Research Article - Neuropsychiatry (2017) Volume 7, Issue 6
Serum Proteome Analysis of Catatonia
- Corresponding Author:
- Tiao-Lai Huang
Department of Psychiatry
Chang Gung Memorial Hospital– Kaohsiung Medical Center
Chang Gung University College of Medicine, 123, Ta-Pei Rd.
Niao-Sung, Kaohsiung 833, Taiwan, R.O.C
Tel: 886-7-7317123 ext. 8753
Fax: 886-7- 7326817
Abstract
Abstract
The exact mechanism of catatonia remains a mystery. We tried to investigate the serum biomarkers for patients with catatonia before and after successful treatment using proteomic analysis. During a three-year period, nine patients with schizophrenia who experienced catatonia and nine healthy controls were recruited. 5 ml of venous blood were collected before (acute phase) and after treatment (recovery phase) of catatonia, relieved by intramuscular lorazepam injection. The serum proteomes of acute and recovery phase were compared using two-dimensional polyacrylamide gel electrophoresis (2D-PAGE), combined with matrixassisted laser desorption ionization (MALDI)-time-of-flight (TOF) TOF mass spectrometry (MS). The findings were then validated with enzyme-linked immunosorbent assay (ELISA) by comparing data of patients and healthy controls. Ten differentially expressed protein spots were identified comparing serum proteome profiles of acute phase and recovery phase of catatonia from 2D PAGE. Alpha-2-HS-glycoprotein (AHSG), plasma retinol-binding protein (RBP4) and serum amyloid P component (APCS) may be potential disease-associated biomarkers for catatonia. In the validation phase, those serum proteins were measured in serum of both acute and recovery phase as well as healthy controls with ELISA. Mann-Whitney U-test showed that patients with catatonia in acute phase (p = 0.006) and recovery phase (p = 0.019) both had significantly higher APCS levels than healthy controls. Using ANCOVA with age adjustment, patients with catatonia in acute phase also had significantly higher APCS level than healthy controls (p = 0.048). Our investigation provided a proteomic study in biomarker research of catatonia. APCS might be an important biomaker for the psychopathology in catatonia of schizophrenia. In future, a larger of sample size will be needed to prove these results.
https://blogum.blogaaja.fi/
https://blogum-1.jimdosite.com/
https://blogummm.edublogs.org/
https://blogummm.websites.co.in/
https://blogum18.wordpress.com/
https://benim-blogum.jigsy.com/
https://fuiegs-symbeaurds-build.yolasite.com/
https://blogum-03.webselfsite.net/
https://blogummm.mystrikingly.com/
https://blogum.splashthat.com/
https://blogum3.webnode.com.tr/
https://blogum.odoo.com/
https://blogum.creatorlink.net/
https://whiteseotr1-s-site.thinkific.com/enrollments
https://blogum.estranky.cz/
https://653ba4fbb538c.site123.me/
https://blogum12m.blogspot.com/
https://blogum.hashnode.dev/
https://whiteseoturkey1.wixsite.com/blogum
https://sites.google.com/view/blogummm/
https://codepen.io/blogum
https://blogumm.livejournal.com/
https://wakelet.com/@blogum82816
https://www.homify.com/users/9538383/blogum
https://lessons.drawspace.com/profile/323613/blogum
https://my.desktopnexus.com/blogum/
https://writeupcafe.com/profile/BLOGUM/
https://www.pearltrees.com/blogum
https://www.easyfie.com/blogum
https://pharmahub.org/members/27615/profile
https://www.zupyak.com/u/blogum/posts
https://www.metroflog.co/blogum
https://www.fuzia.com/fz/blogum-blogum
https://tr.pinterest.com/blogum12/
https://my.getjealous.com/blogum
https://micro.blog/blogum
https://www.tumblr.com/blogummm
https://hub.docker.com/u/blogum
https://fire.blogfree.net/?act=Profile&MID=1342323
https://blogum.pixnet.net/blog
https://www.threadless.com/@blogum/activity
https://blogum.neocities.org/
https://blogum12.amebaownd.com/
https://teletype.in/@blogum
https://ubl.xml.org/users/blogum
https://educatorpages.com/site/blogum/
https://blogum.onlc.fr/
Keywords
Catatonia, Serum amyloid P component, APCS, Biomarker, Schizophrenia
Introduction
Catatonia was first described in 1874 by Karl Ludwig Kahlbaum [1]. It is a behavioral anomaly manifested by conscious and motor disturbances in which patients lose the capability to move normally despite having the full physical capacity to do so. Catatonia had been seen with psychiatric conditions, such as schizophrenia, depressive disorders, bipolar disorder, post-traumatic stress disorder, eating disorders, and autism spectrum disorders, as well as medical conditions, such as autoimmune disorders, paraneoplastic syndromes, infections, focal neurologic lesions, metabolic disturbances, seizure disorders, and alcohol withdrawal [2-9].
In the treatment of catatonia, benzodiazepines are a first-line treatment strategy. Electroconvulsive therapy is another option, often considered if the catatonia is not relieved by benzodiazepines [10,11]. Lorazepam and diazepam could also rapidly relieve catatonia in some of our earlier reports [12-15].
The exact mechanism of catatonia remains unknown. Several systems had been investigated before, such as gamma-aminobutyric acid (GABA), glutamate, and brain-derived neurotrophic factor (BDNF) [16,17]. Data of proteomic analysis of catatonia remains scarce, and could shed new light into better understanding of its pathophysiological mechanism.
Here, we introduced two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) combined with matrix-assisted laser desorption/ ionization-(MALDI) time-of-flight (TOF) mass spectrometry (MS) on catatonia research, in order to get a better understanding of disease mechanisms and response to treatment.
Materials and Methods
▪ Patients with catatonia and healthy controls
This study was conducted from February 2006 to April 2008 and November 2014 to December 2014, including nine patients with catatonia and nine healthy controls from our study grants at the Chang Gung Memorial Hospital in Kaohsiung, Taiwan. Institutional Review Board (IRB) approval was obtained from the hospital’s Ethics Committee. Patients suffering from schizophrenia or schizoaffective disorder and healthy controls were selected according to the results of a Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) [18]. Diagnosis of catatonia was based on the patient meeting at least two of the following five criteria listed in DSM-IV: motor immobility (including waxy flexibility) or stupor, excessive motor activity, extreme negativism or mutism, peculiarities of voluntary movement, and echolalia or echopraxia. The catatonia of all recruited patients was rapidly relieved by 1 or 2 amp intramuscular lorazepam injection. All of the participants were free of heart, liver, renal and metabolic diseases, and they did not take any medication at least one week prior to entering into this study. In patients with catatonia, venous blood (5 mL) were collected before (acute phase) and after treatment which completely relieved of catatonia (recovery phase). In healthy controls, venous blood (5 mL) was collected at baseline. Once the serum specimen had been obtained, they were immediately delivered to the laboratory and stored at -80°C for further analysis.
▪ 2D-PAGE
Isoelectric focusing (IEF) was performed using the 13 cm, pI 4–7 IPG strip (GE Healthcare). The rehydration buffer (8 M urea, 2 % CHAPS, 100 mM DTT, 0.5 % IPG buffer) was added to 200 μg serum protein to a final volume of 250 μl. The first dimension gels were run using a standardized protocol of at 30 V for 10 hr, 500 V for 1hr, 1000 V for 1 hr, 8000 V for 32000 vhr, and finally 30 V until harvest. Gels were taken out from the IEF hold amd moved into glass tube containing equilibration solution. The equilibration solution contains 65 mM DTT and 135 mM IAA, and was individually shaken for 15 min. After equilibration, IPG strips were washed with SDS-polyacrylamide (SDS-PAGE) running buffer and then applied onto the top of 10 % SDS-PAGE gels. The running condition was set at constant 10 mA per gel and with the temperature at 4 ℃ overnight and until to the dye leaves gel. Gels were stained using silver staining method. Protein spots were quantified with ImageMaster 2D Platinum, and differentially expressed protein spots were analyzed using DeCyder software (P < 0.05 in t-test; ratio of acute phase of patient/recovery phase of patient >1.5 or <1.5).
▪ In-gel digestion and protein identification
Protein spots were excised from the gel, and the extract was washed 3 times with 200 μl H2O for 5 min when vortexing. The supernatant was discarded, and 200 μl of destaining agent (0.1 g K3Fe(CN)6 and 0.16 g Na2S2O3 in 10 ml dd H2O) were added, then vortexed for 15 min. The supernatant was again discarded, and the gel was washed 3 times in 200 μl of washing solution I [50 mM NH4HCO3/100% ACN (3:2)] when vortexing for 15 min. The supernatant was again discarded, and 200 μl of 100% ACN was added when vortexing for 10 min. After removing the supernatant, the samples were spin with a vacuum centrifuge for 10 min at room temperature (RT). After adding 6–8 μl of trypsin buffer (25 ng/μl trypsin, 25mM NH4HCO) to cover the gels, the gels were digested in trypsin solution for 16 h at 37°C. The peptides were extracted from the gel with extraction buffer (100% acetonitrile, 1% trifluoroacetic acid), then concentrated with a vacuum centrifuge for 10 min at room temperature (RT), and rehydrated by the addition of 10 μl of deionized water.
Before their MS spectra and MS/MS fragment ion mass were determined with a Bruker MALDITOF/ TOF Analyzer (Bruker Daltonics, Bremen, Germany). All product ions were submitted to a computer database search analysis with the Mascot MS/MS ion search (Matrix Science Inc., MA) by using the SwissProt database (all entries). The Bruker operation system, namely flexControl Version 3.3 (Build 85) and flexAnalysis software were used to create peak lists. MALDI-TOF/TOF data were searched in-house MASCOT software (ver 2.2.04). The following parameters were used: enzyme, trypsin; variable modification, carbamidomethyl and oxidation; mass values, monoisotopic; peptide mass tolerance, ±100 ppm; peptide charge state, 1+; maximum missed cleavage, 1; significance threshold, p < 0.05. Peptide identification and protein assembly were performed in multiple stages. The protein identifications required detection of unique peptides and proteins with more than two spectral counts were selected for further analysis. The peptide mass data of each spot was submitted to the Swiss-Prot 100425 human species bioinformation stations using MASCOT search engines. Proteins identified with a higher MASCOT score in the bovine database than in the human database were considered as serum contamination and removed.
▪ Validation by ELISA analyses
Commercial ELISA kits were used to detect the serum levels of plasma retinol-binding protein (RBP4) (EZHRBP4-18K, Millipore, St. Charles, USA), alpha-2-HS-glycoprotein (fetuin A; AHSG) and serum amyloid P (APCS) (HCVD3MAG-67K, Millipore, St. Charles, USA), according to the manufacturer’s instructions. All analyses were performed at the same laboratory.
▪ Bioinformatic tools for protein searches, networking construction statistical analysis
The protein search used the following databases: NCBI (http://www.ncbi.nlm.nih.gov), and UniProtKB/Swiss-Prot (http://www.uniprot. org/). The combining pathway databases of the PubMed literature (http://www.ncbi.nlm.nih. gov/pubmed/) were used to search the correlated regulatory pathways in catatonic schizophrenia and other related reactions. The MetaCore from GeneGo Inc. (Version 6.5) was applied in thoroughly compiling interaction networks.
Statistical analyses were performed with an SPSS 12.0 package (SPSS, Chicago, IL). Data analysis was performed using nonparametric statistics (Mann-Whitney U-test) and analysis of covariance (ANCOVA) with age adjustment to evaluate the differences between groups for all markers. When p < 0.05 was considered to be statistically significant.
Results and Discussion
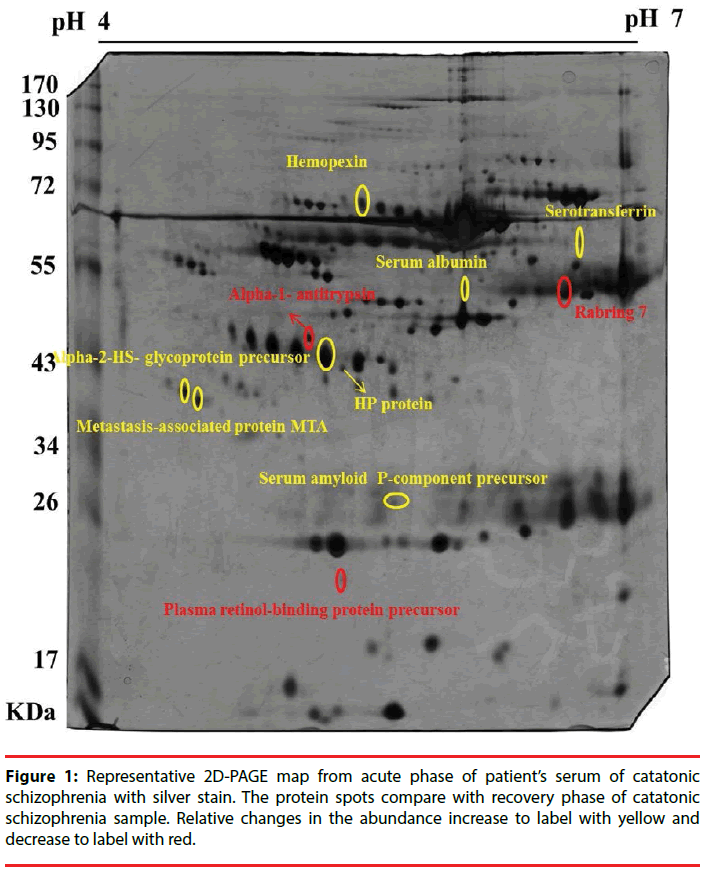
Nine patients with catatonia and nine sexmatched healthy controls were recruited. Figure 1 represents the 2D-PAGE map from patients with catatonia before and after the relief of catatonia with lorazepam. Ten protein spots were differentially expressed before and after treatment in 2D-PAGE maps, and those were identified using the PMF (peptide-mass finger printing) and tandem MS method of MALDI-TOF/TOF MS. Those ten proteins were serotransferrin, AHSG, RBP4, hemopexin, alpha-1- antitrypsin, rabring 7, serum albumin, haptoglobin (HP protein), metastasis-associated protein (MTA) and APCS. The relative changes in the abundance of those protein spots before and after the relief of catatonia were summarized in Table 1.
| Protein name | Mr/pI | Accession name | Protein symbls/ID | Mascot Score | Description | |
|---|---|---|---|---|---|---|
| 1 | Serotransferrin | 48/4.7 | TRFE_HUMAN | TF | 177 | A<R |
| 2 | Alpha-2-HS-glycoprotein | 67/9.3 | FETUA_HUMAN | AHSG | 59 | A<R |
| 3 | Plasma retinol-binding protein | 62/7.1 | RETBP_HUMAN | RBP4 | 74 | A>R |
| 4 | Hemopexin | 95/8.3 | HEMO_HUMAN | HPX | 183 | A<R |
| 5 | alpha-1- antitrypsin | 42/5.4 | A1AT_HUMAN | SERPINA1 | 102 | A>R |
| 6 | Rabring 7 | 33/5.3 | RN115_HUMAN | ZNF 364 | 77 | A>R |
| 7 | Serum albumin | 67/9.3 | ALBU_HUMAN | ALB | 242 | A<R |
| 8 | HP protein | 31/9.2 | HPT_HUMAN | HP | 80 | A<R |
| 9 | Metastasis-associated protein MTA | 167/9.3 | MTA1_HUMAN | MTA1 | 55 | A<R |
| 10 | Serum amyloid P-component | 48/4.7 | SAMP_HUMAN | APCS | 83 | A<R |
Table 1: Relative changes in the abundance of different protein spots with at least 1.5-fold in acute phase of catatonic patient samples (A) and recovery phase of catatonia (R).
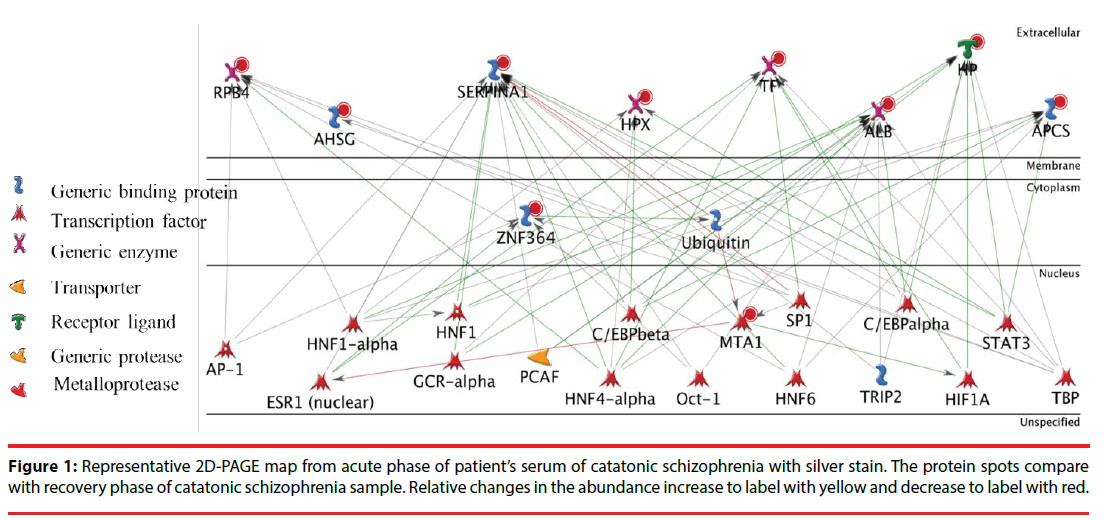
Next, we put these ten identified protein together and tried to assemble protein-protein correlations. Figure 2 shows the result of network construction of ten differentially expressed serum proteins between patients with catatonia and healthy controls. There are two transcription factor, namely TATA-box-binding protein (TBP) and Hepatocyte nuclear factor 4-alpha (HNF4A) in the protein network. TBP and HNF4A are directly related to six proteins, individually. Hepatocyte nuclear factor 1-alpha (HNF1 alpha), Transcription factor Sp1 (SP1) and Enhancer-binding protein beta (C/EBP beta) are also transcription factors which were related to five proteins, individually. The four transcription factors HNF 6, ESR1 (nuclear), C/EBP alpha and STAT 3 were related to four proteins. The eight proteins OCT-1 (transcription factor), GCR-alpha (transcription factor), HIF1A (transcription factor), HNF1 (transcription factor), AP-1 (transcription factor), Ubiquitin (Generic binding protein), TRIP2 (Generic binding protein) and PCAF (Generic enzyme) were related to three proteins. Other 25 proteins were related to two proteins (network not showed).
Due to the limitation of literature search and serum sample amounts, we only chose RPB4, APCS and AHSG to be evaluated and validated for the potential disease-associated biomarkers of catatonia. Table 2 shows the demographic data and serum RBP4, AHSG, APCS levels for all participants. In the protein network we found that RPB4, APCS, and AHSG are frequently related to TBP, HNF4A, HNF1 alpha, SP1 and C/EBP-beta.
| Patient with catatonia (acute phase) | Patient with catatonic (recovery) | Healthy controls | |
|---|---|---|---|
| Total (n=9) men/women=2/7 | Total (n=9) men/women=2/7 | Total (n=9) men/women=2/7 | |
| Age (years) |
50.6 ± 7.2 | - | 37.4 ± 4.1 |
| BMI (kg/㎡) |
21.7 ± 3.5 | - | 21.9 ± 2.4 |
| Illness Duration (years) | 9.2 ± 6.8 | - | - |
| Education (years) |
7.9 ± 6.0 | - | 16.3 ± 2.9 |
| RBP4 levels (ng/mL) | 5.6 ± 2.2 | 5.0 ± 2.4 | 5.8 ± 1.2 |
| APCS levels (ng/mL) | 8637.8 ± 6602.1 | 8853.3 ± 7340.0 | 7828.9 ± 2159.3 |
| AHSG levels (ng/mL) | 338120 ± 262752 | 326157.8 ± 248477.5 | 567586.75 ± 71547.0 |
Abbreviations: BMI=Body Mass Index; RBP4=Plasma retinol-Binding Protein; AHSG= Alpha-2-HS-glycoprotein; APCS=Serum Amyloid P Component
Table 2: The demographic data and serum RBP4, AHSG, APCS levels in all participants.
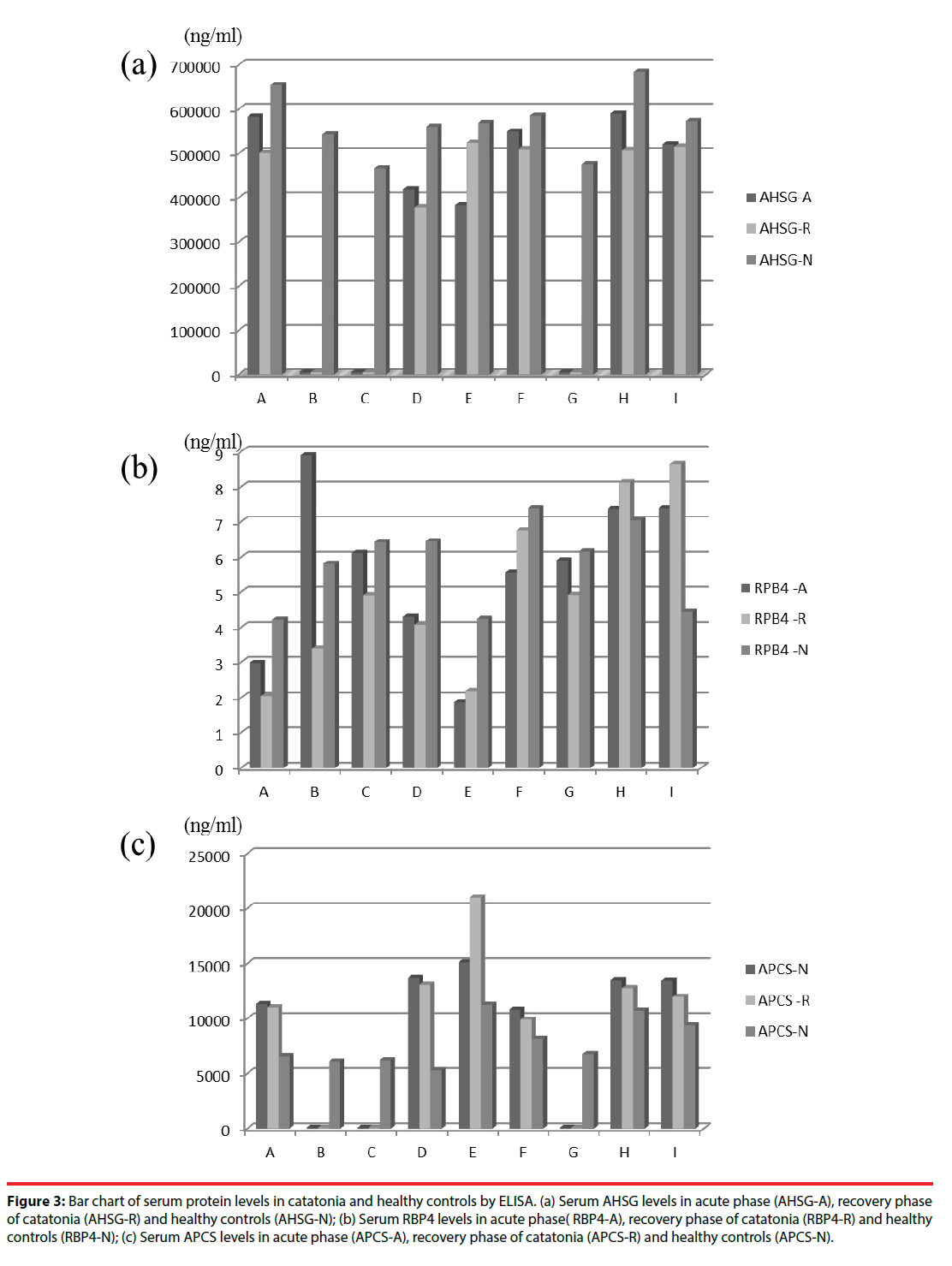
The protein levels of RPB4, APCS, and AHSG of patients with catatonia in acute phase (A) and recovery phase (P), as well as healthy controls (N) were shown in Figure 3. Mann-Whitney U-test showed that patients with catatonia in acute phase (p=0.006) and recovery phase (p=0.019) both had significantly higher APCS levels than healthy controls. Those results showed that serum levels of APCS were increased in patients with catatonia. In addition, using ANCOVA with age adjustment, patients with catatonia in acute phase also had significantly higher APCS level than healthy controls (F=4.479, p=0.048).
APCS levels were also found to be significantly increased of patients suffering from catatonia. RPB4, AHSG were not conspicuously expressed between acute phase, recovery phase of catatonia and healthy control. In Figure 3(a), we found AHSG levels in patients with catatonia (including acute phase and recovery phase) are decreasing compared to healthy controls in all nine cases, but the result is not consistent in statistical analysis (Mann-Whitney U-test). In Figure 3(b), serum RBP4 levels are increased for healthy controls in seven cases (7/9; 77.8 %) but not statistically significant. In Figure 3(c), serum APCS levels are increasing in acute phase, recovery phase of catatonia when compared with healthy controls in six cases, and are compatible with Mann-Whitney U-test and ANCOVA with age adjustment.
Figure 3: Bar chart of serum protein levels in catatonia and healthy controls by ELISA. (a) Serum AHSG levels in acute phase (AHSG-A), recovery phase of catatonia (AHSG-R) and healthy controls (AHSG-N); (b) Serum RBP4 levels in acute phase( RBP4-A), recovery phase of catatonia (RBP4-R) and healthy controls (RBP4-N); (c) Serum APCS levels in acute phase (APCS-A), recovery phase of catatonia (APCS-R) and healthy controls (APCS-N).
Acute-phase proteins are a class of blood proteins whose blood concentrations increase (positive acute phase proteins such as APCS) or decrease (negative acute phase proteins such as AHSG and RBP4) in response to inflammation. In this study, we find APCS is increased in patients with catatonia.
The serum amyloid P component (APCS) is called “amyloid”, a 25kDa pentameric protein first identified as the pentagonal constituent of in vivo pathological deposits the identical serum form of amyloid P component (AP). APCS belongs to pentraxin protein family that exhibit calcium dependant ligand binding, and binds negatively charged carbohydrates. APCS is a heparin- and DNA-binding serum protein and is involved in acute immunological responses [19]. APCS is an acute phase protein, which is synthesized in response to pro-inflammatory cytokines early in the inflammatory response. It interacts with inflammatory and complement factors, acting as an opsonin that is any molecule that enhances phagocytosis by marking an antigen for an immune response or marking dead cells for recycling [20]. Serum amyloid P component is correlated with Alzheimer’s disease and is a normal plasma constituent that is observed in senile plaques and neurofibrillary tangles in brains of Alzheimer’s disease (AD) patients. APCS is believed to stabilize aggregates by preventing proteolytic cleavage and inhibiting fibril removal via the normal protein scavenging mechanisms. In 1996, Nishiyama, et al. have evaluated the AP levels of sera in the AD group was significantly lower than that of the control group. The results suggest that the deposition of AP in senile plaques and neurofibrillary tangles is not due to its overproduction. The production of AP by the liver (hepatocytes), thought to be the only source, may be suppressed in AD patients [21].
Wan, et al. reported that the expression of serum amyloid P-component was significantly higher in the plasma of patients with schizophrenia in comparison with that of healthy controls [22]. Alterations in immune response may be an important component in the etiopathogenesis of schizophrenia. In 2015, Weber et al examined the associations of pentraxin-3 (PTX3) with the onset of schizophrenia. Human APCS is more distantly related to the “long” pentraxins such as PTX3 (cytokine modulated) as well as several neuronal pentraxins. There are preonset serum specimens from 160 US military service members and 160 matched controls. The results display lower serum levels of PTX3 were predictive of schizophrenia and a lower level of inflammatory response showed by PTX3 might be implicated in developing schizophrenia [23]. APCS has also been reported in inflammation disease. In a study carried out by Sezer, et al. reporting that pentraxin-3, fetuin-A, and serum amyloid A all arise together as novel prognostic factors in a group of patients with ischemic stroke. Ischemic stroke is also inflammatory disease [24].
Fetuins (including fetuin-A and fetuin-B) are blood proteins that are made in the liver and secreted into the bloodstream. Fetuin-A (alpha- 2-HS-glycoprotein, AHSG) was originally discovered to be an inhibitor of vascular calcification. AHSG is a negative acute phase proteins had been linked its proinflammatory. Blood levels of AHSG increasing were occurrence of non-alcoholic fatty liver disease and cardiovascular [24,25]. In 2016, Sabine Bahn, et al. reported sex-dependent markers (such as AHSG, β2-microglobulin, CD5L, FASLG receptor, C-reactive protein, trefoil factor 3, cystatin-C) of major depressive disorder were in order of decreasing significance [26].
Retinol-binding protein (RBP) belongs to the lipocalin family and the transport protein for vitamin A in human plasma [27]. Retinolbinding protein delivers retinol from the liver stores to the peripheral tissues. The RBP-retinol complex interacts with transthyretin, which avoids its loss by filtration through the kidney glomeruli in human plasma. When vitamin A blocks secretion of the binding protein posttranslationally is deficient. It results in defective delivery and supply to the epidermal cells. When decreasing of glucose concentration in plasma, retinol-binding protein acts as a signal to other cells [28].
In the RBP4 gene study, autosomal dominant microphthalmia, anophthalmia, and coloboma (MAC) disease is linked to mutations in the RBP4 gene. It means that retinol binding protein has negative effects in transferring vitamin A from maternal liver storage sites to the placenta and is relation to avoid MAC disease [29]. In 2006, Wan, et al. determined the levels of retinol binding protein, no statistical difference was found at gene expression level of schizophrenic patients in comparison with that of healthy controls [22]. Chen and his colleagues (2011) observed schizophrenic patients with metabolic syndrome in higher RBP4 and lower total adiponectin and high molecular weight adiponectin levels. Metabolic adversities are common in patients with schizophrenia. RBP4 have been recently found to be associated with metabolic features in non-psychiatric population [30].
In a study by Wong, et al., some serum acute phase proteins such as retinol-binding protein levels were reduced in male schizophrenic patients [31]. RBP4 has been reported in other psychiatric disease and inflammation disease. In 1992, Maes M, et al. is report lower retinol binding protein plasma levels during depression [32]. Khalid M Alkharfy, et al. also has studying in serum retinol-binding protein 4 as a marker for cardiovascular disease in women [33]. Serum RBP4 levels were significantly elevated in patients with CAD compared to non-CAD patents [34].
In addition, directly related to six proteins, TBP is functioned at the core of the DNAbinding multiprotein factor TFIID. TBP is involved in the activation of eukaryotic genes transcribed by RNA polymerase II, and binding of TFIID to the TATA box is the initial transcriptional step of the pre- initiation complex (PIC). In recent years, evidence has emerged implicating TBP in the molecular mechanism of a number of neurodegenerative and neuropsychiatric diseases. In 1998, Perez, et al. reported that TBP was associated with aggregates in several polyglutamine disorders [35-38]. In 1999, Koide, et al. found that TBP itself could cause neurodegeneration when the polyglutamine stretch near its N-terminal end is expanded [39]. Although no evidence was found for the association of TBP in bipolar disorder, schizophrenia, or Parkinson’s disease, current studies have found suggestion that TBP could play a role in Alzheimer’s disease (AD) [40,41].
Another transcription factor directly related to six proteins, HNF4A is binding to DNA sites required for the transcription of alpha 1- antitrypsin, apolipoprotein CIII, transthyretin genes and HNF1- alpha. HNF4 alpha may be essential for development of the kidney, intestine and liver.
The clinical significance of the HNF4A is associated with a form of diabetes called maturity onset diabetes of the young (MODY) [42] and increased amplification of HNF4A has been observed in colorectal cancer [43].
HNF1 alpha, SP1 and C/EBP beta are three transcription factors and related to five proteins, individually. HNF1 alpha is transcriptional activator that regulates the tissue specific expression of multiple genes and required for the expression of several liver specific genes. HNF- 1 protein is present in clear cell carcinoma of ovary [44,45] and when HNF1A mutations can cause maturity onset diabetes of the monogenic diabetes [46]. SP1 can activate or repress transcription in response to physiological and pathological stimuli. SP1 involved in a variety of processes such as immune responses, cell growth, apoptosis, and differentiation. Alterations of transcription factor SP1 have been linked to different neuropsychiatric diseases. Reduced SP1 protein levels in the prefrontal cortex have been associated with schizophrenia, suggesting that SP1 could be involved in the pathogenesis of disorders with psychotic features [47]. C/EBP beta is playing an important role in the regulation of genes involved in immune and inflammatory responses. C/EBP beta is transcriptional activator and binds to an IL-1 response element in the IL-6 gene. C/EBP-beta could potentially be a marker of in gestational trophoblastic disease [48].
On the other hand, to compare results of literature search and others potential biomarkers from our 2D PAGE results, the higher alpha 1-antitrypsin, haptoglobin, ceruloplasmin and lower retinol binding protein plasma levels were noted during depression [32]. In 1997, Maes M, et al. also reported that plasma complement component 3 (C3C), complement component 4(C4), haptoglobin (Hp), fibrinogen (Fb), hemopexin (Hpx) and alpha 1-acid-glycoprotein (α 1S) were significantly overexpression in schizophrenia than normal controls. Patients of major had higher plasma C3C, C4, α 1S, Hp and Fb than controls. In manic cases showed higher plasma Hp, Hpx Fb, and α 1S than normal subjects [49]. In 2006, Yang, et al. reported that apolipoprotein A-I and transthyretin were found to be significantly decreased in schizophrenia, whereas α1- antitrypsin, haptoglobin α 2 chain, β chain, and complement factor B precursor showed overexpression in schizophrenia [50]. In our previously study had showed significantly lower serum albumin levels in the acute phase of schizophrenia than healthy controls [51].
There are several limitations to the study. The sample size was small. Patients with catatonia were recruited over many years, and only those with favourable outcomes (whose catatonia was relieved rapidly by lorazepam injection), so selection bias might affected our results. Lastly, while the presence and the absence of catatonia was the key factor of the proteomic analysis, the patients’ underlying schizophrenia could be a confounding factor.
Conclusion
This study demonstrates that higher serum levels of APCS in patients with catatonia than healthy controls. The results suggest that inflammation and catatonia are associated. In future, a larger of sample size is needed to validate these results.
Acknowledgement
This work was supported by our study grants from Chang Gung Memorial Hospital (grant numbers including CMRPG840521, CMRPG850491, CMRPG860491 and CMRPG8D0571) in Taiwan.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Funding Statement
N/A
References
- Kahlbaum KL. Catatonia or tension. Berlin: Publisher August Hirshwald (1874).
- Brake JA, Abidi S. A case of adolescent catatonia. J. Can. Acad. Child. Adolesc. Psychiatry 19(2), 138-140 (2010).
- Geoffroy PA, Rolland B, Cottencin O. Catatonia and alcohol withdrawal: a complex and underestimated syndrome. Alcohol. Alcohol 47(3), 288-290 (2012).
- Deuschle M, Lederbogen F. Benzodiazepine withdrawal-induced catatonia. Pharmacopsychiatry 34(1), 41-42 (2001).
- Kanemoto K, Miyamoto T, Abe R. Ictal catatonia as a manifestation of de novo absence status epilepticus following benzodiazepine withdrawal. Seizure 8(6), 364-366 (1999).
- Rosebush PI, Mazurek MF. Catatonia after benzodiazepine withdrawal. J. Clin. Psychopharmacol 16(4), 315-319 (1996).
- Taylor MA,Fink M. Catatonia in psychiatric classification: a home of its own. Am. J. Psychiatry 160(7), 1233-1241 (2003).
- Dhossche DM, Shah A, Wing L. Blueprints for the assessment, treatment, and future study of catatonia in autism spectrum disorders. Int. Rev. Neurobiol 72(1), 267-284 (2006).
- Dhossche DM, van der Steen LF, Shettar SM. Catatonia in autism spectrum disorders: review and case-report. Tijdschr. Psychiatr 57(2), 89-93 (2015).
- Trivedi HK, Mendelowitz AJ, Fink M. Gilles de la Tourette form of catatonia: response to ECT. J. ECT 19(2), 115-117 (2003).
- Carroll BT. Kahlbaum's catatonia revisited. Psychiatry. Clin. Neurosci 55(5), 431-436 (2001).
- Lin CC, Huang TL. Lorazepam-diazepam protocol for catatonia in schizophrenia: a 21-case analysis. Compr. Psychiatry 54(8), 1210-1214 (2013).
- Huang YC, Lin CC, Hung YY, et al. Rapid relief of catatonia in mood disorder by lorazepam and diazepam. Biomed. J 36(1), 35-39 (2013).
- Tsai MC, Huang TL. Lorazepam and diazepam for relieving catatonic features precipitated by initial hemodialysis in a uremic patient: a case report. Prog. Neuropsychopharmacol. Biol. Psychiatry 34(2), 423-424 (2010).
- Huang CE, Huang TL. Intramuscular lorazepam in catatonia in patients with acute renal failure: a report of two cases. Chang. Gung. Med. J 33(1), 106-109 (2010).
- Ahmed AO, Mantini AM, Fridberg DJ, et al. Brain-derived neurotrophic factor (BDNF) and neurocognitive deficits in people with schizophrenia: a meta-analysis. Psychiatry. Res 226(1), 1-13 (2015).
- Lewis DA, Curley AA, Glausier JR, et al. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends. Neurosci 35(1), 57-67 (2012).
- First MB, Spitzer RL, Gibbon M, et al. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders-Clinician Version (SCID-CV). Washington, DC, American Psychiatric Press (1997).
- Gewurz H, Zhang XH, Lint TF. Structure and function of the pentraxins. Curr. Opin. Immunol 7(1), 54-64 (1995).
- Mold C, Baca R, Du Clos TW. Serum amyloid P component and C-reactive protein opsonize apoptotic cells for phagocytosis through Fcgamma receptors. J. Autoimmun 19(3), 147-154 (2002).
- Nishiyama E, Iwamoto N, Kimura M, et al. Serum amyloid P component level in Alzheimer's disease. Dementia 7(5), 256-259 (1996).
- Wan C, La Y, Zhu H, et al. Abnormal changes of plasma acute phase proteins in schizophrenia and the relation between schizophrenia and haptoglobin (Hp) gene. Amino Acids 32(1), 101-108 (2007).
- Weber NS, Larsen RA, Yolken RH, et al. Predictors of the Onset of Schizophrenia in US Military Personnel. J. Nerv. Ment. Dis 203(5), 319-324 (2015).
- Sezer S, Ucar F, Ulusoy EK, et al. Serum amyloid A, fetuin-A, and pentraxin-3 levels in patients with ischemic stroke: novel prognostic biomarkers? Turk. J. Med. Sci 44(1), 16-23 (2014).
- Mukhopadhyay S, Mondal SA, Kumar M, et al. Proinflammatory and antiinflammatory attributes of fetuin-a: a novel hepatokine modulating cardiovascular and glycemic outcomes in metabolic syndrome. Endocr. Pract 20(12), 1345-1351 (2014).
- Ramsey JM, Cooper JD, Bot M, et al. Sex Differences in Serum Markers of Major Depressive Disorder in the Netherlands Study of Depression and Anxiety (NESDA). PLoS. One 11(5), e0156624 (2016).
- Kanai M, Raz A, Goodman DS. Retinol-binding protein: the transport protein for vitamin A in human plasma. J. Clin. Invest 47(9), 2025-2044 (1968).
- Monaco HL, Zanotti G. Three-dimensional structure and active site of three hydrophobic molecule-binding proteins with significant amino acid sequence similarity. Biopolymers 32(4), 457-465 (1992).
- Cowan SW, Newcomer ME, Jones TA. Crystallographic refinement of human serum retinol binding protein at 2A resolution. Proteins 8(1), 44-61 (1990).
- Chen PY, Huang MC, Chiu CC, et al. Association of plasma retinol-binding protein-4, adiponectin, and high molecular weight adiponectin with metabolic adversities in patients with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 35(8), 1927-1932 (2011).
- Wong CT, Tsoi WF, Saha N. Acute phase proteins in male Chinese schizophrenic patients in Singapore. Schizophr. Res 22(2), 165-171 (1996).
- Maes M, Scharpe S, Van Grootel L, et al. Higher alpha 1-antitrypsin, haptoglobin, ceruloplasmin and lower retinol binding protein plasma levels during depression: further evidence for the existence of an inflammatory response during that illness. J. Affect. Disord 24(3), 183-192 (1992).
- Alkharfy KM, Al-Daghri NM, Vanhoutte PM, et al. Serum retinol-binding protein 4 as a marker for cardiovascular disease in women. PLoS. One 7(10), e48612 (2012).
- Lambadiari V, Kadoglou NP, Stasinos V, et al. Serum levels of retinol-binding protein-4 are associated with the presence and severity of coronary artery disease. Cardiovasc. Diabetol 13(1), 121 (2014).
- van Roon-Mom WM, Reid SJ, Faull RL, et al. TATA-binding protein in neurodegenerative disease. Neuroscience 133(4), 863-872 (2005).
- Perez MK, Paulson HL, Pendse SJ, et al. Recruitment and the role of nuclear localization in polyglutamine-mediated aggregation. J. Cell. Biol 143(6), 1457-1470 (1998).
- Uchihara T, Fujigasaki H, Koyano S, et al. Non-expanded polyglutamine proteins in intranuclear inclusions of hereditary ataxias--triple-labeling immunofluorescence study. Acta. Neuropathol 102(2), 149-152 (2001).
- van Roon-Mom WM, Reid SJ, Jones AL, et al. Insoluble TATA-binding protein accumulation in Huntington's disease cortex. Brain Res. Mol. Brain. Res 109(1-2), 1-10 (2002).
- Koide R, Kobayashi S, Shimohata T, et al. A neurological disease caused by an expanded CAG trinucleotide repeat in the TATA-binding protein gene: a new polyglutamine disease? Hum. Mol. Genet 8(11), 2047-2053 (1999).
- Blacker D, Bertram L, Saunders AJ, et al. Results of a high-resolution genome screen of 437 Alzheimer's disease families. Hum. Mol. Genet 12(1), 23-32 (2003).
- Reid SJ, van Roon-Mom WM, Wood PC, et al. TBP, a polyglutamine tract containing protein, accumulates in Alzheimer's disease. Brain Res. Mol. Brain. Res 125(1-2), 120-128 (2004).
- Yamagata K. Roles of HNF1alpha and HNF4alpha in pancreatic beta-cells: lessons from a monogenic form of diabetes (MODY). Vitam. Horm 95(1), 407-423 (2014).
- Zhang B, Wang J, Wang X, et al. Proteogenomic characterization of human colon and rectal cancer. Nature 513(7518), 382-387 (2014).
- Kobel M, Kalloger SE, Carrick J, et al. A limited panel of immunomarkers can reliably distinguish between clear cell and high-grade serous carcinoma of the ovary. Am. J. Surg. Pathol 33(1), 14-21 (2009).
- Offman SL, Longacre TA. Clear cell carcinoma of the female genital tract (not everything is as clear as it seems). Adv. Anat. Pathol 19(5), 296-312 (2012).
- Vaxillaire M, Boccio V, Philippi A, et al. A gene for maturity onset diabetes of the young (MODY) maps to chromosome 12q. Nat. Genet 9(4), 418-423 (1995).
- Fuste M, Pinacho R, Melendez-Perez I, et al. Reduced expression of SP1 and SP4 transcription factors in peripheral blood mononuclear cells in first-episode psychosis. J. Psychiatr. Res 47(11), 1608-1614 (2013).
- Radde J, Loning T, Bamberger AM. Expression pattern of the CCAAT/enhancer-binding protein C/EBP-beta in gestational trophoblastic disease. Int. J. Gynecol. Pathol 23(4), 373-377 (2004).
- Maes M, Delange J, Ranjan R, et al. Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotropic drugs. Psychiatry. Res 66(1), 1-11 (1997).
- Yang Y, Wan C, Li H, et al. Altered levels of acute phase proteins in the plasma of patients with schizophrenia. Anal. Chem 78(11), 3571-3576 (2006).
- Huang TL. Decreased serum albumin levels in Taiwanese patients with schizophrenia. Psychiatry Clin. Neurosci 56(6), 627-630 (2002).