Research Article - (2018) Volume 8, Issue 4
Rosiglitazone Enhances the Phagocytic Ability of Thrombin-Activated Microglia through P38mapk Signaling
- Corresponding Author:
- Guofeng Wu
Postal address: No. 28 Guiyi Street, Guiyang(550004), PR China
Tel: +86-1380-9431-723
Abstract
Objectives
Hematoma formed after intracerebral hemorrhage (ICH) could results in secondary brain damage. In vitro thrombin treatment allows investigation of the pathology and mechanisms behind brain injury, and provides extracellular environment that simulates the conditions that result from ICH. Rosiglitazone treatment protects against brain damage through promoting the phagocytosis of red blood cells by microglia. The present study aimed to explore the underlying molecular mechanisms using the thrombin-induced model of ICH.
Methods
A total of 54 neonatal rats was used in the present study. Primary microglia were obtained from the brain tissues of neonatal SD rats, and then divided into 4 groups: normal control group (NC group), thrombin treatment group(TH group), RSG pretreatment + thrombin treatment group(RSG group), and RSG pretreatment + thrombin treatment together with p38MAPK signaling inhibitor group (SB group). Then, laser scanning confocal microscopywas used to evaluate microglial phagocytosis by observing phagocytizedred fluorescent microspheres. The mRNA and protein expression levels of CD36 were measured by RT-PCR and western blot.
Results
Microglial phagocytosis was increased in the RSG group compared with the other groups, and phagocytosis in the SB group was decreased compared to the RSG group. The RSG group displayed increased expression of CD36 compared to the other groups, both at protein and mRNA levels (P<0.01). The SB group expressed decreased levels of CD36 compared to the RSG group (P<0.01).
Conclusions
Rosiglitazone enhanced thrombin-induced phagocytosis in microglia;and the mechanism might involve increased expression of CD36 on the microglial surface through p38MAPK signaling. Keywords CD36, Intracerebral hemorrhage, Microglial cells, p38MAPK, Rosiglitazone, Thrombin
Keywords
CD36, Intracerebral hemorrhage, Microglial cells, p38MAPK, Rosiglitazone, Thrombin
Introduction
Intracerebral hemorrhage (ICH) is estimated at an overall global incidence of 24.6 per 100,000 person-years [1]. 1-year survival estimated at 46% and 5-year survival estimated at 29% [2]. Secondary injury after ICH is considered to be the main reason for the high morbidity and mortality. Although it has been widely studied in the past few decades, the complexity and variability of the mechanisms of injury after ICH have made it difficult to find effective treatments to improve the prognosis of patients [3,4]. One key process involved is the large amount of thrombin, hemoglobin and ferrous ion cell toxins that are released from lysed red blood cells (RBC) in the hematoma, which forms after ICH. These substances could then lead to secondary brain damage [5-9].
The role of thrombin in brain damage is complex: lower levels may be cytoprotective [10], but high levels cause hydrocephalus and white matter damage after ICH [11,12]. Thrombin induced brain injury has been used as a model in a wide range of experimental studies to reveal much about the pathology and mechanisms behind brain injury after ICH [13,14]. Thrombin has a central role in the coagulation cascade, where it mediates the conversion of fibrinogen to fibrin, the main constituent of a blood clot and activates the blood coagulation factors V, VIII, XI, XIII and the anticoagulant protein C. Thrombin generation results in receptor mediated inflammatory responses, cell proliferation and modulation, cell protection, and apoptosis [10].
Microglia are the immune effector cells of the central nervous system that play important roles in supporting nutrition, protection and restoration [15]. After ICH, activated microglia could recognize and phagocytize red blood cells around the hematoma and toxins released by RBClysis via the scavenger receptor CD36 that expressed onits surface [16]. Study suggests that CD36 only expressed in microglia around hematomas after ICH [17]. This process is a principal method of clearing the RBC around the hematoma after removal of the intracranial hematoma [16-18]. Thereby, microgliareduces secondary brain damage after ICH [19]. The phagocytosis or endocytosis through CD36 on the glial cell surface is regulated by PPARγ to complete removal of necrotic or apoptotic cell remnants [20]. PPAR γ plays an important role in activating microglia/macrophages [21], and increases expression of CD36 on microglia, promotes absorption of hematoma and improves prognosis after ICH [22].
Related research also showed that thrombin and inflammatory factors, such as tumor necrosis factor alpha (TNF α) and interleukin 1 (IL-1) could induce inducible nitric oxide synthase (iNOS) and other reactive oxygen species on microglia surface through the p38MAPK signaling pathway [23]. The p38MAPK is a signaling pathway closely associated with regulation of inflammation and oxidative stress [24]. Microglial phagocytosis in living slice cultures was inhibited by the p38MAPK inhibitor SB203580 but not microglial accumulation in the injured area [25], and Toll/interleukin-1 receptor domain-containing adapter inducing interferon-β (TRIF), upstream of p38MAPK, blocks induction of the interferon response and inhibits microglial phagocytosis of axon debris in vitro [26]. The survival and phagocytosis of activated microglia in vitro was promoted by MAPKs including p38 [27]. Adenosine A1 receptors can also reduce secondary brain damage after ICH via activating the p38MAPK signaling pathway [28]. These data indicated that p38MAPK plays an important role in microglial phagocytosis during clearing hematoma after ICH.
Rosiglitazone (RSG) is a thiazolidinedione drug, which is a PPARγ agonist. This drug could promote the expression of CD36 in microglia, which in turn promotes hematoma absorption and is likely to be protective against brain damage [29,30]. However, the downstream molecular mechanisms, through which does the RSG-PPARγ signal promote the phagocytosis of microglia, are still not well elucidated. It has also been shown that p38MAPK activation plays a key role downstream of the PPARγ signaling [31], indicating that as a PPARγ agonist, RSG might positively regulate the p38MAPK signaling.
Based on these previous studies, we proposed the research hypothesis that in the procoagulant extra-cellular environment resulting from ICH. RSG may increase the phagocytic capacity of microglia through activation of p38MAPK signaling, so as to remove the hematoma in a timely manner and reduce secondary brain damage. To confirm this we used thrombin-activated microglia as a model to observe the influence of RSG on CD36 expression and microglial phagocytosis.
Materials and Methods
▪Mixed culture of neurons and neuroglia
All the animal experiments were approved by the Animal Care We1fare Committee of Guizhou Medical University, China. The 1-2 day neonatal SD rats (provided by the Animal Experimental Center of Guizhou Medical University, China) were killed by CO2 inhalation. Brain tissues were separated from meninges and blood vessels under aseptic conditions, and put into2 ml DMEM/F12 medium (DMEM + 10% FBS + 1% Penicillin-Streptomycin + 1% glutamine) (Gibco, USA).The tissues in medium were digested with equal volume of 0.25% trypsin and then filtrated with a 200-mesh sieve. The filtrate was centrifugedfor 5 min at 1500 rpm, and the collected cells were inoculated into a culture flask (precoated with 0.001% of poly L-lysine (Sigma, USA)) at a density of 2×106, then cultured at 35°C with 5% CO2 for 14 days in a cell incubator.
▪ Purification and identification of microglia
Microglia was purified using a modified method based on a short shaking protocol to prevent damage to the cells (32). A mixture of neurons and neuroglia was cultured for 14 days and then placed on an orbital shaker under aseptic conditions, and shaken for 2 hours (200r/min, 37°C). The microglia fell off into the culture medium during shaking. A small volume of the medium was removed to count the microglia under a microscope. Then 2 ml of the medium was aspirated and labeled with OX-42 (which is murine antibody to the microglial marker CD11b/c; Abcam, USA; 1: 100). A DAB chromogenic reagent kit was also used to visualize the nuclei (CWbiotech, Beijing, China). PBS buffer (0.01 mol/L) was used as a negative control for the OX-42 labeling. The cells were observed under an inverted microscope and imaged.
▪ Cell grouping and treatments
Culture medium containing microglia was added to a six-well plate at a final density of 0.8×106 cells per well. 1.5 ml fresh DMEM/F12 medium was added. After overnight culture, the cells were divided into 4 groups: normal control group (NC group), thrombin treatment group (TH group), RSG pretreatment + thrombin treatment (RSG group) group, and RSG pretreatment + thrombin treatment together with p38MAPK signaling blocker group (SB group).
The TH group was supplemented with 2×104 U/L thrombin (Yige pharmaceutical Co. Ltd, Hunan, China). The RSG group was supplemented with 25 μmol/L RSG (Sigma, USA) for 1 hour and then subjected to 2×104 U/L thrombin treatment, and the SB group was supplemented with10 μmol/L SB203580 (MedchemExpress, USA) for 1 hour, then subjected to 25 μmol/L RSG treatment for 1 hour followed by 2×104 U/L thrombin treatment. The MC group had the same volume of DMEM/F12 medium added as the other groups received during thrombin treatment. The thrombin treatment lasted for 24 hours.
Cells from 6 neonatal SD rats were included in one sample and cells in the same sample were assigned to four groups equally. For western blot and RT-PCR analysis, 4 samples from 24 neonatal rats were used respectively. For the detection of microglial phagocytosis ability, only one sample was used. Cells from the same sample were assigned to four groups equally. A total of 54 neonatal SD rats were used in the present study.
▪ Observation of microglial phagocytosis by laser scanning confocal microscope
The microglia suspension was transferred to a laser confocal petri dish (NEST Biotechnology Co. Ltd., China), and then cultured with different treatments according to the cell grouping. The next day 5 μl red fluorescent microspheres (diameter: 0.5 μm, wave length: 575 nm; Sigma, USA) were added to each dish, and incubation was continued for 4 hours. The microglia were washed with PBS and then stained with DAPI to show the nuclei. After being incubated for 15 minutes in an incubator, the DAPI was discarded and the cells were washed with PBS and this solution was collected. The phagocytized red fluorescent microspheres were observed under a laser scanning confocal microscope (Carl Zeiss, Germany).
▪Western blots to detect CD36 protein expression
Microglia after different treatments according to grouping was lysed with cell lysis buffer and cell debris was collected and cracked to release residual liquid at 4°C and centrifuged at 12000 rpm. The centrifuged supernatant was evaluated by a BCA kit (CWbiotech, Beijing, China) and the total protein in each sample was calculated. An SDS-PAGE gel was made according to instructions of the kit (Solarbio, Beijing, China), and loaded into the electrophoresis system. Samples and standard proteins were loaded into lanes according to scheduled order. Electrophoresis was performed under a 20 mA constant current until the pre dye and marker band had clearly separated. After electrophoresis the SDS-PAGE gel was placed in a trans-blot, with a PVDF membrane, and the protein was transferred under a 200 mA constant current at low temperature for 2 hours. After transferred to membrane, had occurred rabbit anti rat CD36(Abcam, USA) (1:1000) and secondary antibody (Solarbio, Beijing, China) (1:200), were incubated at 4°C overnight. The antibody mixture was then discarded, and the membrane was washed 3 times with washing buffer. After exposure and developing with a high sensitivity chemiluminescence detection kit in a dark room, the resulting image was scanned and saved to computer.
▪ RT-PCR to detect CD36 geneexpression level
Microglia after treatment according to their grouping was extracted for total RNA by Trizol total RNA extract kit (TIANGEN, Beijing, China: wholly-owned subsidiary of QIAGEN, Germany). The resulting extraction was centrifuged at 12000 rpm for 10 min at 4°C, and RNA was precipitated with isopropyl alcohol at room temperature, and then centrifuged at 12000 rpm for 10 min at 4°C. The supernatant was discarded and the pellet dried at room temperature for 5 min; then dissolved in 20 μl DEPC water. 2 μl was removed to measure the RNA purity and concentration by Nanodrop2000 ultraviolet spectrophotometer (Thermo FisherScientific, USA), if the ratio of purity was between 1.8 to 2.2 the sample was used in the experiments. Reverse transcription of cDNA was performed using the PrimeScript ™ RT reagent Kit (TaKaRa, Japan) with gDNA Eraser, steps by following the instructions of the product manual. CD36 primers were synthesized by Invitrogen Beijing Office (Table 1). Using SYBR® Premix Ex Taq ™ II (TliRNaseH Plus) ROX Plus (TaKaRa, Japan) for amplification, the 45 cycles of amplification procedure followed after 95°C for 30 sec with. The melting curve at 60 to 95°C was analyzed for a single-peak at melting curve.
| Primer | Sequence (5'to3') | Product (bp) |
|---|---|---|
| CD36 forward | ATGAGACTGGGACCATCGGC | 123 |
| CD36 reverse | CAACAAACATCACTACTCCAACACC | |
| GAPDH forward | CCTTCCGTGTTCCTACCCC | 131 |
| GAPDH reverse | GCCCAGGATGCCCTTTAGTG |
Table 1: Primers used in this study.
▪ Statistical analysis
SPSS13.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Measurement data were shown as mean ± standard deviation. One-way analysis of variance (ANOVA) was used for mean value comparison among multiple samples; then for post-hoc test, the Student– Newman–Keuls (SNK) method was used if the data met homoscedasticity criteria, otherwise the Dunnett’s T3 test was performed. P < 0.05 meant a significant statistical difference.
Results
▪ Primary microglia culture
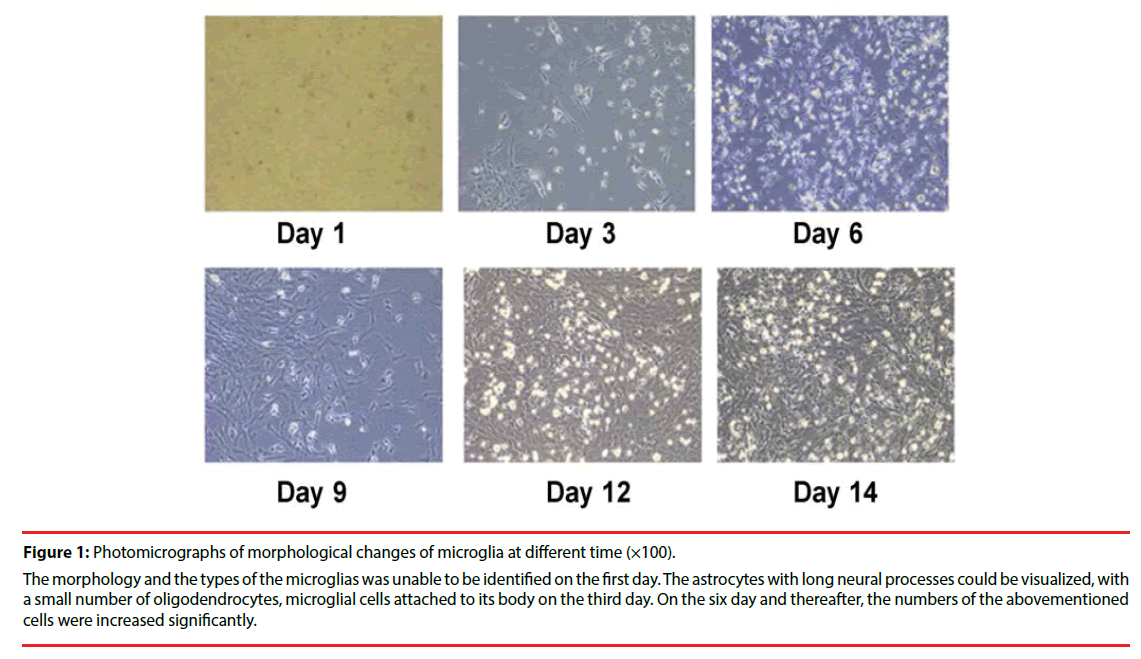
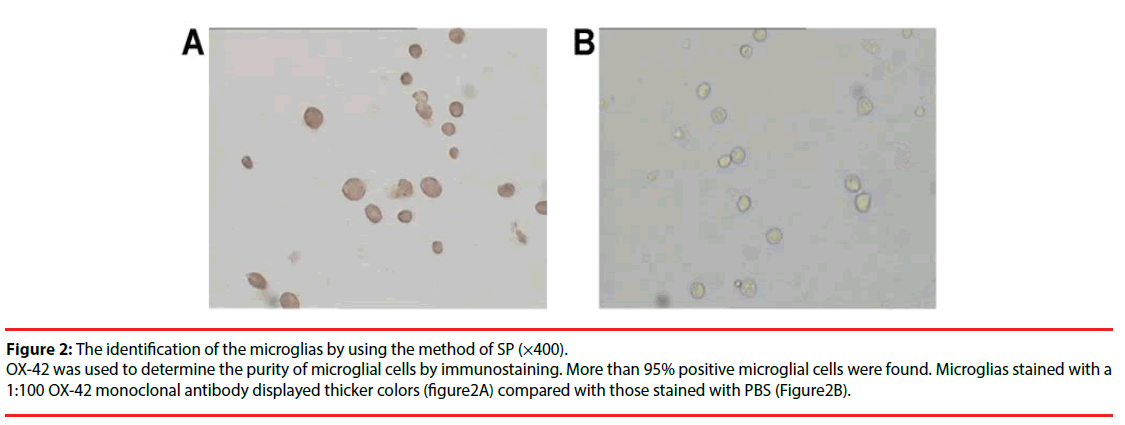
Microglia isolated from the short vibrational method were observed and imaged under a high resolution microscope every 3 days during cell culture (Figure 1). They reached 1.8×106/mm2 at the 14th day of culture. Labeling with OX- 42 to indicate microglia (Figure 2), showed the percentage of positive cells (purity) was above 95%.
Figure 1: Photomicrographs of morphological changes of microglia at different time (×100).
The morphology and the types of the microglias was unable to be identified on the first day. The astrocytes with long neural processes could be visualized, with a small number of oligodendrocytes, microglial cells attached to its body on the third day. On the six day and thereafter, the numbers of the abovementioned cells were increased significantly.
Figure 2: The identification of the microglias by using the method of SP (×400).
OX-42 was used to determine the purity of microglial cells by immunostaining. More than 95% positive microglial cells were found. Microglias stained with a 1:100 OX-42 monoclonal antibody displayed thicker colors (figure2A) compared with those stained with PBS (Figure2B).
▪ Phagocytosis
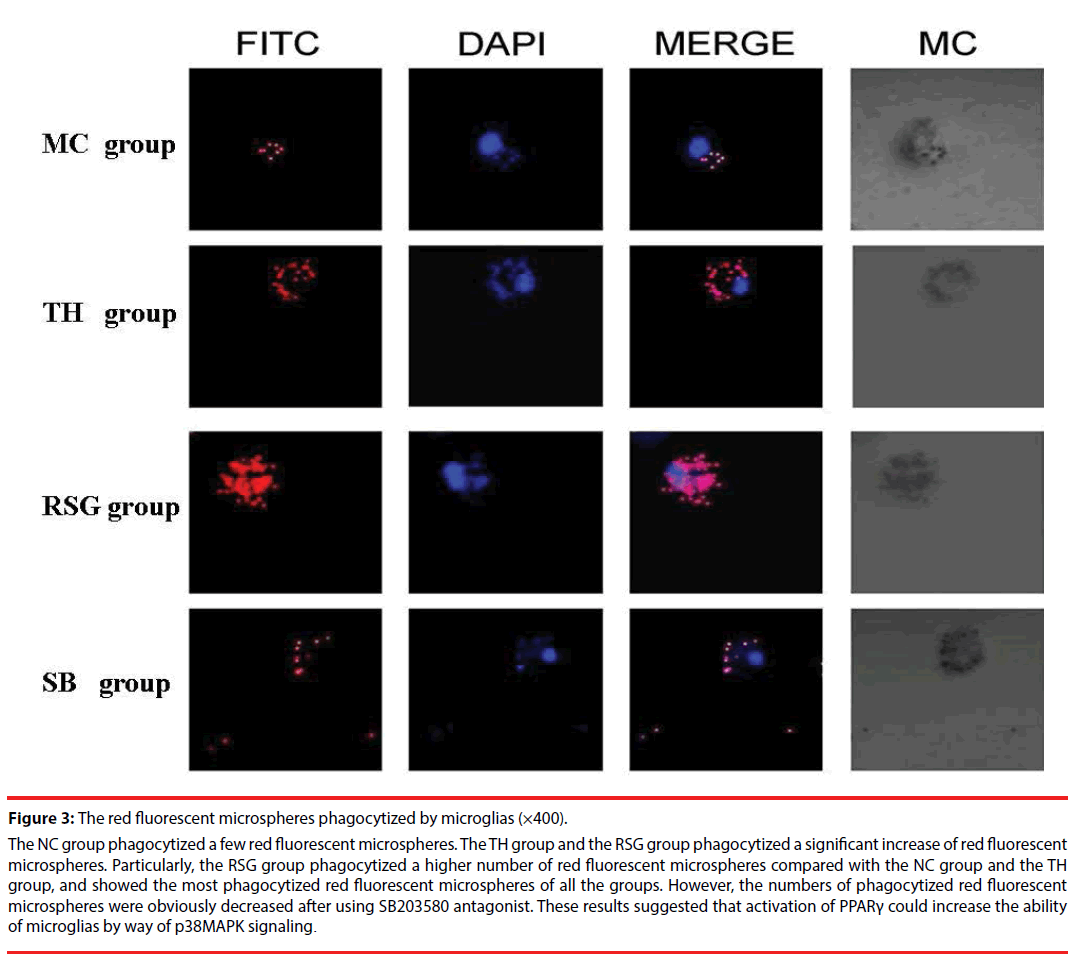
Each group of microglia showed phagocytosis under a 400 times laser scanning confocal microscope, the TH group phagocytized more red fluorescent microspheres than the NC group, the RSG group phagocytized a higher number of red fluorescent microspheres compared with the NC group and the TH group, and showed the most phagocytized red fluorescent microspheres of all the groups. After using SB203580 antagonist the numbers of phagocytized red fluorescent microspheres were obviously decreased (Figure 3).
Figure 3: The red fluorescent microspheres phagocytized by microglias (×400).
The NC group phagocytized a few red fluorescent microspheres. The TH group and the RSG group phagocytized a significant increase of red fluorescent microspheres. Particularly, the RSG group phagocytized a higher number of red fluorescent microspheres compared with the NC group and the TH group, and showed the most phagocytized red fluorescent microspheres of all the groups. However, the numbers of phagocytized red fluorescent microspheres were obviously decreased after using SB203580 antagonist. These results suggested that activation of PPARγ could increase the ability of microglias by way of p38MAPK signaling.
▪ Expression of CD36
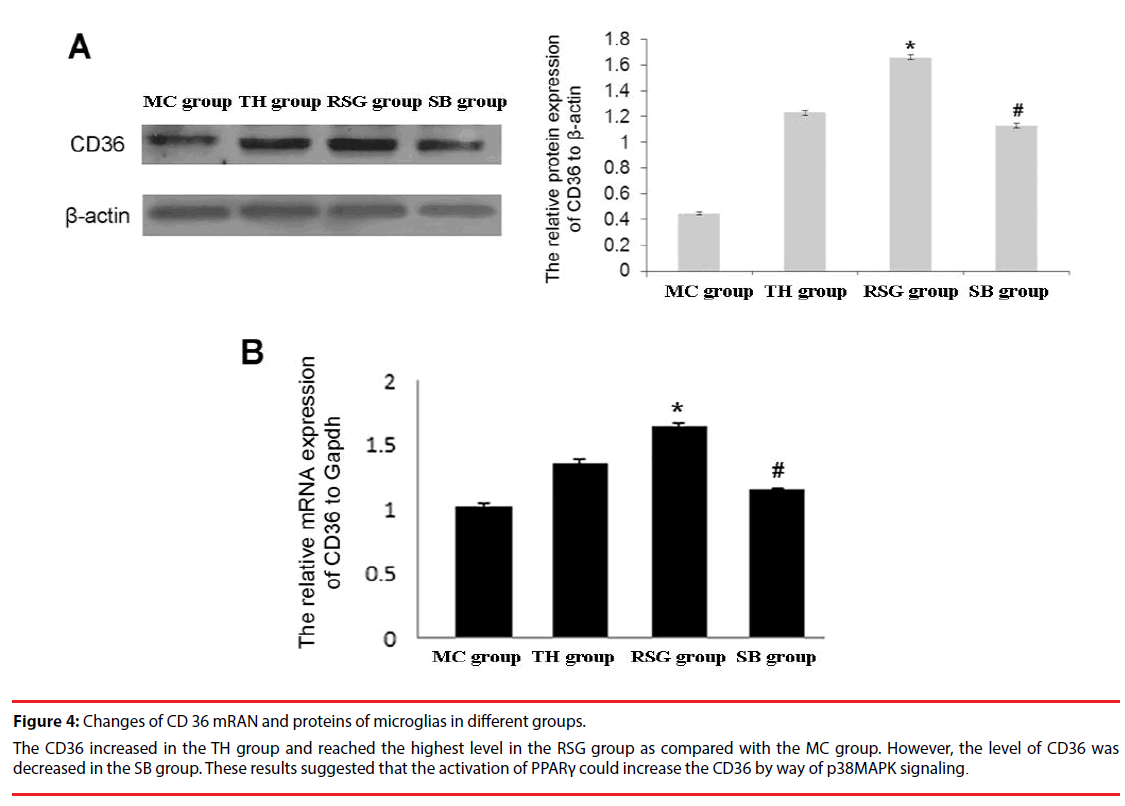
CD36 protein was detected in all 4 groups: the expression level in RSG group was significantly higher than in the TH group (P<0.01), and expression in SB group was obviously lower than in the RSG group (Figure 4A).
Figure 4: Changes of CD 36 mRAN and proteins of microglias in different groups.
The CD36 increased in the TH group and reached the highest level in the RSG group as compared with the MC group. However, the level of CD36 was decreased in the SB group. These results suggested that the activation of PPARγ could increase the CD36 by way of p38MAPK signaling.
CD36 mRNA was detected in all 4 groups: the RSG group also had significantly higher level compared with the TH group (P < 0.01), and expression of SB group was obviously lower than the RSG group (Figure 4B).
Discussions
In the present study, the thrombin -activated microglia was used as a model to observe the influence of RSG on microglial phagocytosis after ICH, and to explore the underlying molecular mechanisms. The results demonstrated that the RSG could enhance the phagocytosis of the microglia, and the phagocytosis of the microglia was inhibted by using SB203580 (an inhibitor of p38 MAPK). The RSG group expressed higher levels of CD36 protein and mRNA than the other groups, which were reversed by SB203580 treatment. Taken together these results suggested RSG increase thrombin-induced phagocytosis in microglia, and the mechanism might involve increased expression of CD36 on the microglia surface through p38MAPK signaling.
The neural protective nature of PPARγ agonists for the clinical treatment of ICH, stroke, spinal cord injury and traumatic brain injury is currently being studied, mostly in terms of the reduction of inflammation [32-36]. Other studies suggest that these agonists may exert neuroprotective effects via the inhibition of neuronal apoptosis and autophagy following experimental traumatic brain injury [37] and decrease matrix metalloproteinase-9 and blood-brain barrier disruption after ICH [38]. However, previous studies have shown that the PPAR γ agonist RSG could promote the expression of CD36 in microglia, which in turn promotes hematoma absorption [29,30]. The present study supports this view as the RSG could increase phagocytosis of thrombinactivated microglia. The mechanism underlying the effects of RSG on the phagocytosis might be through the p38MAPK signaling pathway. Such effects may occur after the RSG upregulated the CD36. Therefore, these results suggest that PPAR γ agonist therapy, if found to be a suitable new treatment, is likely to reduce secondary brain damage after ICH and improve the outcome of the patients with ICH.
The p38MAPK antagonist was used in this study based on the facts that the p38MAPK signaling pathway has an important role in microglial phagocytosis of injured neurons [25-27]. Adenosine A1 receptors could also reduce secondary brain damage after ICH via activating the p38MAPK signaling pathway [28]. It has also been shown that p38MAPK activation plays a key role downstream of the PPARγ signaling [31]. This study supports the view that RSG might positively regulate p38MAPK signaling through PPARγ. However, more studies are needed to fully investigate all the other factors involved in this signaling pathway to fully elucidate the mechanism of RSG promoting thrombin-induced microglia phagocytosis.
Conclusions
PPARγ agonist RSG could promote thrombin-induced microglial phagocytosis, the mechanism of which may be related to the upregulation of the CD36 on the microglial cell surface and the activation of p38MAPK signaling by RSG. Therefore, we hope that PPARγ agonists could function as a new treatment to reduce secondary brain damage after ICH, thereby improving the patient’s cure rate and survival rate.
Acknowledgements
We are grateful for the help provided by the Clinical Research Centre of Affiliated Hospital of Guizhou Medical University. We also wish to thank all the postgraduates that were involved in this study for their hard work.
Source of Funding
This research was supported by the Natural Science Foundation of China (81460185/H0906) and the Guizhou Science and Technology Foundation (2013, 2043), as well as the Project of Guizhou Provincial Talent Team for Science and Technology Innovation ((2014)2040). The Funding body did not take part in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Conflict of Interest
The authors declare that there is no conflict ofinterest in this study.
References
- van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 9(1), 167-176 2010.
- Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 85(1), 660-667 (2014).
- Hemphill JC, 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 46(1), 2032-2060 (2015).
- Fiorella D, Zuckerman SL, Khan IS, et al. Intracerebral Hemorrhage: A Common and Devastating Disease in Need of Better Treatment. World. Neurosurg 84(1), 1136-1141 (2015).
- Xiong XY, Yang QW. Rethinking the roles of inflammation in the intracerebral hemorrhage. Transl. Stroke. Res; 6(1), 339-341 (2015).
- Chen S, Yang Q, Chen G, et al. An update on inflammation in the acute phase of intracerebral hemorrhage. Transl Stroke Res 6(1), 4-8 (2015).
- Hua Y, Nakamura T, Keep RF, et al. Long-term effects of experimental intracerebral hemorrhage: the role of iron. J. Neurosurg 104(1), 305-312 (2006).
- Lee ST, Chu K, Sinn DI, et al. Erythropoietin reduces perihematomal inflammation and cell death with eNOS and STAT3 activations in experimental intracerebral hemorrhage. J. Neurochem 96(1), 1728-1739 (2006).
- Han N, Ding SJ, Wu T, et al.Correlation of free radical level and apoptosis after intracerebral hemorrhage in rats. Neurosci. Bull 24(1), 351-358 (2008).
- Krenzlin H, Lorenz V, Danckwardt S, et al. The Importance of Thrombin in Cerebral Injury and Disease. Int. J. Mol. Sci 17 (2016).
- Gao F, Liu F, Chen Z, et al. Hydrocephalus after intraventricular hemorrhage: the role of thrombin. J. Cereb. Blood. Flow. Metab 34(1), 489-494 (2014).
- Ni W, Gao F, Zheng M, et al. Effects of Aerobic Capacity on Thrombin-Induced Hydrocephalus and White Matter Injury. Acta. Neurochir. Suppl 121(1), 379-384 (2016).
- Tao Y, Li L, Jiang B, et al. Cannabinoid receptor-2 stimulation suppresses neuroinflammation by regulating microglial M1/M2 polarization through the cAMP/PKA pathway in an experimental GMH rat model. Brain. Behav. Immun; 58(1), 118-129 (2016).
- Wu CH, Chen CC, Lai CY, et al. Treatment with TO901317, a synthetic liver X receptor agonist, reduces brain damage and attenuates neuroinflammation in experimental intracerebral hemorrhage. J. Neuroinflammation 13(1), 62 (2016).
- Kettenmann H, Hanisch UK, Noda M, et al. Physiology of microglia. Physiol. Rev 91(1), 461-553 (2011).
- Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 42(1), 1781-1786 (2011).
- Fang H, Chen J, Lin S, et al. CD36-mediated hematoma absorption following intracerebral hemorrhage: negative regulation by TLR4 signaling. J. Immunol 192(1), 5984-5992 (2014).
- Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet. Neurol; 11(1), 720-731 (2012).
- Jesberger JA, Richardson JS. Oxygen free radicals and brain dysfunction. Int. J. Neurosci 57(1), 1-17 (1991).
- Das Gupta S, Sae-tan S, Wahler J, et al. Dietary gamma-Tocopherol-Rich Mixture Inhibits Estrogen-Induced Mammary Tumorigenesis by Modulating Estrogen Metabolism, Antioxidant Response, and PPARgamma. Cancer. Prev. Res. (Phila) 8(1), 807-816 (2015).
- Bernardo A, Ajmone-Cat MA, Gasparini L, et al. Nuclear receptor peroxisome proliferator-activated receptor-gamma is activated in rat microglial cells by the anti-inflammatory drug HCT1026, a derivative of flurbiprofen. J. Neurochem 92(1), 895-903 (2005).
- Flores JJ, Klebe D, Rolland WB, et al.PPARgamma-induced upregulation of CD36 enhances hematoma resolution and attenuates long-term neurological deficits after germinal matrix hemorrhage in neonatal rats. Neurobiol. Dis; 87(1), 124-133 (2016).
- Ryu J, Pyo H, Jou I, et al. Thrombin induces NO release from cultured rat microglia via protein kinase C, mitogen-activated protein kinase, and NF-kappa B. J. Biol. Chem 275(1), 29955-29959 (2000).
- Ma XL, Kumar S, Gao F, et al.Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation 99(1), 1685-1691 (1999).
- Katayama T, Kobayashi H, Okamura T, et al. Accumulating microglia phagocytose injured neurons in hippocampal slice cultures: involvement of p38 MAP kinase. PLoS. One 7(1), e40813 2012.
- Hosmane S, Tegenge MA, Rajbhandari L, et al. Toll/interleukin-1 receptor domain-containing adapter inducing interferon-beta mediates microglial phagocytosis of degenerating axons. J. Neurosci 32(1), 7745-7757 (2012).
- Ohnishi M, Katsuki H, Izumi Y, et al. Mitogen-activated protein kinases support survival of activated microglia that mediate thrombin-induced striatal injury in organotypic slice culture. J. Neurosci. Res 88(1), 2155-2164 (2010).
- Zhai W, Chen D, Shen H, et al. A1 adenosine receptor attenuates intracerebral hemorrhage-induced secondary brain injury in rats by activating the P38-MAPKAP2-Hsp27 pathway. Mol. Brain 9(1), 66 (2016).
- Zhao X, Grotta J, Gonzales N, et al. Hematoma resolution as a therapeutic target: the role of microglia/macrophages. Stroke 40(1), S92-94 .
- Zhao X, Sun G, Zhang J, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann. Neurol 61(1), 352-362 (2007).
- Tamashiro TT, Dalgard CL, Byrnes KR. Primary microglia isolation from mixed glial cell cultures of neonatal rat brain tissue. J. Vis. Exp e3814 (2012).
- Wang S, Awad KS, Elinoff JM, et al. G Protein-coupled Receptor 40 (GPR40) and Peroxisome Proliferator-activated Receptor gamma (PPARgamma): AN INTEGRATED TWO-RECEPTOR SIGNALING PATHWAY. J. Biol. Chem; 290(1), 19544-19557 (2015).
- Zhao XR, Gonzales N, Aronowski J. Pleiotropic role of PPARgamma in intracerebral hemorrhage: an intricate system involving Nrf2, RXR, and NF-kappaB. CNS. Neurosci. Ther 21(1), 357-366 (2015).
- Xu F, Li J, Ni W, Shen YW, et al. Peroxisome proliferator-activated receptor-gamma agonist 15d-prostaglandin J2 mediates neuronal autophagy after cerebral ischemia-reperfusion injury. PLoS. One 8(1), e55080 (2013).
- Gonzales NR, Shah J, Sangha N, et al. Design of a prospective, dose-escalation study evaluating the Safety of Pioglitazone for Hematoma Resolution in Intracerebral Hemorrhage (SHRINC). Int. J. Stroke 8(1), 388-396 (2013).
- Liu H, Rose ME, Culver S, et al. Rosiglitazone attenuates inflammation and CA3 neuronal loss following traumatic brain injury in rats. Biochem. Biophys. Res. Commun 472(1), 648-655 (2016).
- Yao J, Zheng K, Zhang X. Rosiglitazone exerts neuroprotective effects via the suppression of neuronal autophagy and apoptosis in the cortex following traumatic brain injury. Mol. Med. Rep 12(1), 6591-6597 (2015).
- Wu G, Wu J, Jiao Y, et al. Rosiglitazone infusion therapy following minimally invasive surgery for intracerebral hemorrhage evacuation decreases matrix metalloproteinase-9 and blood-brain barrier disruption in rabbits. BMC. Neurol 15(1), 37 (2015).