Review Article - (2019) Volume 9, Issue 1
Role of N-Acylethanolamines in the Neuroinflammation: Ultramicronized Palmitoylethanolamide in the Relief of Chronic Pain and Neurodegenerative Diseases
Enza Palazzo1*, Livio Luongo1, Francesca Guida1, Vito de Novellis1, Serena Boccella1, Claudia Cristiano2, Ida Marabese1 and Sabatino Maione1*
1Department of Experimental Medicine, the University of Campania “L. Vanvitelli”, Naples, Italy
2Department of Pharmacy, the University of Naples “Federico II”, Naples, Italy
- Corresponding Authors:
- Enza Palazzo
Department of Experimental Medicine, the University of Campania “L. Vanvitelli”, via Costantinopoli 16, 80138 Naples, Italy
Fax: +390815667503
Sabatino Maione
Department of Experimental Medicine, the University of Campania “L. Vanvitelli”, via Costantinopoli 16, 80138 Naples, Italy
Fax: +390815667503
Abstract
Pain and neuroinflammation are protective responses aimed at preventing and removing injurious stimuli. However, when prolonged, they can override the bounds of physiological control and become destructive. Chronic pain and neuroinflammation are critical components in the pathophysiology of neurodegenerative diseases, stroke, spinal cord injury, diabetes, and neuropsychiatric disorders. Natural mechanisms, including the production of lipid mediators, represent an endogenous protective process and a program of resolution stimulated and triggered by tissue injury or inflammation. Lipid mediators include N-acylethanolamines (NAEs) such as palmitoylethanolamide (PEA), an endocannabinoid anandamide congener which has shown to be endowed of neuroprotective and antinflammatory properties activated under several pathological states. PEA does not bind the classical cannabinoid receptors but indirectly stimulates the effects of cannabinoids. Its antinflammatory, analgesic and neuroprotective actions have been however associated with peroxisome proliferator-activated receptor-α (PPAR-α) activation. The administration of exogenous PEA requires parenteral routes owing to its lipid structure. The micronized and ultramicronized (m- and um-) formulation permits oral administration increasing the versatility, easiness and compliance of administrations in clinical studies. This review is intended to deal with the effects of m- and um-PEA on chronic pain and neuroinflammation in several animal models of chronic pain and neudegenerative disorders and in clinical studies.
Keywords
Palmitoylethanolamide, Neuroinflammation, Chronic Pain, Neurodegenerative Disorders, Micronized and Ultramicronized Formulation, Peroxisome Proliferator-Activated Receptor-α
Introduction
Acute pain brings an alarm to the body of possible injuries and has normally a protective purpose. Conversely, chronic pain does not convey any useful information and has no any biological benefit. Chronic pain is a major clinical problem that affects up to 30% of persons in the world. It includes neuropathic pain, caused by a dysfunction, damage or degeneration of the sensory nervous system. It gives a feeling of general discomfort and lowers the quality of life. Medications, massage therapy, acupuncture, electrical stimulation, nerve blocks, and surgery are some traditional therapies for chronic pain: all unsatisfactory. So far there are no drugs or treatments able to relieve chronic pain in an effective and definitive way. Increasing evidence suggest that neuroinflammation is a common mechanism of several central nervous system (CNS) diseases including chronic pain [1] but also Alzheimer’s and Parkinson’s diseases, lateral amyotrophic and multiple sclerosis, and psychiatric disorders [2-5]. A bidirectional signaling reciprocally connecting the immune system to the central nervous system promotes the development and maintenance of both, chronic pain and neurodegeneration. The principal cellular players in neuroinflammation are glial cells, whose activation has been shown to be involved in Alzhaimer’s disease, Parkinson,s disease, cerebral ischemia, multiple and amyotrophic lateral sclerosis (MS and SLA), and mood disorders [6-9]. Among glial cells, microglia can either favor the recovery of injury in the CNS by scavenging dead cells and releasing factors promoting neuron survival or, under prolonged activation, induce autoimmune responses, neural death and brain injury [10-13]. Thus, microglia exerts a dual role in neurodegeneration, acting both, as instigators of damage and as guardians of brain homeostasis. In responses to signals generated by immune cells, neurodegeneration or the accumulation of folded proteins, microglia can multiply and assume the proinflammatory phenotype: the activated primed microglia [14]. Primed microglia responds more vigorously to inflammatory signals driving to deleterious consequences. Glia represents also the link between neuroinflammation and neuropathic pain [15]. Microglia in particular proved to be activated under peripheral injury-inducedneuropathic pain [15,16]. Under inflammatory conditions spinal microglia release interleukin- 1β; (IL-1β;), which through the activation of its receptor phosphorylates NR1 subunit of N-methyl-D-aspartate (NMDA) receptors facilitating pain transmission [17]. IL- 1β, whose expression level proved too be increased at spinal and supraspinal level, acts as a neuromodulator increasing synaptic plasticity under inflammatory or neuropathic pain conditions [18,19]. Microglia at dorsal horn level can be also responsible of an up-regulation of purinergic receptors, whose genetic or pharmacological blockade has shown to relieve neuropathic pain [20-22].
Neuroinflammation in chronic pain
Chronic pain represents a substantial and growing therapeutic need. Among all types of chronic pain, neuropathic pain, resulting from damage, degeneration or dysfunction in the sensory system remains almost untreatable. Neuropathic pain is associated with spinal cord injury, multiple sclerosis, stroke, cancer, diabetes and other metabolic diseases. A bidirectional signaling between the immune system and the nervous system contributes to the development and maintenance of neuropathic pain [19,23]. Neuropathic pain development is strictly dependent on Schwann cells, spinal microglia, astrocytes and peripheral immune system elements [24]. Microglia plays a central role in the coordination between the immune system and brain. For example, following infections or nervous system injuries, microglia switch to the “activated” form acquiring an inflammatory cell phenotype. Activated microglia release cytokines such as TNF-α and IL-1β and inflammatory chemokines [25-27] facilitating the recruitment of leukocytes in the brain [28]. Activated microglia undergoes cytoskeleton rearrangements altering the expression pattern of the receptors on the cell surface. These alterations allow microglia to reach the sites of lesions or infections [29] and increase their phagocyte efficiency [30]. If on one hand the activation of microglia and the release of cytokines are aimed to protect against injury, on the other hand the persistent activation of microglia drives to pathological changes such as pain-related affective and cognitive disorders [31]. Activated microglia represents also the convergence point between neuroinflammation and chronic pain. TNF-α and IL-1β released from spinal microglia, act as neuromodulators following peripheral nerve injury and increase synaptic plasticity leading to peripheral and central sensitization, at the base of both, inflammatory and neuropathic pain. Indeed, increased IL-1β/TNF-α released by microglial cells and astrocytes at the spinal cord level, as well as in the brain, were observed in neuropathic mice [18,19]. Il-1β by engaging its own receptors induces phosphorylation of the NR1 subunit of N-methyl-D-aspartate receptors facilitating pain transmission [17]. A cytokinesmediated immune system imbalance has been shown to be associated with neuropathic pain development in both, human and animal studies [32,33]. Oligodendrocytes play also an important role in the induction of neuropathic pain. The over-expression of the oligodendrocyte-derived IL-33 at spinal levels contributes to a further release of proinflammatory cytokines such as TNF-α and IL-1β [34]. Another important role in neuroinflammation at the base of chronic pain appears to be mediated by mast cells, which represent the signaling link between peripheral inflammation and the brain. Indeed mast cells move easily through the blood-brain barrier in normal and under pathological conditions [35]. Mast cells are the player of phagocytosis, antigene presentation, adaptive immune response regulation, IgE switching by B cells [36] and chemokines--induced eosinophil recruitment [37]. Mast cell proliferation and degranulation under pathological conditions involving autoimmune demyelination [38] produce algogenic mediators sensitizing nociceptors and contributing to neuropathic pain [39]. Infiltration and activation of mast cells have been found after spinal cord injury [40,41]. The role of glial and mast cells in neuroinflammation is strengthen by their reciprocal interaction [42,43]. A signal pathway activated by toll like receptors 2 and 4 (TLR2/TLR4) expressed on mast cell surface evokes microglia recruitment [44]. The activation of mast cells also up-regulates CCL5/ RANTE signaling, which in turn induces the switch of microglia into the pro-inflammatory phenotype. Reciprocally microglial release of IL-6 and CCL5 induces the expression of TLR2 and TLR4 on mast cells. The cross-talk between mast cells and microglia involves also the complement system and in particular C5a, which appears to be up- regulated together with its cognate receptor [45] on mast cells, astrocytes and activated microglia. A cross-talk between mast cells and astrocytes and microglia and astrocytes has been also described throughout the involvement of CD40-CD40L and translocator protein, respectively [46,47].
Neuroinflammation in neurogenerative disorders
Apart from common mechanisms, such as the activation of microglia, neuroinflammation occurring in neurodegenerative diseases is however different from that occurring under chronic pain conditions. Causative agents of neurodegeneration have yet to be identified. Neurodegeneration occurs after viral insult and mostly in various ‘neurodegenerative diseases’, generally observed in the elderly, such as Alzheimer’s disease, multiple and amyotrophic lateral sclerosis, Parkinson’s disease, all negatively associated with mental and physical functioning. Viruses are able to injure neurons directly or by apoptosis induction [48] leading to neurodegeneration [49]. Neurodegeneration induced by viruses suggests an important role of immune responses in neuron degeneration [50]. Immune activation in the CNS involves microglia and astrocytes [51] which constitute the resident immune cells of the CNS and play an important role in the regulation of homeostasis of the brain during development, adulthood and aging [52]. Indeed microglia constantly survey the microenvironment surrounding astrocytes and neurons and in response to pathogen invasion or tissue damage promote an inflammatory response engaging the immune system [53]. Inflammation may result in the production of neurotoxic factors amplifying and increasing the persistence of the disease state. These neurotoxic mediators are mainly interleukins and other cytokines which throughout the activation of their own receptors trigger intracellular mechanisms conveying in protein degradation, mitochondria dysfunction, axonal transport impairments and apoptosis [54-56]. The chronic activation of pro-inflammatory signals increases also the vulnerability to neuropsychiatric disorders [57]. The putative mechanism linking the neuroinflammation and depression involves oxidative stress, elevated pro-inflammatory cytokines IL-6 and IL-8, endothelial nitric oxide synthase uncoupling and hyperglutamatergism. Indeed in major depressive disorder increased inflammatory markers are strictly associated with depressive symptoms and high risk of suicide [58].
From molecular mechanisms to putative therapeutic strategies: the lipid mediators
Targeting the specific processes and molecules involved in neuroinflammation may provide new therapeutic opportunities for treating chronic pain and neurodegenerative diseases. Consistently with the crucial role played by nonneuronal cells such as microglia in neuropathic pain, the inhibition of microglial activation by minocycline prevents/delays neuropathic pain development [59-61]. Intrathecal injection of astroglial toxin fluorocitrate [62,63] and L-alpha-aminoadipate [64] also reverses nerve injury- or nerve inflammation-induced mechanical allodynia. Another approach to treat neuroinflammaory mechanisms at the base of chronic pain and neurodegenerative diseases would consist in exploiting and eventually strengthen the endogenous mechanisms able to defend against inflammation. So far a considerable number of “resolution players”, whose stimulation is triggered by tissue inflammation or damage have been identified. These mediators operate an endogenous protective process and their enhancement may represent a natural approach to switch off the neuroinflammatory mechanisms at the base of neurological and psychiatric diseases. Among the several resolution program players, lipid mediators have shown a protective role in inflammatory processes [65,66]. Increasing evidence have suggested that lipid molecules suppress the inflammatory process, rescue homeostasis in damaged tissues and reduce pain sensitivity by affecting neural pathways involved in the processing of nociceptive simuli from the periphery to the CNS. Moreover, the level and/ or the actions of these molecules have shown to be lower in chronic inflammatory disease [67]. Among lipid mediators the fatty acid amides, N-acylethanolamines, are natural compounds which are composed by a fatty acid and ethanolamine. N-acyethanolamines include the endocannabinoid N-arachidonoylethanolamine, anadamide (AEA), and several congeners such as the N-stearoylethanolamine, N-oleylethanolamine (OEA) and N-palmitoylethanolamide (PEA). AEA, OEA and PEA share anabolic and catabolic pathways. They are formed from N-arachidonylphosphatidylethanolamine (NAPE) by several enzymatic pathways: that one involving the NAPE-phospholipase D is the principal one [68]. N-acylethanolamines are hydrolyzed mainly by the fatty acid amide hydrolase (FAAH), an intracellular integral membrane protein belonging to the amidase family of enzymes, which catalyses the hydrolysis of N-acyletethanolamines into the corresponding fatty acids and ethanolamine [69]. Recently, another enzyme not related to FAAH, known as N-acylethanolamine-hydrolyzing acid amidase (NAAA) has been found to hydrolyze preferentially PEA [70]. PEA has been proposed to maintain cellular homeostasis during inflammatory and neurodegenerative conditions and to function as a protective endogenous mediator counteracting inflammation, neuronal damage and pain [71,72]. The role of PEA as a player in the inflammation resolution program is supported by several evidence: i) mast cells and microglia synthesize and metabolize PEA [73,74], ii) PEA negatively modulate mast cell degranulation and microglia activation [75,76] and iii) the concentration of PEA increases in brain areas and spinal cords following peripheral nerve or spinal nerve injury as well as following stroke [77-79]. Being the role of PEA as mediator of resolution of inflammatory process already ascertained, three mechanisms have been proposed for its anti-inflammatory and analgesic effect. The first mechanism, which does not exclude the other two, suggests that PEA acts via an autacoid local inflammation antagonism by down-regulating mast cell degranulation [75]. PEA also acts by enhancing the antiinflammatory and anti-nociceptive effects exerted by AEA, which is often produced together with PEA, and indirectly activates cannabinoid CB1 and CB2 receptors or transient receptor potential vanilloid receptor type 1 (TRPV1) channels, an effect known as the “entourage effect” [80,81], Moreover PEA directly stimulates the nuclear peroxisome proliferator-activated receptor-α (PPAR-α), which clearly mediates many of the anti-inflammatory effects of this compound [82]. However, a yet uncharacterized cannabinoid CB2 receptor-like target [83,84], or the orphan receptor G-protein coupling, GPR55 [85], have been also proposed as target of PEA. A recent study has also suggested a new mechanism of action, through which PEA may affect cannabinoid signaling by upregulating CB2 receptor expression in mononuclear phagocytic cells [86].
The effect of um-PEA on chronic pain
The analgesic property of PEA has emerged in several models of inflammatory pain conditions induced by formalin [83,87], prostaglandins [88], magnesium sulfate [83], kaolin [83,87], carrageenan [76,80,89,90], nerve growth factor [91], and turpentine [92,93]. The antinflammatory and analgesic effects of PEA were initially attributed to peripheral CB2 receptors [83,87,93]. Later on PPARα was proposed to mediate the effect of PEA in inflammatory pain conditions since GW7647, a PPARα selective agonist, mimic, the effect of PEA and since the anti-hyperalgesic effect of PEA was absent in PPARα null mice [90,94]. The action of PEA in inflammation-induced hyperalgesia proved to be also dose dependent [88,95], and to be associated with the suppression of pro-inflammatory enzymes such as cyclooxygenase and endothelial and inducible nitroxide synthases [96]. PEA indeed plays an important role in maintaining cellular homeostasis in inflammatory states. It is produced by microglia and mast cells and, in turn, down-modulates mast cell and microglia activation [77,97,98]. The analgesic properties of PEA have been confirmed in neuropathic pain models [84,98-104]. The levels of PEA proved to be increased in pain-controlling brain areas and in the spinal cord following neuropathic pain development [78]. PEA beneficial actions in neuropathic pain conditions depend always on PPARα activation since they were either absent in PPARα null mice or blocked by PPARα antagonists [100,105]. The analgesic properties of PEA depend also on the modulation of non-neuronal cells in a chronic constriction injury- (CCI) and formalin-induced models of neuropathic pain [98,101]. Moreover, PEA restores the glutamatergic synapse proteins and the changes in amino acid release, the levels of which were deeply altered in the spared nerve injury model of neuropathic pain [103]. PEA also relieved hyperalgesia and allodynia in a model of oxaliplatin-induced neuropathy, either following acute or repeated treatment. In this model of neuropathic pain PEA also produced an improvement in motor coordination [106]. In another study PEA reverted thermal hyperalgesia and mechanical allodynia that developed 14 day after a mild traumatic brain injury model in mice [107]. PEA and anandamide share the activation and desensitization of TRPV1, a non-selective cation channel activated by noxious heat, low pH and capsaicin, the major active constituent of chilli, whose role in pain transmission has been widely reported [108-114]. PEA has shown to enhance the action of AEA on human TRPV1 [115] in a way inhibited by CB2 receptor antagonist [87,93]. Microglia and mast cells have shown to express both, TRPV1 and CB2 receptors, whose activation/desentization may inhibit their activation [86,116]. CB1, PPAR-α and TRPV1 receptors proved to be involved in the anti-hyperalgesic and anti-allodynic effect of PEA in neuropathic pain conditions [84]. Other proposed mechanisms for PEA in counteracting pain include ATP-sensitive K+ channels [88], transient receptor potential (TRP) channels [117], NF-kB [90], Ca2+-activated potassium channels [105] and NR2b subunit of NMDA receptor [103]. Most of the studies about PEA efficacy on pain have been carried out with parenteral administration. The lipid structure of PEA limits the solubility and the oral bioavailability of PEA. The micronization and ultramicronization is a technology often used in the pharmaceutical field to reduce large drug crystals under the micron range (<10 μm). The micronized and ultra-micronized formulation of PEA (m-PEA and um-PEA) facilitates the dissolution and reduces the absorption variability of PEA after oral administration. The analgesic effect of orally administered m-PEA and um- PEA was firstly demonstrated in a carrageenanmodel of inflammatory pain by Impellizzeri et al. [118].
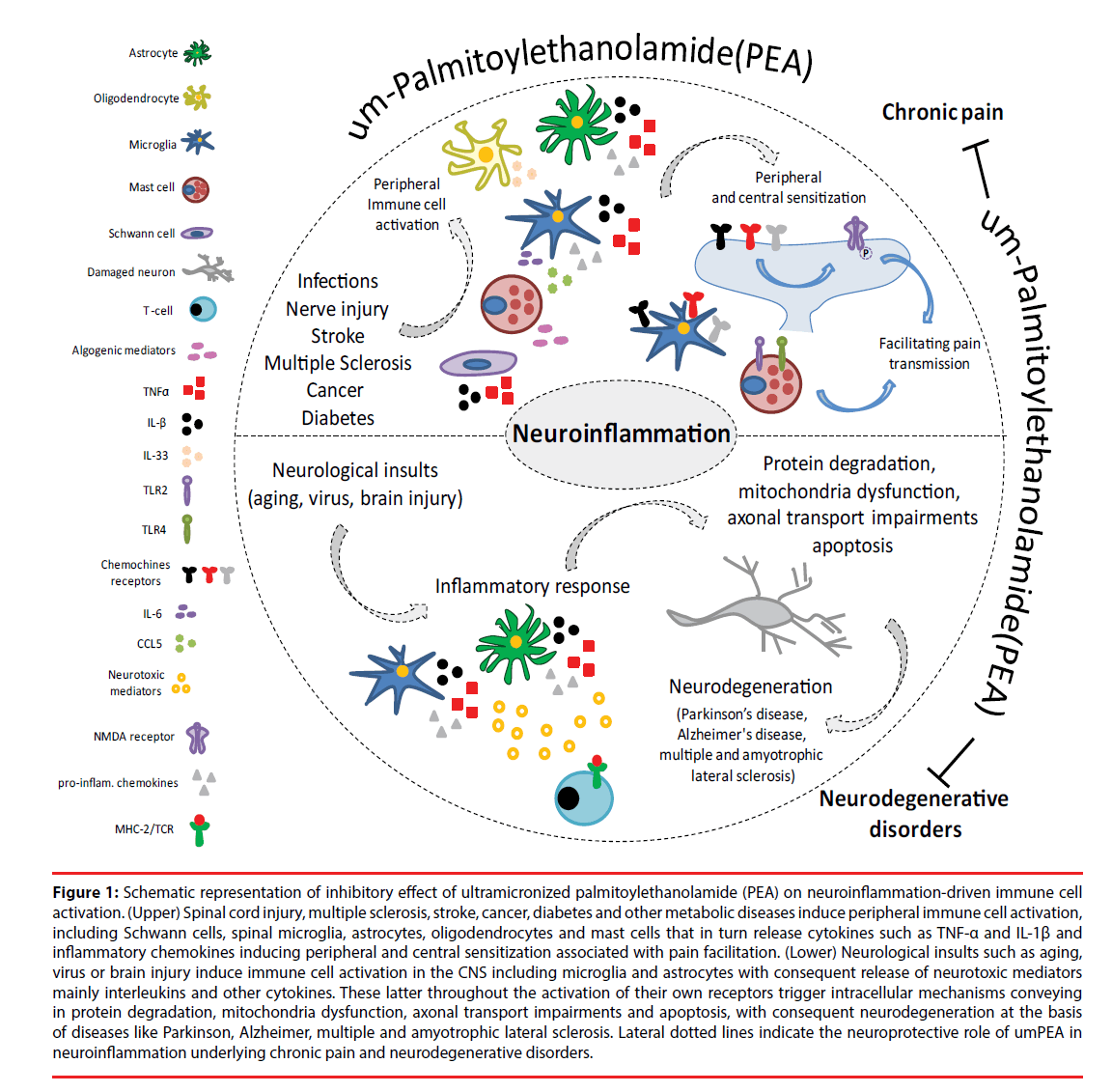
The effects of PEA have been tested in several clinical studies under several different pain conditions. Differently from preclinical studies in which PEA has been administered via parenteral route, in humans the oral administration is the most popular and accepted administration route for its easiness, versatility and the high compliance of the patients. Accordingly, the administration form of PEA was in most case through oral tablets and rarely sachets (sublingual preparations). The first clinical study using m-PEA or um-PEA revealed an analgesic effect in a diabetic patients suffering of peripheral neuropathy [119]. The effectiveness of um-PEA was also demonstrated in patients suffering from lumbosciatalgia or neuropathic pain of different etiologies [120,121]. The study has demonstrated that PEA either alleviated pain scores or potentiated the effect of other concomitant analgesic therapies [121,122]. The analgesic efficacy of PEA was also demonstrated in patients suffering from traumatic neuropathic pain or diabetes [123]. Two recent meta-analysis studies reported that not only PEA was effective in inducing analgesia in several chronic pain conditions but it did not produce adverse reactions worthy of notes [124,125]. Contrariwise, Andresen and colleagues reported that um-PEA did not alleviate pain in patients with spinal cord injuryinduced neuropathic pain, compared to placebotreated patients [126]. The efficacy of PEA in clinical trials and meta-analysis is however reviewed [127,128]. The actions of PEA on the neuroinflammation processes urderlying chronic pain are summarized in Figure 1.
Figure 1: Schematic representation of inhibitory effect of ultramicronized palmitoylethanolamide (PEA) on neuroinflammation-driven immune cell activation. (Upper) Spinal cord injury, multiple sclerosis, stroke, cancer, diabetes and other metabolic diseases induce peripheral immune cell activation, including Schwann cells, spinal microglia, astrocytes, oligodendrocytes and mast cells that in turn release cytokines such as TNF-α and IL-1β and inflammatory chemokines inducing peripheral and central sensitization associated with pain facilitation. (Lower) Neurological insults such as aging, virus or brain injury induce immune cell activation in the CNS including microglia and astrocytes with consequent release of neurotoxic mediators mainly interleukins and other cytokines. These latter throughout the activation of their own receptors trigger intracellular mechanisms conveying in protein degradation, mitochondria dysfunction, axonal transport impairments and apoptosis, with consequent neurodegeneration at the basis of diseases like Parkinson, Alzheimer, multiple and amyotrophic lateral sclerosis. Lateral dotted lines indicate the neuroprotective role of umPEA in neuroinflammation underlying chronic pain and neurodegenerative disorders.
The effect of um-PEA on neurodegenerative diseases
Neurodegeneration, such as that observed in Alzheimer’s and Parkinson’s diseases (AD and PD) and multiple and amyotrophic lateral sclerosis (MS and ALS), consists of a gradual neuronal cell death affecting a specific cellular population of the CNS causing a progressive disability such as cognitive impairments, behavioural and motor dysfunctions up to paralysis. PEA has demonstrated to play a neuroprotective role in several experimental models of neurodegenerative diseases. In animal model of Parkinson’s disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) the chronic treatment with PEA counteracted behavioral impairments, motor dysfunction, the loss of nigrostriatal neurons, astrocytes activation, altered expression of iNOS and microtubule-associated proteins. Such effects were PPARα-dependent [129]. A co-um-PEA/ luteolin composite proved to be neuroprotective in the same Parkinson’s model. It indeed protects against inflammation and increased autophagia [130]. In an AD model produced by i.c.v. injection of amyloid-β 25-35 (Aβ25- 35) peptide PEA ameliorated the behavioral impairments and reduced lipid peroxidation, inducible NO synthase (iNOS) and caspase-3 activation [131]. This effect was reproduced by the GW7647, a PPAR-α agonist, [131]. PEA counteracted the increased expression of glial fibrillary acidic protein (GFAP) and S100β, two typical proteins of astrocytes activation, and that of amyloidogenic proteins BACE1 and APP and phosphorylated τ in a different AD model, consisting of the injection of amyloid-β1-42 (Aβ1-42) peptide into the hippocampus [132].
The treatment with PEA also restored the cognitive behavior and the altered expression of microtubule-associated protein (MAP-2) in a PPARα-dependent way [132]. The co-umPEA/ luteolin composite was also effective in reverting the altered expression of iNOS, GFAP and brainderived neurotrophic factor (BDNF) expression in hippocampal slice culture exposed to Aβ1-42 peptide [133]. Thus, the neuroprotective effect of PEA is based on its capability to revert the altered expression of proteins strictly associated with AD or PD and to down-regulated the activation of pro-inflammatory/pro-apoptotic factors leading to neural loss. PEA has also shown to improve the clinical conditions of a patient suffering from ALS, in particular the respiration and the electromyography [134]. In a more recent clinical study um-PEA counteracted the respiratory worsening and delayed the tracheotomy and death in ALS patients when compared with untreated patients [135]. The neuroprotective effects of PEA have also been shown in several animal models of MS. The levels of PEA proved to be increased in the spinal cord of mice with chronic relapsing experimental autoimmune encephalomyelitis (CREAE), Theiler’s murine encephalomyelitis, virus-induced demyelinating disease (TMEV-IDD) and myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis (MOG-EAE) [136,137]. The plasma levels of PEA were also increased in remitting and secondary progressive MS patients [138]. The more relevant aspect of these studies was that PEA treatment ameliorated motor impairment and spasticity [136,137]. Importantly, the behavioural effect of PEA treatment was associated with a reduced expression of inflammatory cytokines, demyelination and axonal damages [137,139]. Thus, PEA seems to enhance or compensate endogenous defense mechanisms counteracting neuroinflammation and neurodegeneration development. The molecular target through which PEA ameliorates MS and ASL symptoms and slows the progression of the diseases has not been investigated yet. Figure 1 summarizes the different mechanisms through which um-PEA inhibits neuroinflammation processes urderlying neudegenerative diseases.
PEA and Sirtuin-1, sharing a common target or a direct interaction?
Silent information regulator 1 (Sirt1), a NADdependent deacetylase, through deacetylating acetylated histone and other specific substrates plays a relevant role in many physiopathological conditions such as diabetes, cardiovascular disorders, cancer, neurodegeneration and neuropathic pain [140,141]. Abnormal histone acetylation is suggested to be a characteristic gene modification and a transcription factor-mediated epigenetic mechanism underlying neuropathic pain [143-144]. The role of Sirt1 and Sirt1 activator (resveratrol) in alleviating neuropathic pain is more and more emerging [140,142,146]. There are not at the moment clear evidence suggesting that the effects of PEA are mediated thought a PPAR-α-Sirt1 complex. Rather, PEA and Sirt1 share a common mechanism, consisting in the activation of PPAR-α [146-148]. Sirt1 potentiates the effect of PPAR-α, the main target of PEA involved in the antinflammatory, analgesic and neuroprotective effects [82,90,94,100]. Pertinent to this, PEA and Sirt1 could play a PPAR-α mediated sinergic action. Resveratrol as well plays antinflammatory and neuroprotective actions with beneficial effects in neurological disorders including neuropathic pain [140,146,149,150]. Reciprocally, inhibitors of Sirt1 either worsen symptoms of neuropathic pain (and other neurological diseases) or block the effect of Sirt1 or Sirt1 activators [140]. Sirt1, such as PEA, has multitarget-mediated effects which altogether contribute to its protective effects in the nervous system. Studies investigating the neuroprotective effects of PEA in Sirt1 −/− mice or in combination with Sirt1 inhibitors, would certainly clarify whether the neuroprotective effect of PEA involves Sirt1 or a Sirt1/PPAR-α complex. The possible interaction of PEA with Sirt1 is also relevant considering the role of the latter in mitophagy [151]. Mitophagy assures the turnover of defective mitochondria associated with the pathogenesis of several neurological disorders and constitutes a protective mechanism avoiding detrimental consequences on neuron function and plasticity [152,153,154]. Sirt1 controls also the proaptotic gene p53, whose dysregulation is associated with autoimmune diseases [155]. Thus Sirt1 beneficial effect in neuropathic pain is also likely associated with its important role on immune response. Due to its important role in pathological processes including those associated with neurological disease and neuropathic pain development, novel therapeutic strategies aimed at enhancing Sirt1 action may have an unlimited potential [156]. PEA and Sirt1, playing similar effects and sharing a common target, could be exploited (either in combined therapies) to counteract the detrimental processes associated with in neurological disorders including neuropathic pain.
Conclusions
The capability of PEA to modulate protective response in inflammatory, neurodegenerative and chronic pain conditions suggests that endogenous PEA may be a component of the homeostatic system involved in the resolution program of neuroinflammation. Consequently, the administration of exogenous PEA, or the inhibition of PEA degradation may be a therapeutic strategy to counteract the neuroinflammation at the base of several neurological disorders. Alternatively, the discovery of the targets and mechanisms underlying the neuroprotective effects of PEA may drive to the development of novel effective treatment for neurodegenerative and chronic pain diseases. The molecular target through which PEA plays its anti-inflammatory, analgesic and neuroprotective role is PPARα [82,158], even if several indirect mechanisms, known as the “entourage effect” have been demonstrated. The entourage effect includes the endocannabinoid-mediated activation of CB1, CB2 and TRPV1 receptors [114,159] or PPARα-mediated TRPV1 desensitization [60,160]. Moreover, the PPARα direct activation by PEA does not exclude the contribution of the entourage effects thus justifying the multiple and the large spectrum effects of PEA. The development of novel formulations such as m- or um-PEA or the combination with the flavonoid luteolin with antioxidant property has improved the clinical efficacy of PEA. Moreover, PEA was avoided of adverse reactions worthy of notes in clinical studies. Preclinical and clinical studies about PEA efficacy in neurological disease report encouraging outcomes and are considerably multiplying in these last years, which is already a clear sign of its enormous therapeutic potential.
References
- Myers RR, Campana WM, Shubayev VI. The role of neuroinflammation in neuropathic pain: mechanisms and therapeutic targets. Drug. Discov. Today 11(1-2), 8-20 (2006).
- McGeer PL, McGeer EG. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta. Neuropathol 126(4), 479-97 (2013).
- Najjar S, Pearlman DM, Alper K, et al. Neuroinflammation and psychiatric illness. J. Neuroinflammation 10(1), 43 (2013)
- Amor S, Peferoen LA, Vogel DY, et al. Inflammation in neurodegenerative diseases- an update. Immunology 142(2), 151-66 (2014).
- Chen WW, Zhang X, Huang WJ. Role of neuroinflammation in neurodegenerative diseases. Mol. Med. Rep 13(4), 3391-6 (2016).
- Frakes AE, Ferraiuolo L, Haidet-Phillips AM, et al. Microglia induce motor neuron death via the classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron 81(5), 1009-1023 (2014).
- Morales I, Guzmán-Martínez L, Cerda-Troncoso C, et al. Neuroinflammation in the pathogenesis of Alzheimer's disease. A rational framework for the search of novel therapeutic approaches. Front. Cell. Neurosci 8(1), 112 (2014).
- Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat. Rev. Neurosci 15(15), 300-312 (2014).
- Peferoen LA, Vogel DY, Ummenthum K et al. Activation status of human microglia is dependent on lesion formation stage and remyelination in multiple sclerosis. J. Neuropathol. Exp. Neurol 74(1), 48-63 (2015).
- González-Scarano F1, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu. Rev. Neurosci 22(1), 219-240 (1999).
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron 39(6), 889-909 (2003).
- Przedborski S, Vila M, Jackson-Lewis V. Neurodegeneration: what is it and where are we?. J. Clin. Invest 111(1), 3-10 (2003).
- Ghosh A, Roy A, Liu X, et al. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proc. Natl. Acad. Sci. USA 104(47), 18754-18759 (2007).
- Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol 10(4), 217-224 ( 2014).
- Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol. Ther 126(1), 56-68 (2010).
- Luongo L, Petrelli R, Gatta L, et al. 5'-Chloro-5'-deoxy-(±)-ENBA, a potent and selective adenosine A(1) receptor agonist, alleviates neuropathic pain in mice through functional glial and microglial changes without affecting motor or cardiovascular functions. Molecules 17(12), 13712-13726 (2012).
- Guo W, Wang H, Watanabe M, et al. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J. Neurosci 27(22), 6006-6018 (2007).
- Giordano C, Cristino L, Luongo L, et al. TRPV1-dependent and -independent alterations in the limbic cortex of neuropathic mice: impact on glial caspases and pain perception.Cereb. Cortex 22(11), 2495-2518 (2012).
- Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug. Discov 13, 533-548 (2014).
- Chessell IP, Hatcher JP, Bountra C, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 114(3), 386-396 (2005).
- Tsuda M, Kuboyama K, Inoue T, et al. Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol. Pain 11(5), 28 (2009).
- Ballini E, Virginio C, Medhurst SJ, et al. Characterization of three diaminopyrimidines as potent and selective antagonists of P2X3 and P2X2/3 receptors with in vivo efficacy in a pain model. Br. J. Pharmacol 163(6), 1315-1325 (2011).
- Pinho-Ribeiro FA, Verri WA Jr, Chiu IM. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends. Immunol 38(1), 5-9 (2017).
- DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain 90(1-2), 1-6 (2001).
- Clark AK, Wodarski R, Guida F, et al. Cathepsin S release from primary cultured microglia is regulated by the P2X7 receptor. Glia 58(14), 1710-1726 (2010).
- Schwartz M, Kipnis J, Rivest S, et al. How do immune cells support and shape the brain in health, disease, and aging? J. Neurosci 33(45), 17587-17596 (2013).
- Luongo L, Guida F, Imperatore R, et al. The A1 adenosine receptor as a new player in microglia physiology. Glia 62(1), 122-132 (2014).
- Skaper SD, Facci L, Zusso M, et al. Neuroinflammation, Mast Cells, and Glia: Dangerous Liaisons. Neuroscientist 23(5), 478-498 (2017).
- Luongo L, Palazzo E, Tambaro S, et al. 1-(2',4'-dichlorophenyl)-6-methyl-N-cyclohexylamine-1,4-dihydroindeno[1,2-c]pyrazole-3-carboxamide, a novel CB2 agonist, alleviates neuropathic pain through functional microglial changes in mice. Neurobiol. Dis 37(1), 177-185 (2010).
- Russo MV, McGavern DB. Immune Surveillance of the CNS following Infection and Injury. Trends. Immunol 36(10), 637-650 (2015).
- Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci 8(6), 752-758 (2005).
- Disabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J. Neurochem 139 (S2), 136-153 (2016).
- Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J. Neuroimmunol 229(1-2), 26-50 (2010).
- Machelska H, Celik MÖ. Recent advances in understanding neuropathic pain: glia, sex differences, and epigenetics. F1000Res 5(1), 2743 (2016).
- Zarpelon AC, Rodrigues FC, Lopes AH, et al. Spinal cord oligodendrocyte-derived alarmin IL-33 mediates neuropathic pain. FASEB. J 30(1), 54-65 (2016).
- Silverman AJ, Sutherland AK, Wilhelm M, et al. Mast cells migrate from blood to brain. J. Neurosci 20(1), 401-408 (2000).
- Gauchat JF, Henchoz S, Mazzei G, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature 365(6444), 340-343 (1993).
- Wardlaw AJ, Moqbel R, Cromwell O, et al. Platelet-activating factor. A potent chemotactic and chemokinetic factor for human eosinophils. J. Clin. Invest 78(6), 1701-1706 (1986).
- Brenner T, Soffer D, Shalit M, et al. Mast cells in experimental allergic encephalomyelitis: characterization, distribution in the CNS and in vitro activation by myelin basic protein and neuropeptides. J. Neurol. Sci 122(1), 210-213 (1994).
- Xanthos DN, Gaderer S, Drdla R, et al. Central nervous system mast cells in peripheral inflammatory nociception. Mol. Pain 7(1), 42 (2011).
- Fiala M, Chattopadhay M, La Cava A, et al. IL-17A is increased in the serum and in spinal cord CD8 and mast cells of ALS patients. J. Neuroinflammation 7(1), 76 (2010).
- Esposito E, Paterniti I, Mazzon E, et al. Effects of palmitoylethanolamide on release of mast cell peptidases and neurotrophic factors after spinal cord injury. Brain Behav. Immun 25(6), 1099-1112 (2011).
- Skaper SD. Mast Cell - Glia Dialogue in Chronic Pain and Neuropathic Pain: Blood-Brain Barrier Implications. CNS. Neurol. Disord. Drug. Targets 15(9), 1072-1078 (2016).
- Skaper SD, Facci L, Zusso M, et al. An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front. Cell. Neurosci 12(1), 72 (2018).
- Pietrzak A, Wierzbicki M, Wiktorska M, et al. Surface TLR2 and TLR4 expression on mature rat mast cells can be affected by some bacterial components and proinflammatory cytokines. Mediators. Inflamm 2011(1), 427-473 (2011).
- Gasque P, Singhrao SK, Neal JW, et al. Expression of the receptor for complement C5a (CD88) is up-regulated on reactive astrocytes, microglia, and endothelial cells in the inflamed human central nervous system. Am. J. Pathol 150(1), 31-41 (1997).
- Kim DY, Hong GU, Ro JY. Signal pathways in astrocytes activated by cross-talk between of astrocytes and mast cells through CD40-CD40L. J. Neuroinflammation 8(1), 25 (2011).
- Wang M,Wang X, Zhao L, et al. Macroglia-microglia interactions via TSPO signaling regulates microglial activation in the mouse retina. J. Neurosci 34(10), 3793-3806 (2014).
- Amor S, Puentes F, Baker D, et al. Inflammation in neurodegenerative diseases. Immunology 129(2), 154-69 (2010).
- Shinya K, Shimada A, Ito T, et al. Avian influenza virus intranasally inoculated infects the central nervous system of mice through the general visceral afferent nerve. Arch. Virol 145(1), 187-195 (2000).
- Czirr E, Wyss-Coray T. The immunology of neurodegeneration. J. Clin. Invest 122(4), 1156-1163 (2012).
- Perry VH, Teeling J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin. Immunopathol 35(5), 601-612 (2013).
- Schwartz M, Kipnis J, Rivest S, et al. How do immune cells support and shape the brain in health, disease, and aging?. J. Neurosci 33(45), 17587-17596 (2013).
- Wyss-Coray T1, Mucke L. Inflammation in neurodegenerative disease--a double-edged sword. Neuron 35(3), 419-432 (2002)
- Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science 296(5575), 1991-1995 (2002).
- Chevalier-Larsen E, Holzbaur EL. Axonal transport and neurodegenerative disease. Biochim. Biophys. Acta 1762(11-12), 1094-108 (2006).
- Capuron L, Su S, Miller AH, et al. Depressive symptoms and metabolic syndrome: is inflammation the underlying link?. Biol. Psychiatry 64(10), 896-900 (2008)
- Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum. Mol. Genet 18(2), 169-176 (2009).
- Kessler D, Sharp D, Lewis G. Screening for depression in primary care. Br. J. Gen. Pract 55(518), 659-660 (2005).
- Raghavendra V1, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J. Pharmacol. Exp. Ther 306(2), 624-630 (2003).
- Hua XY, Svensson CI, Matsui T, et al. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur. J. Neurosci 22(10), 2431-2440 (2005).
- Ledeboer A1, Sloane EM, Milligan ED, et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 115(1-2), 71-83 (2005).
- Milligan ED, Twining C, Chacur M, et al. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J. Neurosci 23(3), 1026-1040 (2003).
- Hosoi R, Okada M, Hatazawa J, et al. Effect of astrocytic energy metabolism depressant on 14C-acetate uptake in intact rat brain. J. Cereb. Blood. Flow. Metab 24(2), 188-190 (2004).
- Zhuang ZY1, Wen YR, Zhang DR, et al. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J. Neurosci 26(13), 3551-360 (2006).
- Buckley CD, Gilroy DW, Serhan CN, et al. The resolution of inflammation. Nat. Rev. Immunol 13(1), 59-66 (2013).
- Piomelli D, Sasso O. Peripheral gating of pain signals by endogenous lipid mediators. Nat. Neurosci 17(2), 164-174 (2014).
- Morris T, Stables M, Colville-Nash P, et al. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc. Natl. Acad. Sci. USA 107(19), 8842-8847 (2010).
- Okamoto Y, Morishita J, Tsuboi K, et al. Molecular characterization of a phospholipase D generating anandamide and its congeners. J. Biol. Chem 279(7), 5298-5305 (2004).
- Cravatt BF, Giang DK, Mayfield SP, et al. Molecular characterization of an enzyme that degradesneuromodulatory fatty-acid amides. Nature 384(6604), 83-87 (1996).
- Ueda N, Yamanaka K, Yamamoto S. Purification and characterization of an acid amidase selective for N-palmitoylethanolamine, a putative endogenous anti-inflammatory substance. J. Biol. Chem 276(38), 35552-35557 (2001).
- Skaper SD, Facci L. Mast cell-glia axis in neuroinflammation and therapeutic potential of the anandamide congener palmitoylethanolamide. Philos. Trans. R. Soc. Lond. B. Biol. Sci 367(1607), 3312-332 (2012).
- Skaper SD, Facci L, Barbierato M, et al. Palmitoylethanolami-ne and Neuroinflammation: a Novel Therapeutic Strategy of Resolution. Mol. Neurobiol 52(2), 1034-1042 (2015).
- Bisogno T, Maurelli S, Melck D, et al. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J. Biol. Chem 272(6), 3315-3323 (1997).
- Muccioli GG, Stella N. Microglia produce and hydrolyze palmitoylethanolamide. Neuropharmacology 54(1), 16-22 (2008).
- Aloe L, Probert L, Kollias G, et al. Level of nerve growth factor and distribution of mast cells in the synovium of tumour necrosis factor transgenic arthritic mice. Int. J. Tissue React 15(1), 139-143 (1993).
- De Filippis D, Luongo L, Cipriano M, et al. Palmitoylethanolamide reduces granuloma-induced hyperalgesia by modulation of mast cell activation in rats. Mol. Pain 7(1), 3 (2011).
- Franklin A, Parmentier-Batteur S, Walter L, et al. Palmitoylethanolamide increases after focal cerebral ischemia and potentiates microglial cell motility. J. Neurosci 23(21), 7767-7775 (2003).
- Petrosino S, Palazzo E, de Novellis V, et al. Changes in spinal and supraspinal endocannabinoid levels in neuropathic rats. Neuropharmacology 52(2), 415-422 (2007).
- Jhaveri MD, Richardson D, Robinson I, et al. Inhibition of fatty acid amide hydrolase and cyclooxygenase-2 increases levels of endocannabinoid related molecules and produces analgesia via peroxisome proliferator-activated receptor-alpha in a model of inflammatory pain. Neuropharmacology 55(1), 85-93 (2008).
- Conti S, Costa B, Colleoni M, et al. Anti-inflammatory action of endocannabinoid palmitoylethanolamide and the synthetic cannabinoid nabilone in a model of acute inflammation in the rat. Br. J. Pharmacol 135(1), 181-187 (2002).
- Smart D, Jonsson KO, Vandevoorde S et al. ‘Entourage’ effects of N-acyl ethanolaminesat human vanilloid receptors. Comparison of effects upon anandamide induced vanilloid receptoractivation and upon anandamide metabolism. Brit. J. Pharmacol 136(3), 452-458 (2002).
- Lo Verme J, Fu J, Astarita G, et al.The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol 67(1), 15-19 (2005).
- Calignano A, La Rana G, Piomelli D. Antinociceptive activity of the endogenous fatty acid amide, palmitylethanolamide. Eur. J. Pharmacol 419(2-3), 191-198 (2001).
- Costa B, Comelli F, Bettoni I, et al. The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB(1), TRPV1 and PPAR gamma receptors and neurotrophic factors. Pain 139(1), 541-550 (2008).
- Ryberg E, Larsson N, Sjögren S et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol 152(7), 1092-1101 (2007).
- Guida F, Luongo L, Boccella S, et al. Palmitoylethanolamide induces microglia changes associated with increased migration and phagocytic activity: involvement of the CB2 receptor. Sci. Rep 7(1), 375 (2017).
- Calignano A, La Rana G, Giuffrida A, et al. Control of pain initiation by endogenous cannabinoids. Nature 394(6690), 277-281 (1998).
- Romero TR, Galdino GS, Silva GC, et al. Involvement of the L-arginine/nitricoxide/cyclic guanosine monophosphate pathway in peripheral antinociception induced by N-palmitoyl-ethanolamine in rats. J. Neurosci. Res 90(7), 1474-1479 (2012).
- Mazzari S, Canella R, Petrelli L, et al. N-(2-hydroxyethyl)hexadecanamide is orally active in reducing edema formation and inflammatory hyperalgesia by down-modulatingmast cell activation. Eur. J. Pharmacol 300(3), 227-236 (1996).
- D’Agostino G, La Rana G, Russo R, et al. Central administration of palmitoylethanolamide reduces hyperalgesia in mice via inhibition of NF-kappaB nuclear signalling in dorsal root ganglia. Eur. J. Pharmacol 613(1-3), 54-59 (2009).
- Farquhar-Smith WP, Rice AS. A novel neuroimmune mechanism in cannabinoid-mediated attenuation of nerve growth factor-induced hyperalgesia. Anesthesiology 99(6), 1391-401 (2003).
- Jaggar SI, Hasnie FS, Sellaturay S, et al. The antihyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain 76(1), 189-199 (1998).
- Farquhar-Smith WP, Rice AS. Administration of endocannabinoids prevents a referred hyperalgesia associated with inflammation of the urinary bladder. Anesthesiology 94(3), 507-513 (2001).
- Lo Verme J, Russo R, La Rana G, et al. Rapid broad-spectrum analgesia through activation ofperoxisome proliferator- activated receptor-alpha. J. Pharmacol. Exp. Ther 319(3), 1051-1061 (2006).
- Romero TR, Duarte ID. N-palmitoylethanolamine (PEA) induces peripheral antinociceptive effect by ATP-sensitive K+-channel activation. J. Pharmacol. Sci 118(2), 156-160 (2012).
- Costa B, Conti S, Giagnoni G, et al. Therapeutic effect of the endogenous fatty acid amide, palmitoylethanolamide, in rat acute inflammation: inhibition of nitric oxide and cyclooxygenasesystems. Br. J. Pharmacol 137(4), 413-420 (2002).
- Facci L, Dal Toso R, Romanello S et al. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc. Natl. Acad. Sci. USA 92(8), 3376-3380 (1995).
- Luongo L, Guida F, Boccella S, et al. Palmitoylethanolamide reduces formalin-induced neuropathic-like behaviourthrough spinal glial/microglial phenotypical changes in mice. CNS. Neurol. Disord. Drug. Targets 12(1), 45-54 (2013).
- Wallace VC, Segerdahl AR, Lambert DM, et al. The effect of the palmitoylethanolamide analogue, palmitoylallylamide (L-29) on pain behaviour in rodent models of neuropathy. Br. J. Pharmacol 151(7), 1117-1128 (2007).
- Di Cesare Mannelli L, D’Agostino G, Pacini A, et al. Palmitoylethanolamide is a disease-modifying agent in peripheral neuropathy: Pain relief and neuroprotection share a PPAR-alpha mediated mechanism. Mediators. Inflamm 2013(328797), 1-12 (2013).
- Bettoni I, Comelli F, Colombo A, et al. Non-neuronal cell modulation relieves neuropathic pain: Efficacy of the endogenous lipid palmitoylethanolamide. CNS Neurol. Disord. Drug. Targets 12(1), 34-44 (2013).
- Donvito G, Bettoni I, Comelli F, et al. Palmitoylethanolamide relieves pain and preserves pancreatic islet cells in a murine model of diabetes. CNS. Neurol. Disord. Drug. Targets 14(4), 452-62 (2015).
- Guida F, Luongo L, Marmo F, et al. Palmitoylethanolamide reduces pain-related behaviors andrestores glutamatergic synapses homeostasis in the medial prefrontalcortex of neuropathic mice. Mol. Brain 8(1), 47 (2015).
- Seol TK, Lee W, Park S, et al. Effect of palmitoylethanolamide on inflammatory and neuropathic pain in rats. Korean. J. Anesthesiol 70(5), 561-566 (2017).
- de Novellis V, Luongo L, Guida F, et al. Effects of intra-ventrolateral periaqueductal grey palmitoylethanolamide on thermoceptive threshold and rostral ventromedial medulla cell activity. Eur. J. Pharmacol 676(1-3), 41-50 (2012).
- Di Cesare Mannelli L, Pacini A, Corti F, et al. Antineuropathic profile of N-palmitoylethanolamine in a rat model of oxaliplatin-induced neurotoxicity. PloSOne 10(6), e0128080 (2015).
- Guida F, Boccella S, Iannotta M, et al. Palmitoylethanolamide Reduces Neuropsychiatric Behaviors by Restoring Cortical Electrophysiological Activity in a Mouse Model of Mild Traumatic Brain Injury. Front. Pharmacol 8(1), 95 (2017).
- Zygmunt PM, Petersson J, Andersson DA, et al. Vanilloid receptors on sensory nerves mediatethe vasod ilator action of anandamide. Nature 400(6743), 452-457 (1999).
- Palazzo E, Rossi F, Maione S. Role of TRPV1 receptors in descending modulation of pain. Mol. Cell. Endocrinol 286(1-2), 79-83 (2008).
- Palazzo E, Luongo L, de Novellis V, et al. Moving towards supraspinal TRPV1 receptors for chronic pain relief. Mol. Pain 6(1), 66 (2010).
- Palazzo E, Luongo L, de Novellis V, et al. Transient receptor potential vanilloid type 1 and pain development. Curr. Opin. Pharmacol 12(1), 9-17 (2012).
- Palazzo E, Rossi F, de Novellis V et al. Endogenous modulators of TRP channels. Curr. Top. Med. Chem 13(3), 398-407 (2013).
- Starowicz K, Makuch W, Osikowicz M, et al. Spinal anandamide produces analgesia in neuropathic rats: possible CB(1)- and TRPV1-mediated mechanisms. Neuropharmacology 62(4), 1746-1755 (2012).
- Petrosino S, Schiano Moriello A, Cerrato S, et al. The anti-inïìÃâammatory mediator palmitoylethanolamide enhances the levels of 2 -arachidonoyl-glycerol and potentiates its actions at transient receptor potential vanilloid type-1 channels. Br. J. Pharmacol 173(7), 1154-1162 (2016).
- De Petrocellis L, Davis JB, Di Marzo V. Palmitoylethanolamide enhances anandamide stimulation of human vanilloid VR1 receptors. FEBS. Lett 506(3), 253-256 (2001).
- Bíró T, Maurer M, Modarres S, et al. Characterization of functional vanilloid receptors expressed by mast cells. Blood 91(4), 1332-1340 (1998).
- Lowin T, Apitz M, Anders S, et al. Anti-inflammatory effects of N-acylethanolamines in rheumatoid arthritis synovial cells are mediated by TRPV1 and TRPA1 in a COX-2 dependent manner. Arthritis Res. Ther 17(1), 321 (2015).
- Impellizzeri D, Bruschetta G, Cordaro M, et al. Micronized/ultramicronized palmitoylethanolamidedisplays superior oral efïìÃÂcacy compared to non-micronized palmitoylethanolamide in a rat model of inïìÃâammatory pain. J. NeuroinïìÃâammation 11(1), 136 (2014).
- SchiïìÃÂlliti C, Cucinotta L, Fedele V, et al. Micronized p almitoylethanolamide reduces the symptoms ofneuropathic pain in diabetic pat ients. Pain Res. Treat 2014(1), 849623 (2014).
- [120] Domínguez CM, Martín AD, Ferrer FG, et al. N-palmitoylethanolamide in the treatment of neuropathic pain associated with lumbosciatica. Pain Manag. 2, 119–124 (2012).
- Gatti A, Lazzari M, Gianfelice V, et al. Palmitoylethanolamide in the treatment of chronic paincaused by different etiopathogenesis. Pain. Med 13(9), 1121-1130 (2012).
- Petrosino S, Di Marzo V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol 174(11), 1349-1365 (2017).
- Cocito D, Peci E, Ciaramitaro P, et al. Short-term efïìÃÂcacy of ultramicronized palmitoylethanolamide inperipheral neuropathic pain. Pain Res. Treat 2014(1), 8545603 (2014).
- Paladini A, Fusco M, Cenacchi T, et al. Palmitoylethanolamide, a Special Food for Medical Purposes,in the Treatment of Chronic Pain: A Pooled Data Meta-analysis. Pain. Physician 19(2), 11-24 (2016).
- Artukoglu BB, Beyer C, Zuloff-Shani A, et al. Efficacy of Palmitoylethanolamide for Pain: A Meta-Analysis. Pain. Physician 20(5), 353-362 (2017).
- Andresen SR, Bing J, Hansen RM, et al. Ultramicronized palmitoylethanolamide inspinal cord injury neuropathic pain: a randomized, double-blind,placebo-controlled trial. Pain 157(9), 2097-2103 (2016).
- Hesselink JM, Hekker TA. Therapeutic utility of palmitoylethanolamide in the treatment of neuropathic pain associated with various pathological conditions: a case series. J. Pain. Res 5(1), 437-42 (2012).
- Gabrielsson L, Mattsson S, Fowler CJ. Palmitoylethanolamidefor the treatment of pain: pharmacokinetics, safety and efïìÃÂcacy. Br. J. Clin. Pharmacol 82(4), 932-942 (2016).
- Esposito E, Impellizzeri D, Mazzon E, et al. Neuroprotective activities of palmitoylethanolamide in an animal model of Parkinson's disease. PLoSOne 7(8), e41880 (2012).
- Siracusa R, Paterniti I, Impellizzeri D, et al. The association of palmi toylethanolamide withluteolin decreases neuroinïìÃâammation and stimulates autophagy inParkinson ’s disease model. CNS. Neurol. Disord. Drug. Targets 14(10), 1350-1365 (2015).
- D’Agostino G, Russo R, Avagliano C, et al. Palmitoylethanolamide protects against the amyloid-β25 -35 -induced learning and memory impairment in mice, an experimental model of Alzheimer disease. Neuropsychopharmacology 37(1), 1784-1792 (2012).
- Scuderi C, Esposito G, Blasio A, et al. Palmitoylethanolamide counteracts reactive astrogliosis induced by β-amyloid peptide. J. Cell Mol. Med 15(12), 2664-2674 (2011).
- Paterniti I, Cordaro M, Campolo M, et al. Neuroprotection by association ofpalmitoylethanolamide with luteolin in experimental Alzheimer’sdisease models: the control of neuroinïìÃâammation. CNS. Neurol. Disord. Drug. Targets 13(9), 1530-1541 (2014).
- Clemente S. Amyotrophic lateral sclerosis treatment with ultramicronized palmitoylethanolamide: a case report. CNS. Neurol. Disord. Drug. Targets 11(7), 933-936 (2012).
- Palma E, Reyes-Ruiz JM, Lopergolo D, et al. Acetylcholine receptors from human muscle aspharmacological targets for ALS therapy. Proc. Natl. Acad. Sci. USA 113(11), 3060-3065 (2016).
- Baker D, Pryce G, Croxford JL, et al. Endocannabinoids control spasticity in a multiple sclerosis model. FASEB. J 15(2), 300-302 (2001).
- Loría F, Petrosino S, Mestre L, et al. Study of the regulation of the endocannabinoid systemin a virus model of multiple sclerosis reveals a therapeutic effect ofpalmitoylethanolamide. Eur. J. Neurosci 28(4), 633-641 (2008).
- Jean-Gilles L, Feng S, Tench CR, et al. Plasma endocannabinoid levels in multiple sclerosis. J. Neurol. Sci 287(1-2), 212-215 (2009).
- Rahimi A, Faizi M, Talebi F, et al. Interaction between the protective effects of cannabidiol andpalmitoylethanolamide in experimental model of multiple sclerosisin C57BL/6 mice. Neuroscience 290(1), 279-287 (2015).
- Shao H, Xue Q, Zhang F, et al. Spinal SIRT1 activation attenuates neuropathic pain in mice. PLoSOne 9(6), e100938 (2014).
- Qadir MI, Anwar S. Sirtuins in Brain Aging and Neurological Disorders. Crit. Rev. Eukaryot. Gene. Expr 27(4), 321-329 (2017).
- Zhou CH, Zhang MX, Zhou SS, et al. SIRT1 attenuates neuropathic pain by epigenetic regulation of mGluR1/5 expressions in type 2 diabetic rats. Pain 158(1), 130-139 (2017).
- Uchida H, Ma L, Ueda H. Epigenetic gene silencing underlies C-fiber dysfunctions in neuropathic pain. J. Neurosci 30(13), 4806-4814 (2010).
- Uchida H, Matsushita Y, Ueda H. Epigenetic regulation of BDNF expression in the primary sensory neurons after peripheral nerve injury: implications in the development of neuropathic pain. Neuroscience 240(1), 147-154 (2013).
- Kiguchi N, Kobayashi Y, Maeda T, et al. Epigenetic augmentation of the macrophage inflammatory protein 2/C-X-C chemokine receptor type 2 axis through histone H3 acetylation in injured peripheral nerves elicits neuropathic pain. J. Pharmacol. Exp. Ther 340(3), 577-587 (2012).
- Cheng W, Wang JF, Yang CX, et al. Intrathecal Injection of Resveratrol Attenuates Burn Injury Pain by Activating Spinal Sirtuin 1. Pharmacogn. Mag 12(S2), 201-205 (2016).
- Picard F, Kurtev M, Chung N, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429(6993), 771-776 (2004).
- Sugden MC, Caton PW, Holness MJ. PPAR control: it's SIRTainly as easy as PGC. J. Endocrinol 204(2), 93-104 (2010).
- Min SW, Sohn PD, Cho SH, et al. Sirtuins in neurodegenerative diseases: an update on potential mechanisms. Front. Aging Neurosci 5(1), 53 (2013).
- Singh N, Agrawal M, Doré S. Neuroprotective properties and mechanisms of resveratrol in in vitro and in vivo experimental cerebral stroke models. ACS Chem. Neurosci 4(8), 1151-1162 (2013).
- Tang BL. Sirt1 and the Mitochondria. Mol. Cells 39(2), 87-95 (2016).
- de Castro IP, Martins LM, Tufi R. Mitochondrial quality control and neurological disease: an emerging connection. Expert Rev. Mol. Med 12(1), e12 (2010).
- Martins IJ. Defective Interplay between Adipose Tissue and Immune System Induces Non Alcoholic Fatty Liver Disease. Updates. Nutr. Disord. Ther 1(1), 3 (2017).
- Martins IJ. Nutritional and Genotoxic Stress Contributes to Diabetes and Neurodegenerative Diseases such as Parkinson's and Alzheimer's Diseases: 3rd Edtn. Frontiers in Clinical Drug Research -CNS and Neurological Disorders (2015).
- Martins IJ. Nutrition Therapy Regulates Caffeine Metabolism with Relevance to NAFLD and Induction of Type 3 Diabetes. J. Diabetes. Metab. Disord 4(1), 019 (2017).
- Martins IJ. Autoimmune disease and mitochondrial dysfunction in chronic diseases. Res. Chronic. Dis 1(1), 10-12 (2017).
- Mattace Raso G, Russo R, Calignano A, et al. Palmitoylethanolamide in CNS health and disease. Pharmacol. Res 86(1), 32-41 (2014).
- Boccella S, Cristiano C, Romano R, et al. Ultramicronized palmitoylethanolamide rescues the cognitive decline-associated loss of neural plasticity in the neuropathic mouse entorhinal cortex-dentate gyrus pathway. Neurobiol Dis. in press (2018).
- Di Marzo V, Melck D, Orlando P, et al. Palmitoylethanolamide inhibits the expression of fattyacid amide hydrolase and enhances the anti-proliferative eff ect ofanandamide in human breast cancer cells. Biochem. J 358(1), 249-255 (2001).