Research Article - (2018) Volume 8, Issue 3
Resting-State Functional Connectivity within Default Mode Network in Chinese-speaking Children with Specific Learning Disabilities
- Corresponding Authors:
- Hsiao-Lan Sharon Wang, PhD
Department of Special Education, National Taiwan Normal University162, Heping East Road Section 1, Taipei, Taiwan
Tel: +886-2-77345020
Fax: +886-2-23413061
Natalie Yu-Hsien Wang, PhD
Research Center for Information Technology Innovation, Academic Sinica, Taiwan, No. 128, Section 2, Academia Rd, Taipei City, 115, Taiwan
Abstract
ABSTRACT
Objectives:
Learning is a complex process requiring support from various cognitive domains. Neuroimaging studies of clinical populations have linked various cognitive impairments with dysfunctions of the default mode network. This study investigates whether there is a fundamental deficit contributing to various sub-types of specific learning disability in children.
Methods:
Resting-state functional connectivity of the default mode network was examined using seedbased correlation and network-based statistical analyses.
Results:
The seed-based functional connectivity analysis revealed reduced functional connectivity in left superior frontal gyrus within the default mode network in children with specific learning disability, compared with typically developing children. Further, network-based statistical analysis showed reduced connectivity between superior frontal gyrus and sub-network regions, such as bilateral inferior frontal gyrus and bilateral posterior cingulate cortex, associated with executive function.
Conclusion:
This study reveals that, in addition to the specific difficulties for the sub-types of SLD, reduced executive function is a fundamental deficit that generalized across children with difficulties in reading and/or math. The study also contributes to the understanding of the role of the default mode network in specific learning disability. These findings can lead to further clinical implications, including early diagnosis and development of effective interventions.
Keywords
Specific learning disabilities (SLD), Frontal-lobe dysfunction, Resting-State functional magnetic resonance imaging (Rs-Fmri), Functional connectivity (FC), Network-Based statistical analysis (NBS)
Introduction
Schooling children who are diagnosed with specific learning disabilities (SLD) often struggle with various areas of academic performance due to a number of cognitive processing difficulties [1,2]. In the situation where the academic performance of a child fells below the agematched level, potential learning disabilities can be identified by several standardized measures of cognitive abilities [3-5]. For example, based on the Diagnostic and Statistical Manual of Mental Disorders [6], selective impairments are found in numerical and arithmetical cognition [7,8] and language abilities [9-11] in children with SLD. That is, depending on different sub-types of SLD, inputting information into the central neural system, retrieving information from the brain, and outputting the formulated information may be negatively affected. However, the difficulties demonstrated by children with SLD may result from fundamental deficits in cognitive abilities, such as auditory perception [12-14], visual perception [15-17], temporal processing [18,19], abstraction [20], memory [21-23], or motor functioning [24,25].
Since SLD are directly related to the complex processes and functions of the central neural system and affect the ability to store, process, or communication information [26], it is important to further investigate the core deficits of SLD with neuroimaging techniques. Functional magnetic resonance imaging (fMRI) studies have provided consistent evidence on atypical activations among children with SLD in various cognitive tasks [27-30]. Nevertheless, in recent years, attention has been paid to potential functional alterations in large-scale brain networks and how the alteration(s) within these networks may contribute to the observed cognitive deficits [31-33]. Default mode network (DMN), one of the well-established large-scale networks, is a set of brain regions that ‘co-active’ when the brain does not actively engage in goal-directed cognitive tasks and the co-activation is suppressed during task performance [34]. The DMN was defined by an emergent body of evidence on restingstate fMRI (rs-fMRI) showing high degree of functional connectivity between three major brain regions, including precuneus/posterior cingulate cortex (PCC), lateral and inferior parietal cortex, and medial prefrontal cortex (mPFC) [34,35]. Functional connectivity between brain regions plays an important role in cognitive processes. It has been reported that the DMN was involved in the processing of self-referential information, including the monitoring, evaluation, and integration of selfrelated stimuli [36], mind-wandering [37,38], executive function [39], memory [40,41], semantic classification [42] and processing speed [43]. Taken together, these findings indicate that the DMN may have a significant impact on sophisticated learning processes.
Previous studies have reported dysfunction of regions that are part of the default mode network in the performance of non-socioemotional tasks, such as such as a visually-guided motor task [44], a spatial working memory task [45] and a verbal learning test [46]. In other words, the high resting metabolic activity, which presumably supports complex mental activities, may also be impaired in children with SLD. However, the role of DMN in SLD has been overlooked. The current study, therefore, sets out to investigate 1) whether atypical activation(s) and/or connectivity(ies) of the DMN may be observed among children with SLD in an unconstrained resting-state, and 2) to determine whether the atypical functional connectivity, if any, are pervasive across the entire network or restricted to a regional specific area within the network. To achieve the goals, DMN functionality was studied at rest using functional connectivity MRI (fcMRI). The technique was used to assess the pattern of spontaneous occurring fluctuations in the blood oxygenation level-dependent (BOLD) signal within the default network, providing a measure of functional organization and functional engagement of this network at rest. Base on previous evidence, it was hypothesized that regions associated memory and executive function are two components affecting all subtypes of learning disabilities. Consequently, it was expected that different pattern(s) activation and functional connectivity between children with and without SLD would appear in regions involved in memory (i.e. hippocampus & precuneus) and executive functioning (i.e. prefrontal cortex).
Methods
▪ Participants
Twenty-two 10- to 11-year-old schooling children in Taipei City volunteered for the study, including twelve children with specific learning disabilities (SLD group) and ten age-matched typically developing children (control group). Children with SLD demonstrated evident academic learning difficulties (e.g. reading and writing impairments) and had been two-year behind the typically developing students. They were evaluated and identified by the Special Education Division, Department of Education of Taipei City Government. None of the children with SLD had any other neurological or psychiatric disorder. All participants and their parents signed written consents prior to the data collection to ensure their understanding of and willingness to participate the study.
Children’s intelligence quotient (IQ) was assessed using the Wechsler Intelligence Scale for Children [47]. The IQ assessments generated overall score and four sub-score indicating verbal concept formation, memory span, reasoning, and attention. The overall performances of the two groups on IQ assessment were compared using t-tests. No significant between-group difference was found in IQ, t (20) = .66, p = .16. However, children with SLD were significantly worse than the control at verbal concept formation, t (20) = 2.57, p = .21. Demographic information and the assessment outcomes are shown in Table 1.
| SLD | control | p-value | ||||
|---|---|---|---|---|---|---|
| Age (years) | 9.59 | (0.52) | 9.45 | (0.68) | > 0.05 | |
| Age range | 8.58 – 10.50 | 8.58 – 10.17 | - | |||
| Gender (male / female) | 10 / 2 | 6 / 4 | > 0.05 | |||
| year(s) of education | 3.57 | (1.27) | 3.40 | (0.55) | > 0.05 | |
| IQ (overall) | 97.60 | (9.56) | 106.51 | (10.51) | > 0.05 | |
| IQ-Similarities | 8.50 | (3.16) | 12.56 | (3.32) | = .021 | |
| IQ-Digit Span | 10.13 | (3.76) | 12.89 | (3.76) | > 0.05 | |
| IQ-Matrix Reasoning | 8.63 | (1.60) | 9.67 | (4.18) | > 0.05 | |
| IQ- Symbol Search | 10.00 | (2.27) | 11.56 | (2.40) | > 0.05 | |
Table 1: Demographic and clinical characteristics of the two groups of participants. Group means are shown with standard deviations in brackets.
▪ MRI Data acquisition
Structural and functional MRI data of all participants were acquired using a 3-Tesla scanner (MAGNETOM Prisma, Siemens, Germany) with a 32-channel head coil. During the scan, participant’s head was fixed to the location of the coil with sponges to minimize motion artifacts. Using an auto-align technique; the images acquired were parallel to anterior-commissureposterior- commissure line. In a single session, each participant underwent the structural scan, which took about 3 mins and 36 secs, prior to the restingstate fMRI acquisition, which was about 6 mins and 40 secs. The total scan time for a participant was approximately 10 mins.
For the referential anatomy, a magnetizationprepared rapid gradient echo (MPRAGE) sequence was applied to acquisition of a whole brain high-resolution T1-weighted image in a coronal view. The sequence parameters were TR/ TE = 2000 ms/ 2.98 ms, inversion time = 900 ms, field of view (FOV) = 192 mm × 256 mm, matrix size = 192 x 256, in-plane spatial resolution = 1 mm × 1 mm, and slice thickness = 1 mm without gaps.
The resting-state fMRI was performed with a gradient-echo echo-planar imaging (EPI) sequence. Before starting the image acquisition, the participants were instructed to relax with their eyes closed while remained awake and think of nothing in particular throughout the scan. Wakefulness was monitored throughout the scan via an intercom linked to the scanner chamber. The fMRI acquisition parameters were TR/TE = 2000 ms / 30 ms, FOV = 192 mm × 192 mm, matrix size = 64 × 64, in-plane spatial resolution = 3 mm × 3 mm, slice thickness = 3 mm, interleaved scanning, and flip angle = 90°. For each participant, thirty-seven trans-axial slices with no gap were acquired to cover the whole brain volume. Each resting-state fMRI run contained 200 image volumes.
▪ Functional MRI preprocessing
Data preprocessing was carried out using Data Processing Assistant for Resting-State fMRI [48]. Functional images were realigned to correct for motion, corrected for errors in slice timing, spatially transformed to standard stereotaxic space (based on the Montreal Neurologic Institute coordinate system), and smoothed with a 6-mm full-width half-maximum Gaussian kernel. There was no participant with movement greater than 3 mm of translation or 3° of rotation. Also, no significant difference between the total range of movement across any axis of translation or rotation between the two groups was found.
▪ Seed-based functional connectivity (FC) analysis
Functional connectivity analysis was conducted with a seed-region approach using the posterior cingulate cortex (PCC) as defined in the automated anatomical labeling atlas (AAL) [49]. For each region of interest (ROI), analyses for individual participants were conducted using the General Linear Model (GLM) with the time series for the ROI and for the nuisance covariates (time series regressor for global signal intensity, white matter, cerebrospinal fluid, & six motion parameters) as predictors. These nuisance signals are typically adjusted for in resting-state functional connectivity studies due to their reflection of global signal fluctuations of non-neuronal origins (e.g. physiological artifacts were linked with variables, including cardiac and respiratory cycles, CSF motion, and scanner drift) [50]. Data was then bandpass filtered from 0.01 to 0.12 Hz in order to remove low frequency noise, including slow scanner drifts and influences of higher frequencies reflecting cardiac and respiratory signals [51]. To enforce a Gaussian distribution of the correlation data, the Pearson’s correlation r was then transformed into z-scores using the Fisher z-transformation.
▪ Network-based statistical (NBS) analysis
Using the functional connectivity toolbox [52], each of the 90 brain regions, 45 in each hemisphere, defined by AAL template was considered a node [53]. The functional connectivity of the brain could be represented as an edge. To obtain the mean time series of each region, the time series of all voxels a particular region were averaged. Then, multiple linear regression models were used to further remove multiple sources of variance of the BOLD signal from the mean time series. The regressors were composed of the estimated profiles of head motion, three for translation and three for rotation, and the global brain activity. The raw mean time series of the corresponding regions were replaced by the residuals of this regression. Further, Pearson’s correlation coefficients between the residual time series of each possible pair of the 90 regions were computed to create a symmetric correlation matrix (i.e., functional connectivity matrix) for each subject, in which the nodes represented brain regions and edges represented the undirected connection. Finally, the connectivity matrix was used for the networkbased statistical [54] analysis.
The network-based statistics was used to identify potential alteration(s) of connectivity in any connected sub-networks in children with SLD [54]. The NBS dealt with the multiple comparison problem posed by connectomics data through evaluating the null hypothesis at the level of interconnected sub-networks. In this method, the identification of sub-networks was similar to the recognition of significant clusters of activation in the cluster-based thresholding strategies used in the voxel-wised MRI studies [55,56]. The NBS analysis attempted to identify potential connected structures formed by an appropriately chosen set of supra-threshold links. The topological extent of such structure was then applied to determine its significance. To establish a set of supra-threshold links, the primary threshold (i.e., test statistic) for each pair-wise association was computed. The null distribution of the number of edges was empirically obtained using a nonparametric permutation (5000 permutations) to assess the significance of each connected edge.
Results
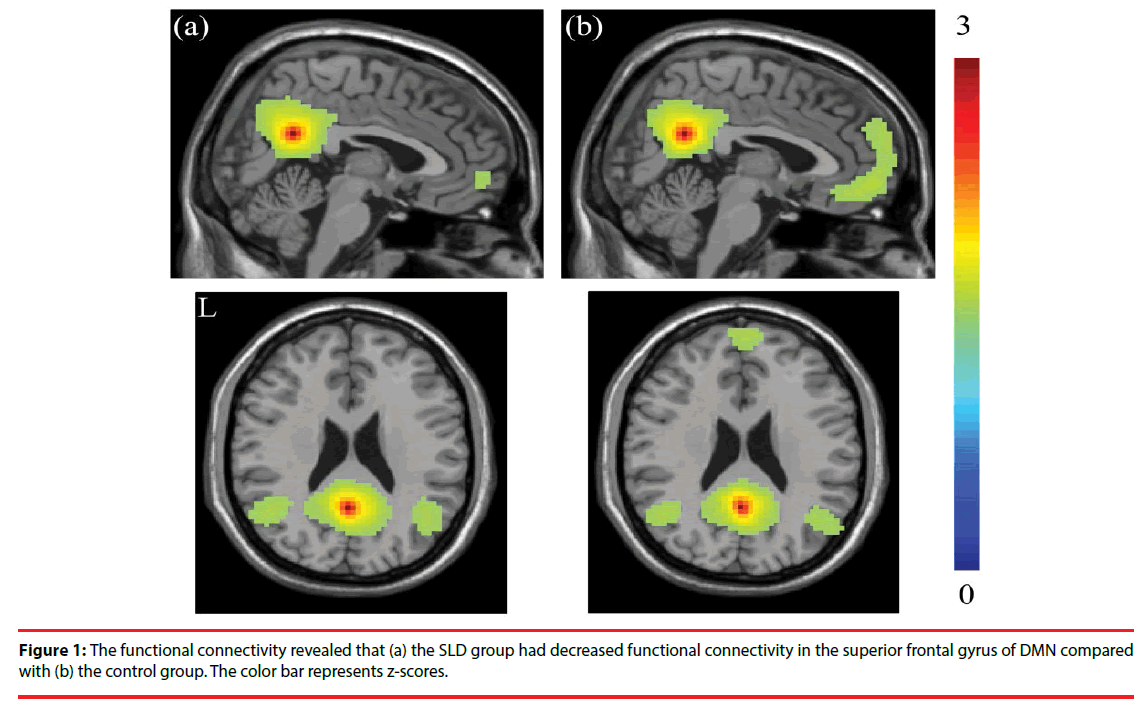
The seed-based functional connectivity analysis revealed, compared with the control group, decreased connectivity in the frontal gyrus within the DMN in the children SLD, especially in superior frontal gyrus. The resting-state functional activations of the two groups are shown in Figure 1. The seed-based correlation analysis reported the decrease of functional connectivity in the frontal gyrus within DMN reached significance (p < 0.001), especially in superior frontal gyrus.
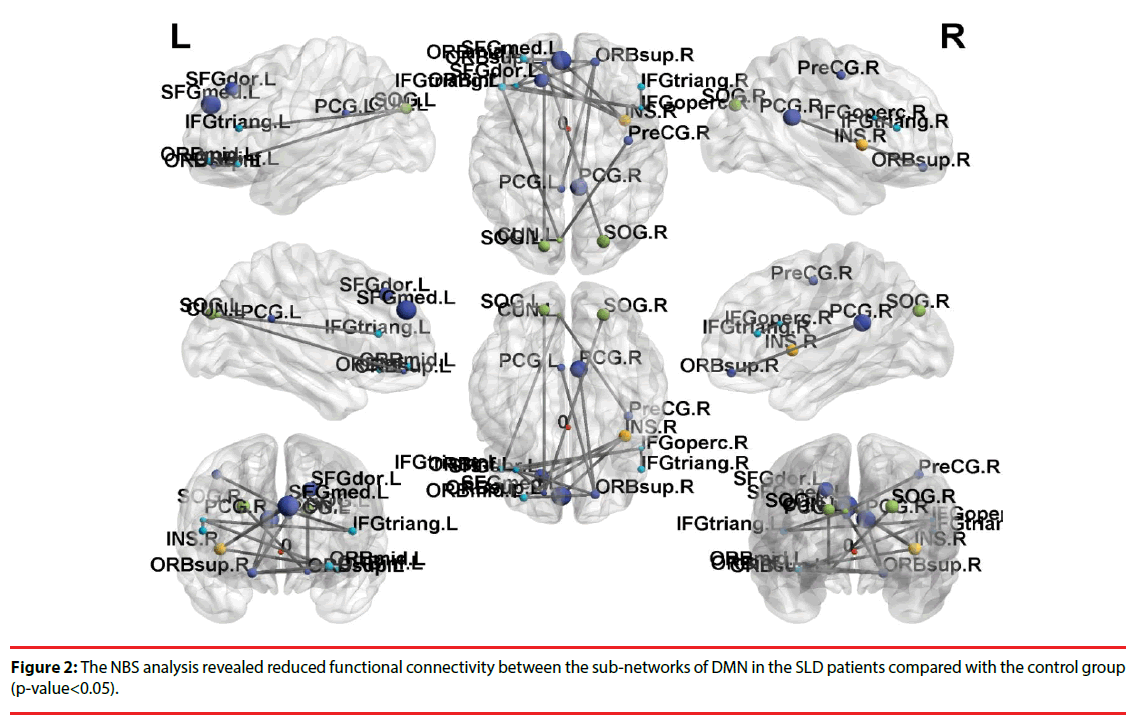
Further, the results of NBS analysis revealed that weakened connectivity within sub-networks in the children with SLD, compared with the control group (p-value < 0.05). Significant disruption within sub-network was found in the frontal gyrus among the SLD group, especially in superior frontal gyrus. The weakened connectivity within sub-networks (Figure 2) comprised edges between the left superior frontal gyrus (SFG) and the bilateral inferior frontal gyrus (IFG), bilateral posterior cingulate cortex (PCG), and bilateral superior occipital gyrus (SOG); between orbital part of superior frontal gyrus (ORB) and bilateral PCG; between the bilateral IFG and bilateral SOG; between the rights IFG to left IFG.
Discussion
The study contributes to our understanding of whether the DMN plays a role in SLD. The study acquired resting-state functional connectivity data from children with SLD and typically developing children. Our findings revealed decreased functional activation in the superior frontal gyrus as well as reduced functional connectivity within the sub-network of DMN. Despite other cognitive abilities linked with the frontal gyrus, this brain region has been closely associated with executive function [56]. Indeed, impaired cognitive functions have been constantly identified among various populations with developmental disorders [58,59], leading to reduced executive function and control of attention.
The results of the current study support the predictions that functional connectivity in children with SLD is distinctive from typically developing children and the difference can be observed within the DMN. This evidence is partially in line with previous studies [60] in terms of disrupted functional connectivity in children with various sub-types of learning disability. Despite the disrupted regions reported in the previous studies have been inconsistent within and across sub-types of learning difficulties, dysfunctions of the frontal regions have been constantly identified. Taking children with reading difficulties for example, Farris, Odegard, Miller, Ring, Allen, and Black [61] reported reduced functional connectivity between the inferior frontal regions using rs-fMRI whilst reduced connectivity between left posterior temporal areas and left inferior frontal gyrus was demonstrated by Schurz et al. [31]. Children with dyscalculia, on the other hand, have also demonstrated dysfunction in areas within the frontal lobes [62,63] along with other regions, such as anterior cingulate and parietal cortices. Variations of sub-type of SLD may account for the discrepancies reported in the previous and the current study. However, the potential factor(s) leading to within sub-type variations is yet to be investigated. In the studies mentioned above, including the current study, participants were children of different age range. It is possible that functional connectivity between regions change as age, further evidence is required to confirm/dismiss this potential developmental factor. Another potential factor leading to the discrepancies is severity of learning disabilities. It is widely reported that the effectiveness of interventions largely depends on level of severity of learning disabilities [64,65]. However, this issue receives little attention in neuroimaging studies in SLD.
Further, we demonstrated reduced connectivity between bilateral IFG and between SFG and IFG and PCG. Comprehensive evidence has linked IFG with executive function whilst SFG and PCG are reported to involve in the executive control of attention [66] and working memory [67] respectively. Therefore, reduced functional connectivity in these regions within the DMN may be considered as additional evidence showing that the disadvantage for children with SLD lies in impairments of executive functioning. Also, the current evidence may account for the mild attention and working memory deficit observed among children with SLD. A large body of evidence has associated human frontal lobe with this particular cognitive ability, some earlier literature even referred tasks involving executive function as ‘frontal lobe task’ [68]. The important perspective of executive function in relation to learning is regulating goal-oriented behavior. Several studies have demonstrated executive function deficits among children with learning disabilities [69,70]. Some even suggest that executive function may be a predictor for learning ability among children [71]. Intriguingly, with this particular cognitive deficit presented in all sub-types of SLD, the underlying neural mechanisms seem to vary across groups. Further research is necessary to identify potential neural markers for SLD subtypes.
There are however a few limitations in the current study, which are also found in previous studies involving children with SLD. Due to recruitment limitation, acquiring a large sample size was a challenge. Consequently, within this small group, we could not further examine functional connectivity within different subtypes. Moreover, detailed cognitive profiles and level of SLD severity of the participants were not available. Cognitive abilities along with severity can account for not only the potential individual differences but also the variation of functional connectivity reported by different studies.
Conclusions
The DMN is a large-scale brain network involving in complex cognitive processes that support learning ability. The study employed multimodal functional MRI techniques and analyses to investigate the role of DMN in children with SLD. Among children with SLD, atypical functional connectivity was identified within the SFG as well as between SFG and other regions associated with executive control. Our findings advance the field by providing evidence showing that the impaired neural mechanism underlying SLD was largely associated with reduced executive function. The current study has a couple of clinical implications. Firstly, atypical functional connectivity within the DMN may be a potential neural marker for clinical diagnosis. Secondly, interventions based on training of executive function could be a promising approach to improve learning performance. Nevertheless, investigators should also consider the importance of establishing complete cognitive profile for each individual, as it could offer valuable accounts for individual variations. For future research, multimodal imaging technique can be used to evaluate effectiveness of interventions.
References
- Cole M, Kraft MB. Specific learning disability. Cortex 1(3), 302-313 (1964).
- Johnson ES, Humphrey M, Mellara DF, et al. Cognitive processing deficits and students with specific learning disabilities: a selective meta-analysis of the literature. Learning. Disability. Quarterly 33, 3-18 (2010).
- Lerner JW. Learning disabilities: theories, diagnosis, and teaching strategies. Houghton Mifflin, Boston.
- Lorusso ML, Vernice M, Dieterich M, et al. The process and criteria for diagnosing specific learning disorders: indications from the Consensus Conference promoted by the Italian National Institute of Health. Annali. dell'Istituto. Superiore di. Sanità 50(1), 77-89 (2014).
- Poletti M. WISC-IV intellectual profiles in Italian children with specific learning disorder and related impairments in reading, written expression, and mathematics. J. Learn. Disabil 49(3), 320-335 (2014).
- Association AP. Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Author, Arlington, VA.
- Geary DC, Hamson CO, Hoard MK. Numerical and arithmetical cognition: a longitudinal study of process and concept deficits in children with learning disability. J. Exp. Child Psychol 77(3), 236-263 (2000).
- Mussolin C, Mejias S, Noël MP. Symbolic and nonsymbolic number comparison in children with and without dyscalculia. Cognition 115(1), 10-25 (2010).
- Kan PF, Windsor J. Word learning in children with primary language impairment: a meta-analysis. J. Speech. Lang. Hear. Res 53, 739-756 (2010).
- Krishnan S, Watkins KE, Bishop DVM. Neurobiological basis of language learning difficulties. Trends. Cogn. Sci 20(9), 701-714 (2016).
- Melby-Lervag M, Lyster SA, Hulme C. Phonological skills and their role in learning to read: a meta-analytic review. Psychological. Bulletin 138(2), 322-352 (2012).
- Bretherton L, Holmes VM. The relationship between auditory temporal processing, phonemic awareness, and reading disability. J. Exp. Child Psychol 84(3), 218-243 (2003).
- Dawes P, Bishop D. Auditory processing disorder in relation to developmental disorders of language, communication and attention: a review and critique. Int. J. Lang. Commun. Diso 44(4), 440-465 (2009).
- Talla P. Auditory temporal perception, phonics, and reading disabilities in children. Brain. And. Language 9(2), 182-198 (1980).
- Bowan MD. Learning disabilities, dyslexia, and vision: a subject review--a rebuttal, literature review, and commentary. Optometry (St. Louis, Mo.) 73(9), 553-575 (2002).
- Franceschini S, Gori S, Ruffino M, et al. A causal link between visual spatial attention and reading acquisition. Current. Biology 22(9), 814-819 (2012).
- Skoyles J, Skottun BC. On the prevalence of magnocellular deficits in the visual system of non-dyslexic individuals. Brain. And. Language 88(1), 79-82 (2004).
- Hari R, Renvall H. Impaired processing of rapid stimulus sequences in dyslexia. Trends. Cogn. Sci 5(12), 525-532 (2001).
- Landerl K, Willburger E. Temporal processing, attention, and learning disorders. LEARN. INDIVID DIFFER 20(5), 393-401 (2010).
- Kasirer A, Mashal N. Comprehension and generation of metaphoric language in children, adolescents, and adults with dyslexia. Dyslexia 23, 99-118 (2017).
- Cowan N, Hogan TP, Alt M, et al. Short-term memory in childhood dyslexia: deficient serial order in multiple modalities. Dyslexia 23, 209-233 (2017).
- Cowan N, Alloway T. The development of memory in infancy and childhood. In: Development. Of. Working. Memory. In. Childhood. Psychology Press, Hove, East Sussex, UK, 303-342 (2009).
- Gallinat E, Spaulding TJ. Differences in the performance of children with specific language impairment and their typically developing peers on nonverbal cognitive tests: A meta-analysis. J. Speech. Lang. Hear. Res 57, 1363-1382 (2014).
- Geary DC. Mathematics and learning disabilities. J. Learning. Disabilities 37(1), 4-15 (2004).
- Smith-Spark JH, Henry LA, Messer DJ, et al. Executive functions in adults with developmental dyslexia. Res. Dev. Disabil 53, 323-341 (2016).
- Berninger V, Richards T. Brain literacy for educators and psychologists. Academic Press, New York.
- Backes W, Vuurman E, Wennekes R, et al. Atypical brain activation of reading processes in children with developmental dyslexia. J. Child. Neurol 17, 867-871 (2002).
- Beneventi H, Tønnessen FE, Ersland L. Dyslexic children show short-term memory deficits in phonological storage and serial rehearsal: An fMRI study. Int. J. Dev. Neurosci 119(11), 2017-2043 (2009).
- Richlan F, Kronbichler M, Wimmer H. Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage 56(3), 1735-1742 (2011).
- Urion DK, Huff HV, Carullo MP. MRI in assessing children with learning disability, focal findings, and reduced automaticity. Neurology 85(7), 604-609 (2015).
- Schurz M, Wimmer H, Richlan F, et al. Resting-state and task-based functional brain connectivity in developmental dyslexia. Cerebral. Cortex 25(10), 3502-3514 (2015).
- Richards T, Berninger V. Abnormal fMRI connectivity in children with dyslexia during a phoneme task: Before but not after treatment. J. of. Neurolinguistics 21(4), 294-304 (2008).
- Richlan F. Developmental dyslexia: dysfunction of a left hemisphere reading network. Front. Hum. Neurosci 6, 120 (2012).
- Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. PNAS 98(2), 676-82 (2001).
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: A review on resting-state fMRI functional connectivity. Europe. Neuropsychopharmacology 20(8), 519-534 (2010).
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129(3), 564-583 (2006).
- Mason MF, Norton M, van Horn JD, et al. Wandering minds: the default network and stimulus-independent thought. Science 351(5810), 393-395 (2007).
- Sood A, Jones DT. On mind wandering, attention, brain networks, and meditation. Explore (NY) 9(3), 136-141 (2013).
- Gerlach KD, Spreng RN, Gilmore AW, et al. Solving future problems: Default network and executive activity associated with goal-directed mental simulations. NeuroImage 55(4), 1816-1824 (2011).
- Andreasen NC, O’Leary DS, Cizadlo T, et al. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am. J. Psychiatry 152(11), 1576-1585 (1995).
- Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci 21(3), 489-510 (2009).
- Lustig C, Z Snyder A, Bhakta M, et al. Functional deactivations: change with age and dementia of the Alzheimer type. PNAS 100, 14504-14509 (2003).
- Li SC, Lindenberger U, Hommel B, Aschersleben G, et al. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychological. Science 15, 155-163 (2004).
- Müller R-A, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cerebral. Cortex 21(10), 2233-2243 (2011).
- Luna B, Minshew NJ, Garver KE, et al. Neocortical system abnormalities in autism An fMRI study of spatial working memory. Neurology 59(6), 834-840 (2002).
- Haznedar MM, Buchsbaum MS, Wei T-C, et al. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am. J. Psychiatry 157(12), 1994-2001 (2000).
- Wechsler D. Wechsler Intelligence Scale for Children. Pearson, Bloomington, MN (2014).
- Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Front. Syst. Neurosci, 4, 13 (2010).
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15(1), 273-289 (2002).
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci 8(9), 700-711 (2007).
- Cordes D, Haughton VM, Arfanakis K, et al. Frequencies contributing to functional connectivity in the cerebral cortex in "resting-state" data. AJNR. Am. J. Neuroradiol 22(7), 1326-1333 (2001).
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain. connectivity 2(3), 125-141 (2012).
- Behzadi Y, Restom K, Liau J, et al. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37(1), 90-101 (2007).
- Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage 53(4), 1197-1207 (2010).
- Behrens TE, Sporns O. Human connectomics. Curr. Opin. Neurobiol, 22(1), 144-153 (2012).
- Sporns O, Tononi G, Kotter R. The human connectome: a structural description of the human brain. PLoS. Comput. Biol 1(4), e42 (2005).
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychology 16(1), 17-42 (2006).
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. J. Child Psychol. Psychiatry 37(1), 51-87 (1996).
- Johnson ES, Mellard DF, Byrd SE. Challenges with SLD identification: What is the SLD problem? TEC. Plus 3(1), Article 3 (2006).
- Pugh KR, Mencl WE, Jenner AR, et al. Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment. Retard. Dev. Disabil. Res. Rev 6(3), 207-213 (2000).
- Farris EA, Odegard TN, Miller HL, et al. Functional connectivity between the left and right inferior frontal lobes in a small sample of children with and without reading difficulties. Neurocase 17(5), 425-439 (2011).
- Davis N, Cannistraci CJ, Rogers BP, et al. The neural correlates of calculation ability in children: an fMRI study. Magnetic. Resonance. imaging 27(9), 1187-1197 (2009).
- Kucian K, Loenneker T, Dietrich T, et al. Impaired neural networks for approximate calculation in dyscalculic children: a functional MRI study. Behav. Brain. Funct 2(1), 31 (2006).
- Fletcher JM, Lyon GR, Fuchs LS, Barnes MA. Learning Disabilities from Identification to Intervention. Guilford, New York.
- Lyon GR. Learning disabilities. The. Future. Of. children 6(1), 54-76 (1996).
- Boisgueheneuc F Du, Levy R, Volle E, et al. Functions of the left superior frontal gyrus in humans: A lesion study. Brain 129(12), 3315-3328 (2006).
- Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain 137, 12-32 (2014).
- Shallice T, Burgess P. Frontal lobe function and dysfunction. In: Higher-order cognitive impairments and frontal lobe lesions in man. Levin HS, Eisenberg HM, Benton AL (Eds.). . Oxford University Press, New York, 125-138 (1991).
- Alahmadi NA. Cognitive control in children with learning disabilities: neuromarker for deficient executive functions. NeuroReport 28(11), 638-644 (2017).
- Helland T, Asbjornsen A. Digit span in dyslexia: Variations according to language comprehension and mathematics skills. J. Clin. Exp .Neuropsychol 26(1), 31-42 (2004).
- Bull R, Scerif G. Executive functioning as a predictor of children’s mathematics ability: Inhibition, switching, and working memory. Developmental. neuropsychology 19, 273-297 (2001).