergoing dialysis.
Keywords
Dialysis, Anatomical structural covariance network, Striatal network, Ventral striatum, Network neurodegeneration
Abbreviations
SCNS, Structural Covariance Networks; GM, Gray Matter; CVVLT, Chinese Version Of The Verbal Learning Test; CVVLT-30s, 30-Second Delay; CVVLT-10m, 10-Minute Recall; CVVLT-Total, Total Correct Scores; GDS, Geriatric Depression Scale; FOV, Field Of View; MNI, Montreal Neurological Institute; VSI, Inferior Ventral Striatum; VSS, Superior Ventral Striatum; DC, Dorsal Caudate; DCP, Dorsal Caudal Putamen; DRP, Dorsal Rostral Putamen; VRP, Ventral Rostral Putamen
Introduction
Patients with end-stage renal disease (ESRD) undergoing dialysis are at higher risks of cognitive impairment and dementia [1,2]. Based on the literature review, the risk factors for predicting cognitive dysfunctions [3] include advanced aging, low hemoglobin level, malnutrition, low or high body mass index, dialysis adequacy, primary etiology for dialysis [4] and low educational level [5]. Aside from these systemic factors, patients with end-stage renal disease often encountered stroke, syncope or cardiovascular events that may lead to dementia [6]. A portion of ESRD patients with dementia may experience slowly progressive cognitive decline but the intra-cerebral network alterations were not fully established.
Changes in dopaminergic integrity within the fronto-striatal circuits have been associated with motor deficits, parkinsonism, executive dysfunctions and depression [7]. Similarly, these neurobehavioral presentations have been demonstrated in the literature of ESRD patients [3,8,9]. Evidence linking uremic toxins and brain dopaminergic deficits [10,11] has been supported by decreased levels of the monoaminergic amino acid precursor [12-14]. In clinical presentations, the dopaminergic dysregulations have led to higher rates of restless leg syndrome [15], parkinsonian syndrome [16] and depression [17,18] in uremia patients. It will be of clinical relevance to understand whether the dopaminergic-related network may modulate the degenerative scaffold in ESRD patients.
In recent years, researchers have started to conceptualize the functional connectivity of neural circuits associated with different sub-regions of the striatum. For instance, emotion/motivation- related function has been attributed to the ventral striatum [19], and executive dysfunction has been reported in patients showing putamen or caudate damage [7,20]. In 2006, a seed-based correlation model defining six striatal sub-regions in the Talairach space was proposed [21]. This model may be ideal to elucidate the relationships between dopaminergic pathways and neurobehavioral presentations in ESRD patients showing progressive cognitive declines [21]. Recent research has also suggested that highly-related brain regions show covariance in morphometric characteristics, so called structural covariance [22]. With careful control of confounding factors [23,24] it may be possible to use the structural covariance networks (SCNs) to test the clinical significance of striatal networks in dialysis patients.
The survey of intracerebral status in ESRD patients can be complex as the physical intactness, biological risk factors, treatment procedure and the uremic toxin may modulate the cognitive outcomes. The ideal model as how to control these factors may depend on the study purposes. The main purpose of this study is to understand whether the striatal network may be a target network of involvement in patients with ESRD. In such context, the study population included patients with ESRD under dialysis and a clinical stage of mild dementia. If the striatal network was involved in the degenerative brain network in ESRD, we hypothesized that the peak voxels of degeneration may explain the cognitive performance per se.
Based on the biological properties and a literature review, we hypothesized that selective striatal SCNs may be involved in modulating the executive and emotional changes in ESRD patients. We compared the gray matter (GM) atrophy topography and the striatal SCN patterns between the patients and controls group, explored whether the striatal SCN may predict the degenerative processes and correlated with the neurobehavioral outcomes.
Materials and Methods
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Chang Gung Memorial Hospital. All of the study participants were referred to the Cognition and Aging Center, Department of General Neurology, Kaohsiung Chang Gung Memorial Hospital for neurobehavioral evaluations. Adults older than 20 years of age were eligible for the study if they: (1) had ESRD and had been treated with outpatient dialysis for at least 90 days; (2) had a stable physical status as confirmed by the treating physicians; (3) had progressive neurobehavioral complaints; (4) were able to provide written informed consent, and (5) were accompanied by a reliable caregiver who could clearly delineate the patient’s daily functions. The rationale for setting an interval of 90-day criteria at outpatient dialysis clinics was to ensure that the patient had steady physical intactness so that he cognitive results and neuroimaging status were adequately measured and also reflect the steady status of everyday cognition.
The diagnosis of neurobehavioral deficits in the patients included either the presence of cognitive dysfunction or depressive state. The cognitive dysfunctions were confirmed by a one-hour interview with a behavioral neurologist and a clinical dementia rating score of 0.5 or more [25]. The clinical diagnosis of depression was made after completing a half-hour structured interview according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria [26]. The exclusion criteria were a past history of clinical stroke, a modified Hachinski ischemic score > 4 and the presence of territorial infarctions, micro-bleeds or high intensity due to calcification in brain magnetic resonance imaging.
After screening according to the inclusion and exclusion criteria, a total of 27 patients completed the study protocol (15 males, 12 females), including 19 on hemodialysis and eight on peritoneal dialysis. The study also included 27 age- and gender-matched cognitively normal controls for comparisons.
Study Working Scheme
The working scheme was as follows. First, we compared neuropsychiatric data between the two diagnostic groups (patients and controls). Next, the striatal SCN was established by seed-based correlation analysis [21]. Differences in each seed regional volume were compared between the two diagnostic groups and also correlated with the neuropsychiatric data. In order to evaluate the protective effect mediated by dopamine, only the covariance strength showing controls > patients was considered to be statistically significant. The significant peak clusters were selected and the volumes were also correlated with the cognitive test scores to evaluate the clinical relevance.
Neurobehavioral Data
After enrolment, demographic data were recorded and neuropsychiatric tests [27] were performed to assess memory, language and executive function. Verbal and non-verbal episodic memory was assessed using a Chinese version of the Verbal Learning Test (CVVLT) [28] and the Rey-Osterrieth Complex Figure Test after a 10-minute delay. The CVVLT consisted of four registration trials (T1 to T4), 30-second delay (CVVLT-30s) and 10-minute recall (CVVLT- 10m). Total correct scores (CVVLT-total) from T1 to T4 were also calculated. The Verbal Fluency Test and 16-item Boston Naming Test were used to test language ability. Specific tests to analyze executive function including backwardspan, design fluency, Stroop Interference, and Modified Trails B tests were also performed. The 15-item Geriatric Depression Scale (GDS) was used to grade the severity of depression (Spreen and Strauss, NY: Oxford University Press).
Image Acquisition
Magnetic resonance images were acquired using a 3.0T MRI scanner (Excite, GE Medical Systems, Milwaukee, WI, USA). The clinical protocols of an axial fast spin-echo T2-weighted image (4200 ms/102 ms/2[TR/TE/NEX]; field of view (FOV), 240 mm × 240 mm; matrix, 320 × 224; and section thickness, 5 mm) were used to exclude intracerebral lesions. Structural images were acquired to construct the SCNs using the following protocols: a T1-weighted, inversionrecovery- prepared, three-dimensional, gradientrecalled acquisition in a steady-state sequence with a repetition time/echo time/inversion time of 8,600 ms/minimal/450 ms, a 256 × 256 mm field of view, and a 1-mm slice sagittal thickness with a resolution of 0.5 × 0.5 × 1 mm3.
Data Analysis for Neuroimaging Biomarkers
Image preprocessing and statistical analysis were performed using SPM 8 (SPM8, Wellcome Trust Centre of Cognitive Neurology, University College London, UK, http://www.fil.ion.ucl. ac.uk/spm/). The T1 images were reoriented, realigned, and normalized using the standard Montreal Neurological Institute (MNI) space. The images were then segmented into GM and white matter. Related tissue segments were used to create a custom template using diffeomorphic anatomical registration using an exponentiated lie algebra approach. The modulated and warped images were then smoothed using a Gaussian kernel of 8 mm full width at half maximum.
Images Analysis
Using voxel-based morphometry, [29] direct comparisons between the GM volume of the patients and controls were performed. T contrasts were used to identify voxels that showed between-group significance. The threshold was set at p < 0.01, corrected for a false discovery rate and a cluster size > 100 voxels.
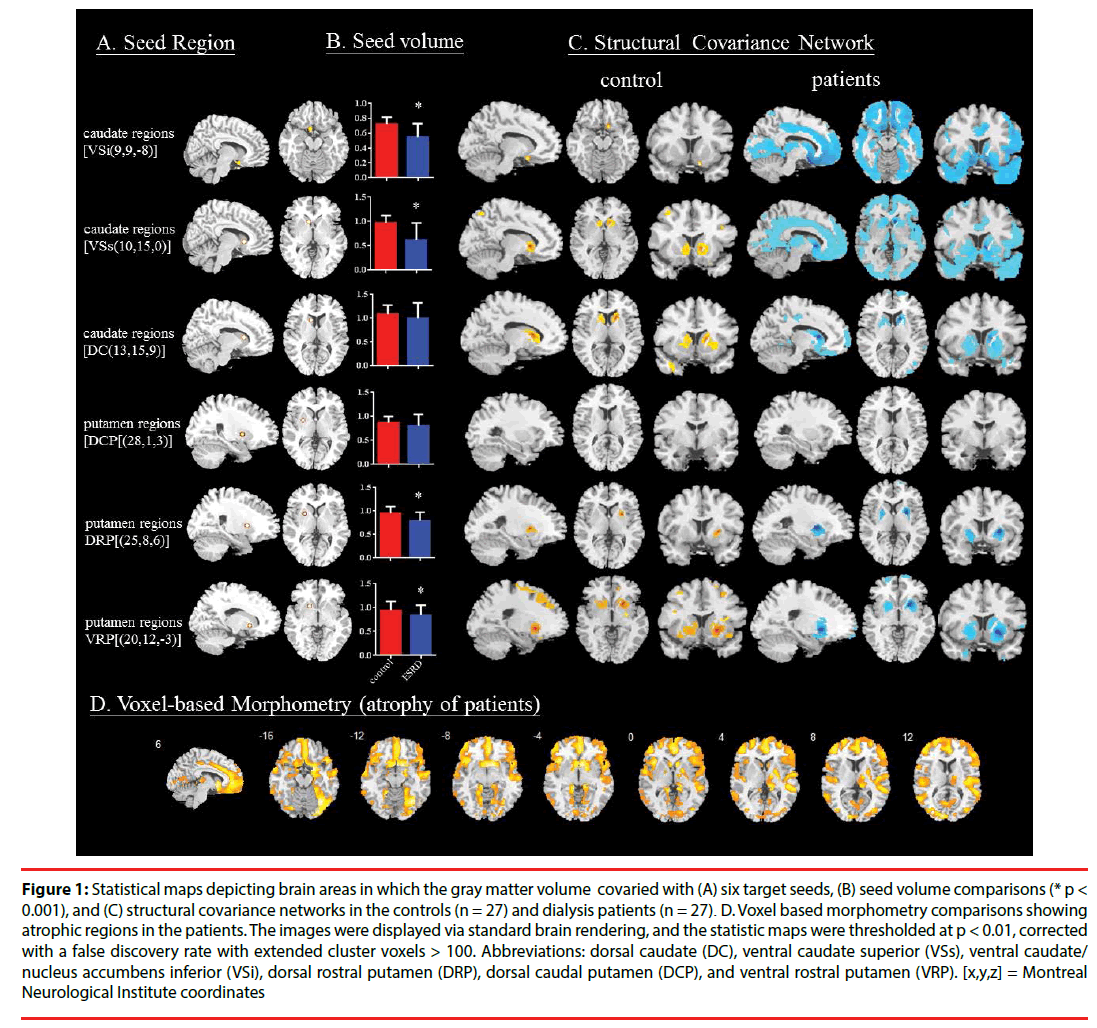
To investigate the SCN patterns, six regions of interest (Postuma and Dagher, 2006), representing seeds, were selected from the 54 preprocessed images. The seeds and MNI coordinates were as follows (Figure 1A): inferior ventral striatum (VSi, coordinates: [9,9,-8], superior ventral striatum [VSs, coordinates: 10,15,0]; dorsal caudate [DC, coordinates: 13,15,9]; dorsal caudal putamen [DCP; coordinates: 28,1,3]; dorsal rostral putamen [DRP; coordinates: 25,8,6] and ventral rostral putamen [VRP, coordinates: 20,12,-3]).
Figure 1: Statistical maps depicting brain areas in which the gray matter volume covaried with (A) six target seeds, (B) seed volume comparisons (* p < 0.001), and (C) structural covariance networks in the controls (n = 27) and dialysis patients (n = 27). D. Voxel based morphometry comparisons showing atrophic regions in the patients. The images were displayed via standard brain rendering, and the statistic maps were thresholded at p < 0.01, corrected with a false discovery rate with extended cluster voxels > 100. Abbreviations: dorsal caudate (DC), ventral caudate superior (VSs), ventral caudate/ nucleus accumbens inferior (VSi), dorsal rostral putamen (DRP), dorsal caudal putamen (DCP), and ventral rostral putamen (VRP). [x,y,z] = Montreal Neurological Institute coordinates
From the modified GM images, the GM volumes of a 4-mm radius sphere around the seed coordinates were calculated, followed by six separate correlation analyses using the extracted GM volumes as the covariates of interest, to form the SCN. The two diagnostic groups were modeled separately. Specific T contrasts were used to identify voxels that showed positive correlations for each seed. The results reflected the SCNs anchored by each seed. The threshold was set at p < 0.01, corrected for a false discovery rate with a cluster size > 100 voxels.
In addition, to investigate whether the diagnosis interfered with SCN covariance strength, voxels showing significant differences in the regression slopes in each seed-peak cluster correlation were compared that pointed to interactions between controls > patients. Specific T contrasts were established to map the voxels that expressed significant between-diagnosis associations.
For the peak clusters showing significant between-group differences, a 4-mm radius sphere was placed on the peak voxel, and the GM volumes were then calculated. To evaluate the clinical significance of the seed or the identified peak voxel, we used correlation analysis with the neuropsychiatric data or GDS scores as outcome measures. The threshold was set at p < 0.05 with multiple corrections.
Statistical Analysis
Clinical data were expressed as mean ± standard deviation. The Student’s t test was used to compare continuous variables, and chi-square test for categorical variables. Spearman’s correlation analysis was used to analyze the seed or cluster volume to predict the cognitive or GDS score. Specific to the memory domain, we calculated the retention rate in the Verbal Memory Test [28]. All statistical analyses were conducted using SPSS software (SPSS version 20.0 for Windows®, SPSS Inc., Chicago, IL). Statistical significance was set at p <0.05.
Results
▪ Demographic data and cognitive data
There were no significant differences in gender, age and educational level between the patients and controls (Table 1). The neuropsychiatric results suggested that the patients had significantly lower verbal memory scores at the end of the learning curve and shorter and longer delay recall than the controls. However, differences in the retention rates of CVVLT (CVVLT-30s/CVVLT-T4 [controls 0.98 ± 0.15; patients 0.85 ± 0.32; p = 0.08]; and CVVLT-10m/ CVVLT-T4 [controls 0.93 ± 0.17; patients 0.78 ± 0.36; p = 0.078]) were not significant. The patients also had lower visual memory scores and required a longer time to complete the Modified Trails B test. There were no significant differences in speech and language ability between the patients and controls.
| Group | Control (n=27) | Dialysis (n=27) | P value |
|---|---|---|---|
| Age | 64.3(4.1) | 64.2(9.5) | 0.94 |
| Education (year) | 10.6(4.3) | 9.6(4.6) | 0.39 |
| Sex (male/female) | 15/12 | 15/12 | 1 |
| Chinese Version Verbal Learning Test | |||
| Total CVLT (T1 to T4) | 26.0(5.0) | 22.4(6.6) | 0.04 |
| T1 | 4.8(2.0) | 4.1(1.5) | 0.21 |
| T2 | 6.3(1.5) | 5.4(2.1) | 0.07 |
| T3 | 7.2(1.2) | 6.4(1.7) | 0.06 |
| T4 | 7.7(1.1) | 6.6(1.9) | 0.01 |
| 30 s free recall | 7.5(1.3) | 5.8(2.7) | 0.01 |
| 10 min free recall | 7.2(1.5) | 5.4(2.9) | 0.01 |
| Visual memory | |||
| Modified Rey–Osterrieth recall (17) | 11.6(3.1) | 7.9(5.5) | <0.01 |
| Visual function | |||
| Modified Rey–Osterrieth copy (17) | 16.8(0.7) | 15.7(3.0) | 0.06 |
| Visual object and space perception(10) | 8.0(2.1) | 6.7(2.8) | 0.08 |
| Speech and language ability | |||
| Semantic fluency (Fruit) | 13.3(3.4) | 12.2(3.8) | 0.26 |
| Boston Naming Test (16) | 14.9(1.4) | 13.9(3.7) | 0.21 |
| Executive function | |||
| Digit backward | 4.6(1.6) | 4.0(1.7) | 0.24 |
| Stroop test (1 min) | 36.5(10.0) | 31.5(17.7) | 0.22 |
| Design fluency | 6.4(3.1) | 5.3(2.7) | 0.17 |
| Modified Trails B test time | 49.0(29.6) | 76.7(40.7) | 0.01 |
| Correct line in Modified Trails B (14) | 13.70(13.36) | 10.2(4.6) | 0.23 |
| Geriatric depression scale | 1.63 (1.9) | 4.83 (4.09) | 0.001 |
Table 1: Neuropsychiatric data between two groups.
A direct comparison between all test scores between the hemodialysis and peritoneal dialysis showed no significant differences. Aetiologies of ESRD included type 2 diabetes (n=14), use of nephrotoxic medication (n=8), malignancy hypertension (n=4) and primary kidney disease (n=1). The hemodialysis adequacy using Kt/V and urea reduction ratio suggested adequate dialysis in the patient group (Kt/V; 1.82 ± 0.29, reference range >1.2; Urea reduction ratio 0.77 ± 0.05, reference range >65%).
▪ Patterns of Striatal SCNs
In the striatal model, the VSi, VSs, VRP and DRP seeds had a significantly smaller volume in the patients than in the controls (all p < 0.001; (Figure 1B)). The SCN patterns and related clusters in each diagnosis are shown in Figure 1C and Supplementary Tables 1-12 (Figure 1C) (Supplementary Table 1-12). Based on the equal size of each group the patients had larger SCN topography, and qualitative differences were most evident in the ventral caudate-connected network. A direct comparison of voxel-based morphometry suggested diffuse GM atrophy in the patients (Figure 1D, Supplementary Table 13). The topography was highly coherent with the network interconnected with the VSi or VSs-anchored SCNs (Figure 1C). Meanwhile, striatal atrophy (Figure 1D) was mainly located symmetrically in the ventral striatum and caudate head.
▪ Peak clusters showing significant covariance strength interactions between groups
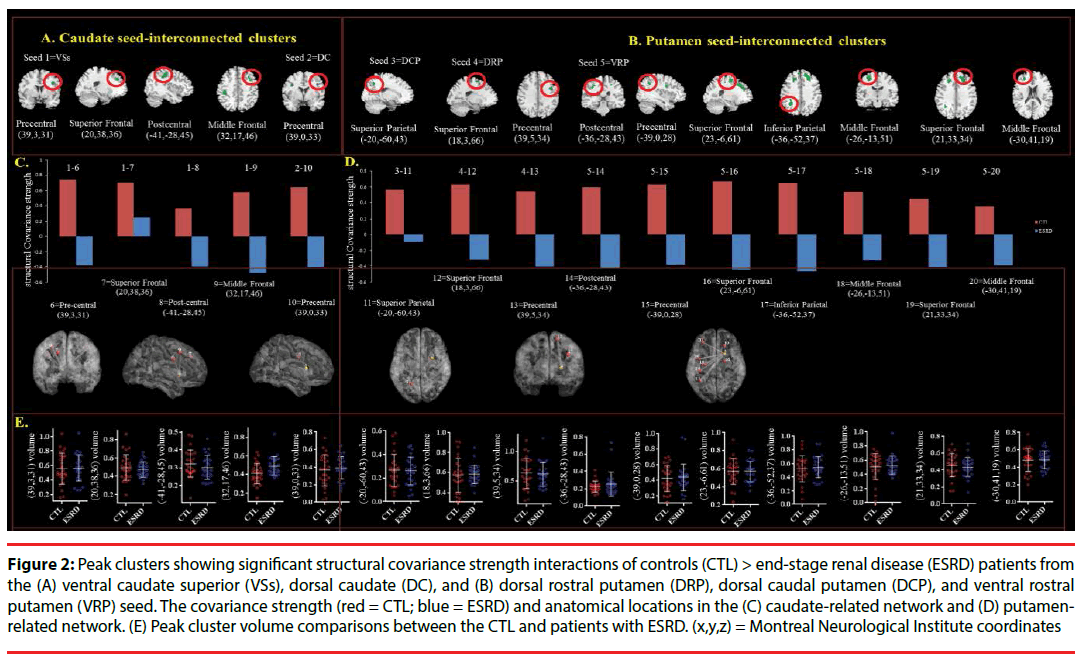
For each seed, we explored diagnostic groupinteractions with regards to the peak cluster topography showing smaller structural covariance strength in the patients ((Figure 2) (Table 2)). A total of 15 peak clusters were significant (Figure 2A: VSs and DC seeds; Figure 2B: DCP, DRP, VRP seeds) (Figure 2A) (Figure 2B). The covariance strength and anatomical regions are shown in Figure 2C and 2D (Figure 2C) (Figure 2D). However, there were no significant differences in peak cluster volumes between the patients and controls (Figure 2E).
Figure 2: Peak clusters showing significant structural covariance strength interactions of controls (CTL) > end-stage renal disease (ESRD) patients from the (A) ventral caudate superior (VSs), dorsal caudate (DC), and (B) dorsal rostral putamen (DRP), dorsal caudal putamen (DCP), and ventral rostral putamen (VRP) seed. The covariance strength (red = CTL; blue = ESRD) and anatomical locations in the (C) caudate-related network and (D) putamenrelated network. (E) Peak cluster volume comparisons between the CTL and patients with ESRD. (x,y,z) = Montreal Neurological Institute coordinates
| Seed Regions | Peak regions | Stereotaxic coordinates | Extent | MaxT | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||||
| Superior Ventral Striatum | Precentral | 39 | 3 | 31 | 355 | 5.15 | <0.001 | |||
| Superior Frontal | 20 | 38 | 36 | 155 | 4.83 | <0.001 | ||||
| Postcentral | -41 | -28 | 45 | 682 | 4.52 | <0.001 | ||||
| Middle Frontal | 32 | 17 | 46 | 186 | 4.22 | <0.001 | ||||
| Dorsal Caudate | Precentral | 39 | 0 | 33 | 118 | 4.37 | <0.001 | |||
| Dorsal Caudal Putamen | Superior Parietal | -20 | -60 | 43 | 149 | 4.23 | <0.001 | |||
| Dorsal rostral putamen | Superior Frontal | 18 | 3 | 66 | 210 | 3.96 | <0.001 | |||
| Precentral | 39 | 5 | 34 | 196 | 4.31 | <0.001 | ||||
| Ventral Rostral Putamen | Postcentral | -36 | -28 | 43 | 103 | 3.51 | <0.001 | |||
| Precentral | -39 | 0 | 28 | 110 | 3.9 | <0.001 | ||||
| Superior Frontal | 23 | -6 | 61 | 337 | 4.37 | <0.001 | ||||
| Inferior Parietal | -36 | -52 | 37 | 391 | 5.15 | <0.001 | ||||
| Middle Frontal | -26 | -13 | 51 | 480 | 5.18 | <0.001 | ||||
| Superior Frontal | 21 | 33 | 34 | 1648 | 5.46 | <0.001 | ||||
| Middle Frontal | -30 | 41 | 19 | 1375 | 5.58 | <0.001 | ||||
Max T is the maximum T statistic for each local maximum. P<0.001with cluster size=100
Table 2: Interactions showing greater structural covariance strength in the controls (compared with dialysis group).
▪ Relationships between seed volume and cognitive score
We explored whether the seed volumes were related to the neuropsychiatric test scores in each group (Table 3). Only domains showing significantly lower scores in the patients were selected (Table 1). The VSi volume predicted all of the test scores, while the VSs and DC seeds also showed significance in the patients. Paradoxically, an inverse relationship was found in the DCP between the volume and verbal memory scores in the controls.
| Anatomy | Caudate | Putamen | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seed | Ventral inferior | Ventral superior | Dorsal | Dorsal caudal | Dorsal rostral | Ventral rostral | ||||||
| Diagnostic groups | Control | Patients | Control | Patient | Control | Patients | Control | Patient | Control | Patient | Control | Patient |
| 30 second delay | 0.261 | 0.571 | 0.149 | 0.443 | -0.298 | 0.157 | -0.295 | 0.062 | -0.111 | 0.280 | 0.119 | 0.083 |
| 0.189 | 0.004 | 0.459 | 0.034 | 0.131 | 0.474 | 0.135 | 0.778 | 0.582 | 0.196 | 0.553 | 0.707 | |
| 10 minute delay | 0.161 | 0.538 | -0.031 | 0.644 | -0.231 | 0.375 | -0.395 | -0.157 | -0.283 | 0.086 | 0.061 | 0.265 |
| 0.421 | 0.008 | 0.879 | 0.001 | 0.246 | 0.078 | 0.041 | 0.473 | 0.153 | 0.697 | 0.761 | 0.222 | |
| Visual memory | -0.493 | 0.485 | 0.228 | 0.608 | 0.225 | 0.425 | -0.110 | -0.037 | 0.190 | 0.085 | 0.376 | 0.337 |
| 0.009 | 0.019 | 0.253 | 0.002 | 0.258 | 0.043 | 0.585 | 0.866 | 0.342 | 0.699 | 0.053 | 0.115 | |
| Trail making test time (second) | -0.290 | -0.680 | -0.081 | -0.655 | -0.046 | -0.451 | -0.160 | 0.090 | 0.142 | -0.241 | 0.121 | -0.369 |
| 0.143 | <0.0001 | 0.688 | 0.001 | 0.819 | 0.031 | 0.426 | 0.682 | 0.481 | 0.267 | 0.547 | 0.083 | |
| Geriatric depression scale |
-0.57 | -0.510 | 0.048 | -0.352 | -0.02 | 0.25 | -0.007 | -0.211 | 0.177 | -0.288 | 0.303 | -0.388 |
| 0.777 | 0.013 | 0.811 | 0.100 | 0.922 | 0.91 | 0.971 | 0.334 | 0.377 | 0.182 | 0.125 | 0.68 | |
Table 3: Correlations between test scores and seed volumes in the striatal model.
▪ Clinical significance of peak clusters showing diagnostic-group interactions
In the patients, the clinical significance of the aforementioned 15 peak clusters showing diagnostic-group interactions (Figure 2) were also evaluated using correlation analysis with cognitive tests (Table 4). None of the peak cluster volumes predicted the neurobehavior data.
| MNI coordinate | 30 second delay | 10 minute delay | Visual Memory | Trail Making test time | Geriatric depression scale | |
|---|---|---|---|---|---|---|
| Caudate-connected peak clusters | ||||||
| Precentral | (39,3,31) | 0.115 | 0.130 | -0.017 | 0.203 | 0.043 |
| Superior frontal | (20,38,36) | 0.273 | 0.285 | 0.376 | -0.300 | -0.388 |
| Post-central | (-41,-28,45) | -0.362 | -0.398 | -0.293 | 0.148 | 0.243 |
| Middle Frontal | (32,17,46) | 0.064 | -0.141 | -0.204 | 0.299 | 0.058 |
| Precentral | (39,0,33) | 0.045 | -0.043 | -0.052 | 0.177 | 0.061 |
| Putamen connected peak clusters | ||||||
| Superior parietal | (-20,-60,43) | -0.261 | -0.369 | -0.400 | 0.236 | 0.212 |
| Superior Frontal | (18,3,66) | 0.013 | 0.104 | 0.410 | -0.393 | -0.083 |
| Precentral | (39,5,34) | 0.257 | 0.186 | 0.154 | 0.088 | 0.026 |
| Postcentral | (-36,-28,43) | -0.226 | -0.295 | -0.272 | 0.261 | 0.156 |
| Precentral | (-39,0,28) | 0.002 | -0.062 | -0.099 | 0.174 | 0.062 |
| Superior Frontal | (23,-6,61) | 0.054 | -0.048 | 0.144 | -0.108 | -0.001 |
| Inferior Parietal | (-36,-28,43) | -0.226 | -0.495 | -0.272 | 0.261 | 0.017 |
| Middle Frontal | (-26,-13,51) | 0.077 | 0.062 | 0.203 | -0.143 | -0.210 |
| Superior Frontal | (21,33,34) | 0.127 | -0.006 | -0.017 | 0.042 | 0.053 |
| Middle Frontal | (-30,41,19) | -0.203 | -0.223 | -0.323 | 0.261 | 0.03 |
Table 4: Correlations between test scores and seed volumes in the striatal model.
Discussion
This study provides evidence that the striatal networks were involved in the degenerative process in ESRD patients with dialysis. From the clinical perspective, the neuropsychiatric comparisons showed that the verbal episodic memory dysfunction in the dialysis patients were related to the memory registration processes. The patients also showed a lower visual memory recall, longer time to complete the Trail making test and higher GDS scores and the treatment itself was not associated with the cognitive deficits. From the striatal seed analysis, the VSi and VSs volumes are smaller in the patients. The correlation analysis supported the clinical significance of the caudate regions, especially the VSi. Lastly, our results validated the importance of ventral striatum interconnected networks in patients with dialysis. The lower covariance strength in the patients suggested the GM degenerative patterns may be mediated in part by the striatal seed-interconnected circuit.
▪ The brain network affected most by dialysis was the ventral-striatal degenerative network
The functional studies have shown that structural covariance analysis is a particularly suitable technique to detect structural manifestations of persistent functional and trophic cross talk in different brain networks [30-33]. Based on the spatial distribution, the SCNs anchored by the caudate seed were significantly wider in the patients or compared with those anchored by the putamen seed. Specifically in the VSs or VSi seed, the coherent spatial distribution with the GM atrophic map (Figure 1D) suggested the importance of these two striatal hubs in mediating the cortical degenerative processes.
In human brain, the segregation of corticostriatal connections and modulation by dopaminergic projections have been confirmed by several diffusion tensor imaging studies [34,35]. Our SCN pattern results may provide evidence that the dopaminergic network serves as a predictive biomarker for clinical outcomes in ESRD patients undergoing dialysis. Interestingly, the pairwise correlations of the ventral-striatal seeds (i.e. VSs, Vsi) that determined the global GM degenerative properties suggested that pathological alterations are mediated by these two striatal hubs. In the patients, the volumes of VSs and VSi were also smaller and correlated with the neurobehavioral profiles. Whether these pathological alterations are related to the treatment or by other co-morbidities is difficult to ascertain in this study. As there were no significant differences in peak cluster volumes between the patients and control, the clinical weighting of the seed regions may outweigh the effect of the interconnected cortical hubs.
▪ Striatal network in the dialysis patients that modulated cognitive symptoms
Based on a recent meta-analysis [3], patients undergoing dialysis tend to show cognitive impairments in orientation, attention, memory and executive function compared to the general population. Our study provided possible mechanisms for verbal episodic memory deficits in these patients. The lower learning curve suggested memory deficits started from the encoding processes [36]. Aging may be a confounding factor for such encoding deficits [37], however the effect was likely to be negligible here seeing as the ages of the two groups was similar. We also excluded those showing clinical evidence of structural lesions either according to their medical history or in neuroimaging. As such, it is possible that the deficits were caused by diseaserelated functional disconnectivities in the brain. Unlike those reported in hepatic encephalopathy [38], functional connectivity abnormalities of the basal ganglia-thalamocortical circuits have not been fully explored in dialysis patients. In a review of the literature related to dialysis, we found reports of aberrant functional connectivity in the default mode network, the medial frontal lobe and thalamus [2,39-47].
▪ Depression/executive speed/memory and ventral inferior striatum in dialysis patients
The ventral striatal seeds were related to the verbal or visual episodic memory scores in our ESRD patients. A recent report suggested that ventral striatal iron accumulation is linked to demyelination and impairment in declarative memory in the elderly [48]. Patients with hemodialysis have a significantly higher incidence of iron deposition in the striatum areas [49]. Whether the changes of ventral striatum and memory deficits were related to higher portions of hemodialysis patients required further explorations. VSs volume was associated with the processing time of the Trail Making test in our dialysis patients and the role of VSs in decision making/executive control has been reported [50,51].
In addition to the aforementioned cognitive deficits, our patients also scored higher in the GDS compared to the controls. Higher GDS can be related to poor physical health, perception of not being healthy, old age [52], cognitive dysfunction [53] or emotional dysregulations [21]. The GDS scores were significantly related to the VSi volume in this study, consistent with a study positing the association between ventral striatal circuits and emotional responses [21].
▪ Changes of structural covariance strength and GM degeneration
Theoretically, structural covariance strength represents direct strengthening of a “physical” interconnection among key hubs [54]. As we only explored the covariance interactions showing controls > patients, the results may provide possible pathological mechanisms between SCNs and GM atrophy in dialysis patients. The lower covariance strength in the patients reinforced segregated and less integrated components in the brain networks. Alternatively, stronger structural covariance in the controls may reflect the modulatory role of dopaminergic transmitters that provide a protective role via offering greater regional connectivity at the network level.
Study Limitations
There are several limitations to this study, including a relatively small sample size. The results should be considered as exploratory, linking dopamine deficits, neurobehavioral outcomes and network modulation in dialysis patients. Nonetheless, these spatially-scattered cortical hubs still add evidence to the structuralfunctional alterations in patients undergoing dialysis and explain why GM degeneration may occur in ESRD patients. Second, we only reported structural covariance strength showing controls > patients. The expression of dopamine has been reported to be affected by gene-environment interactions, types of uremic toxin, hemodialysis adequacy and a hyper- or hypo-dopaminergic state may mediate the clinical symptoms [55-57]. Although the seeds were confined to the striatum, our group stratification may have involved factors other than those mediated by dopamine. Further longitudinal studies may help to validate the specificity of the striatal network in grading disease severity. Third, although the striatum is considered to be a seed, the results of our analysis do not directly imply the top-down relationship driven by the predefined seed. Instead, our results support a higher clinical hierarchy of the striatal seeds in predicting symptoms and the relationships with cortical atrophy. Lastly, the influence of dialysis method has been emphasized in the literature [41,43,47] however, patients with hemodialysis and peritoneal dialysis showed no cognitive differences in this study. The cross sectional design as this study was not able to answer how treatment per se may modulate the brain network. In the future, we will perform a longitudinal analysis and explore whether the decline of cognition may be directly related to the treatment procedure or the dialysis adequacy.
Conclusion
Our analysis supports the clinical role of ventral-striatal SCNs in modulating the cortical degenerative scaffold in dialysis patients. In these patients, lower covariance strength was found in the ventral-striatum interconnected networks, and the clinical correlation suggested early ventral striatal disintegrity which influenced the dopamine pathways and modulated the severity of memory, executive and depressive symptoms.
Author’s Contributions
YYC, YCC, CWH and CCC are the physician for the principal diagnosis in this study for cognitive deficits and were responsible for the ideal generation, study conception, grants support, design and drafted manuscript.
JBC, CYL WCL, CCL, SWH, WCL, and LCL contributed to the administrative support in hospital for survey work, the interpretation of the clinical or neuroimaging results and critical intelligence of the manuscript.
All the authors have read and approved the final manuscript.
Acknowledgements
The authors wish to thank the patients and their caregivers for their time and commitment to this research and Dr. Ben-Chung Cheng from Kaohsiung Chang Gung Memorial Hospital and Dr. Yu-Tzu Chang from National Cheng Kung University College of Medicine and Hospital for referring patients undergoing hemodialysis and peritoneal dialysis and Dr. Nai-Ching Chen for study executive coordination.
This work was supported by grants CMRPG8E0541 from Chang Gung Memorial Hospital, and 104-2314-B-182A-026-MY2 from the National Science Council to CCC for MRI acquisition, model establishment and clinical data collection and analysis.
The funder had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Ethical approval and consent to participate
The study was approved by the Institutional Review Board of Chang Gung Memorial Hospital and the patients provided their informed consent. The methods were carried out in accordance with the relevant guidelines and regulations.
References
- Miwa K, Tanaka M, Okazaki S, et al. Chronic kidney disease is associated with dementia independent of cerebral small-vessel disease. Neurology 82(1), 1051-1057 (2014).
- Ma X, Jiang G, Li S, et al. Aberrant functional connectome in neurologically asymptomatic patients with end-stage renal disease. PloS. one 10(1), e0121085 (2015).
- O'Lone E, Connors M, Masson P, et al. Cognition in People With End-Stage Kidney Disease Treated With Hemodialysis: A Systematic Review and Meta-analysis. Am. J. Kidney. Dis 67(1), 925-935 (2016).
- Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology 67(1), 216-223 (2006).
- Sarnak MJ, Tighiouart H, Scott TM, et al. Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology 80(1), 471-480 (2013).
- Sud M, Tangri N, Pintilie M, et al. Risk of end-stage renal disease and death after cardiovascular events in chronic kidney disease. Circulation 130(1), 458-465 (2014).
- Chang CC, Hsu JL, Chang WN, et al. Metabolic Covariant Network in Relation to Nigrostriatal Degeneration in Carbon Monoxide Intoxication-Related Parkinsonism. Front. Neurosci 10(1), 187 (2016a).
- Elias MF, Dore GA, Davey A. Kidney disease and cognitive function. Contrib. Nephrol 179(1), 42-57 (2013).
- Schneider SM, Kielstein JT, Braverman J, et al. Cognitive Function in Patients With Chronic Kidney Disease: Challenges in Neuropsychological Assessments. Semin. Nephrol 35(1), 304-310 (2015).
- Biasioli S, D'Andrea G, Chiaramonte S, et al. The role of neurotransmitters in the genesis of uremic encephalopathy. Int. J. Artif. Organs 7(1), 101-106 (1984).
- Adachi N, Lei B, Deshpande G, et al. Uraemia suppresses central dopaminergic metabolism and impairs motor activity in rats. Intensive. Care. Med 27(1), 1655-1660 (2001).
- Ali F, Tayeb O, Attallah A. Plasma and brain catecholamines in experimental uremia: acute and chronic studies. Life. Sci 37(1), 1757-1764 (1985).
- Biasioli S, D'Andrea G, Feriani M, et al. Uremic encephalopathy: an updating. Clin. Nephrol 25(1), 57-63 (1986).
- Schmid G, Bahner U, Peschkes J, et al. Neurotransmitter and monoaminergic amino acid precursor levels in rat brain: effects of chronic renal failure and of malnutrition. Miner. Electrolyte. Metab 22(1), 115-118 (1996).
- Kavanagh D, Siddiqui S, Geddes CC. Restless legs syndrome in patients on dialysis. Am. J. kidney. Dis 43(1), 763-771 (2004).
- Ishii K, Shioya A, Nemoto K, et al. Decreased dopamine transporter and receptor ligand binding in Parkinsonism with diabetic uremic syndrome. Ann. Nucl. Med 30(1), 320-324 (2016).
- Su SF, Ng HY, Huang TL, et al. Survey of depression by Beck Depression Inventory in uremic patients undergoing hemodialysis and hemodiafiltration. Ther. Apher. Dial 16(1), 573-579 (2012).
- Hsu HJ, Yen CH, Chen CK, et al. Association between uremic toxins and depression in patients with chronic kidney disease undergoing maintenance hemodialysis. Gen. Hosp. Psychiatry 35(1), 23-27 (2013).
- O'Doherty J, Dayan P, Schultz J, et al. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 304(1), 452-454 (2004).
- Chang CC, Hsiao IT, Huang SH, et al. F-FP-(+)-DTBZ positron emission tomography detection of monoaminergic deficient network in patients with carbon monoxide related parkinsonism. Eur. J. Neurol 22(1), 845-852 (2015).
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb. Cortex 16(1), 1508-1521 (2006).
- Mechelli A, Friston KJ, Frackowiak RS, et al. Structural covariance in the human cortex. J. Neurosci 25(1), 8303-8310 (2005).
- Lin PH, Tsai SJ, Huang CW, et al. Dose-dependent genotype effects of BDNF Val66Met polymorphism on default mode network in early stage Alzheimer's disease. Oncotarget 7(1), 54200-54214 (2016a).
- Huang CW, Hsu SW, Tsai SJ, et al. Genetic effect of interleukin-1 beta (C-511T) polymorphism on the structural covariance network and white matter integrity in Alzheimer's disease. J. Neuroinflammation 14(1), 12 (2017).
- Morris JC The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43(1), 2412-2414 (1993).
- Zimmerman M, Emmert-Aronson BO, Brown TA. Concordance between a simpler definition of major depressive disorder and Diagnostic and statistical manual of mental disorders, fourth edition: an independent replication in an outpatient sample. Compr. Psychiatry 52(1), 261-264 (2011).
- Chang CC, Chang WN, Lui CC, et al. Clinical significance of the pallidoreticular pathway in patients with carbon monoxide intoxication. Brain 134(1), 3632-3646 (2011).
- Chang CC, Kramer JH, Lin KN, et al. Validating the Chinese version of the Verbal Learning Test for screening Alzheimer's disease. J. Int. Neuropsychol. Soc 16(1), 244-251 (2010).
- Ashburner J, Friston KJ (2000) Voxel-based morphometry--the methods. NeuroImage 11(1), 805-821.
- Zielinski BA, Anderson JS, Froehlich AL, et al. scMRI reveals large-scale brain network abnormalities in autism. PloS. one 7(1), e49172 (2012).
- Soriano-Mas C, Harrison BJ, Pujol J, et al. Structural covariance of the neostriatum with regional gray matter volumes. Brain. Struct. Funct 218(1), 697-709 (2013).
- Spreng RN, Turner GR. Structural covariance of the default network in healthy and pathological aging. J. Neurosci 33(1), 15226-15234 (2013).
- Montembeault M, Rouleau I, Provost JS, et al. Altered Gray Matter Structural Covariance Networks in Early Stages of Alzheimer's Disease. Cereb. Cortex 26(1), 2650-2662 (2016).
- Lehericy S, Ducros M, Van de Moortele PF, et al. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann. Neurol 55(1), 522-529 (2004).
- Leh SE, Ptito A, Chakravarty MM, et al. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neurosci. Lett 419(1), 113-118 (2007).
- Hudon C, Belleville S, Gauthier S. The association between depressive and cognitive symptoms in amnestic mild cognitive impairment. Int. Psychogeriatr 20(1), 710-723 (2008).
- Chen NC, Lin YI, Chang CC, et al. Learning and error patterns in the Chinese Verbal Learning Test in subjects with mild cognitive impairment and normal elderly. Acta. Neurol. Taiwan 20(1), 114-124 (2011).
- Zhang LJ, Zheng G, Zhang L, et al. Altered brain functional connectivity in patients with cirrhosis and minimal hepatic encephalopathy: a functional MR imaging study. Radiology 265(1), 528-536 (2012).
- Liang X, Wen J, Ni L, et al. Altered pattern of spontaneous brain activity in the patients with end-stage renal disease: a resting-state functional MRI study with regional homogeneity analysis. PloS. one 8(1), e71507 (2013).
- Ni L, Wen J, Zhang LJ, et al. Aberrant default-mode functional connectivity in patients with end-stage renal disease: a resting-state functional MR imaging study. Radiology 271(1), 543-552 (2014).
- Zheng G, Wen J, Zhang L, et al. Altered brain functional connectivity in hemodialysis patients with end-stage renal disease: a resting-state functional MR imaging study. Metab. Brain. Dis 29(1), 777-786 (2014).
- Chen HJ, Qi R, Kong X, et al. The impact of hemodialysis on cognitive dysfunction in patients with end-stage renal disease: a resting-state functional MRI study. Metab. Brain. Dis 30(1), 1247-1256 (2015).
- Zhang XD, Wen JQ, Xu Q, et al. Altered long- and short-range functional connectivity in the patients with end-stage renal disease: a resting-state functional MRI study. Metab. Brain. Dis 30(1), 1175-1186 (2015).
- Li S, Ma X, Huang R, et al. Abnormal degree centrality in neurologically asymptomatic patients with end-stage renal disease: A resting-state fMRI study. Clin. Neurophysiol 127(1), 602-609 (2016).
- Luo S, Qi RF, Wen JQ, et al. Abnormal Intrinsic Brain Activity Patterns in Patients with End-Stage Renal Disease Undergoing Peritoneal Dialysis: A Resting-State Functional MR Imaging Study. Radiology 278(1), 181-189 (2016).
- Ma X, Tian J, Wu Z, et al. Spatial Disassociation of Disrupted Functional Connectivity for the Default Mode Network in Patients with End-Stage Renal Disease. PloS. one 11(1), e0161392 (2016).
- Zhang LJ, Wen J, Liang X, et al. Brain Default Mode Network Changes after Renal Transplantation: A Diffusion-Tensor Imaging and Resting-State Functional MR Imaging Study. Radiology 278(1), 485-495 (2016).
- Steiger TK, Weiskopf N, Bunzeck N. Iron Level and Myelin Content in the Ventral Striatum Predict Memory Performance in the Aging Brain. J. Neurosci 36(1), 3552-3558 (2016).
- Chai C, Yan S, Chu Z, et al. Quantitative measurement of brain iron deposition in patients with haemodialysis using susceptibility mapping. Metab. Brain. Dis 30(1), 563-571 (2015).
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci 20(1), 2369-2382 (2000).
- Haber SN. The primate basal ganglia: parallel and integrative networks. 26(1), 317-330 (2003). J. Chem. Neuroanat
- Almeida OP, Norman P, Hankey GJ, et al. The association between C-reactive protein concentration and depression in later life is due to poor physical health: results from the Health in Men Study (HIMS). Psychol. Med 37(1), 1775-1786 (2007).
- Park JI, Park TW, Yang JC, et al. Factors associated with depression among elderly Koreans: the role of chronic illness, subjective health status, and cognitive impairment. Psychogeriatrics 16(1), 62-69 (2016).
- Chang TI, Tabada GH, Yang J, et al. Visit-to-visit variability of blood pressure and death, end-stage renal disease, and cardiovascular events in patients with chronic kidney disease. J. Hypertens 34(1), 244-252 (2016b).
- Hoth KF, Paul RH, Williams LM, et al. Associations between the COMT Val/Met polymorphism, early life stress, and personality among healthy adults. Neuropsychiatr. Dis. Treat 2(1), 219-225 (2006).
- Lin PH, Tsai SJ, Huang CW, et al. Dose-dependent genotype effects of BDNF Val66Met polymorphism on default mode network in early stage Alzheimer's disease. Oncotarget 7(1), 54200-54214 (2016b).
- Spreen O, Strauss E. Geriatric depression scale (GDS). A Compendium of Neuropsychological Tests. Oxford University Press, NY (1998).