Research Article - Neuropsychiatry (2017) Volume 7, Issue 6
Protective Effects of Preactivated and Disaggregated Shape-Changed Platelets and Human Embryonic Stem Cell-Derived Exosomes Improve Neurological Function and Attenuate Brain Infarct after Acute Ischemic Stroke
- Corresponding Authors:
- Sarah Chua
Division of Cardiology
Department of Internal Medicine Kaohsiung
Chang Gung Memorial Hospital. No. 123, Dapi Road,
Niaosong District, Kaohsiung City, 83301, Taiwan
Tel: +886-7-7317123
Fax: +886-7-7322402
Hon-Kan Yip
Division of Cardiology
Department of Internal Medicine Kaohsiung
Chang Gung Memorial Hospital. No. 123, Dapi Road,
Niaosong District, Kaohsiung City, 83301, Taiwan
Tel: +886-7-7317123
Fax: +886-7-7322402
Abstract
Abstract
Background: This investigation tested the hypothesis that preactivated and disaggregated shape-changed platelets (PreD-SCP) and human embryonic stem cell-derived exosomes (hESC-Exo) treatments can reduce brain-infarct area (BIA) in rat with preservation of neurological function following acute ischemic stroke (AIS) induced by left middle-cerebral artery occlusion.
Materials and Methods: Adult-male Sprague-Dawley rats (n=24) were randomly divided into group 1 (sham-control), 2 (AIS), 3 [AIS + PreD-SCP (3.0x108 cells)] and 4 (AIS + hESC-Exo, 100 μg). PreD-SCP and hESC-Exo were administered intravenously at 2/6/24 hours after the AIS procedure for animals in group 3 and 4, respectively. All animals were euthanized by day-28 post-AIS.
Results: Brain infarct area (BIA) measured by histopathology was significantly larger in group 2 than that in other groups, and significantly larger in group 3 and 4 than that in group 1 (p<0.01), but it showed no difference between group 3 and 4. The improvement in neurological function displayed a pattern opposite to that of BIA by days 3/7/14/28 after AIS in the four groups (all p<0.01). The protein expressions of inflammation (MMP-9/TNF-α/Cox-2/iNOS), oxidative stress (oxidized protein/NOX-1/NOX-2) and apoptosis (cleaved-caspase-3/PARP/mitochondrial-Bax) exhibited a pattern identical to that of BIA among the four groups (all p<0.01). The cellular levels of inflammatory (CD14+/CD68+/GFAP+), DNA-damage/apoptotic (γ-H2AX+/apoptotic nuclei) biomarkers exhibited an identical pattern, whereas the neuron/myelin-sheath integrity markers (NeuN+/BMP+ cells) expressed an opposite pattern compared to that of BIA in the four groups (all p<0.05). The expressions of endothelial cell markers at cellular (CD31+/vWF+) and protein (CD31/eNOS/vWF) levels and the number of small vessels showed an opposite pattern compared to that of BIA in all groups, whereas the endothelial progenitor cell markers at cellular/protein (CXCR4/SDF-1α) levels progressively increased from groups 1 to 4 (all p<0.001).
Conclusion: PreD-SCP and hESC-Exo treatments significantly protected the brain from AIS damage andimproved neurological outcome.
Keywords
Acute ischemic stroke, Preactivated and disaggregated shape-changed platelets, Human embryonic stem cell-derived exosome, Inflammation, Oxidative stress, Angiogenesis
Abbreviations
APQ4: Aquaporin4; Casp 3: Caspase 3; eNOS: Endothelial Nitric Oxide Synthase; GFAP: Glial Fluorescent Acid Protein; hESC-Exo: Human Embryonic Stem Cell-Derived Exosome; iNOS: Inducible Nitric Oxide Synthase; MBP: Myelin Basic Protein; Mito: Mitochondrial; MMP-9: Matrix Metalloproteinase 9; PARP: Poly (ADPRibose) Polymerase; PreD-SCP: Pre-Activated And Disaggregated Shape-Changed Platelet; SDF-1α: Stromal Cell-Derived Factor 1-α; TNF-α: Tumor Necrosis Factor-α; VEGF: Vascular Endothelial Growth Factor; vWF: Von Willebrand Factor
Introduction
Although stroke is the third most notorious killer worldwide, effective treatment for acute stroke has been very limited [1,2]. In spite of remarkable advances in our understanding of the pathophysiology of stroke over the past two decades, tissue-type plasminogen activator (tPA) is still the only golden standard for treating patients with acute ischemic stroke (IS). However, its narrow treatment window of 3 to 4.5 hours following acute IS onset [3] has been reported to limit its wide clinical application (i.e. <10% of stroke patients actually received tPA) [2,4]. Importantly, tPA has serious side effects, including intracranial hemorrhage, cytotoxicity and increased permeability of neurovascular unit with development of cerebral edema [5]. Accordingly, to find a safe and effective treatment for patients after acute IS is paramount to scientists and physicians. However, prior to finding an innovative treatment for acute IS, to understand the pathophysiological pathway and the underlying mechanisms of brain tissue damage after acute IS is utmost important.
Studies have previously established that brain injury following IS results from a complex series of pathophysiological events including excitotoxicity, oxidative/nitrative stress, inflammation and cellular apoptosis in ischemic neuron [6-10]. These cascades cause microglial activation and the release of pro-inflammatory cytokines, which in turn, further enhance inflammation via recruitment of inflammatory and immune cells into the brain lesion participate in brain damage [7,11,12].
Growing evidence has demonstrated the anti-inflammatory, immunomodulatory and angiogenic capacities of mesenchymal stem cell (MSC)-derived exosomes (Exo) [13- 15]. Intriguingly, these studies have further demonstrated that MSC-derived Exo therapy significantly reduced ischemia-related and ischemia-reperfusion caused organ dysfunction [13-15]. Limited data has shown that embryonic stem cell (ESC)-derived MSC therapy effectively improved neurological function in rodent after acute IS [16]. However, a major concern for ESC-derived MSC therapy is tumorigenesis that results from elusive differentiation of these MSCs. On the other hand, ESC-derived Exo has not such adverse effects to be reported. However, there was no additional information to address the impacts of ESC-derived Exo on reducing brain infarct size and preserving neurological function after acute IS.
We have recently shown that pre-activated and disaggregated shape-changed platelets (PreDSCP) treatment attenuated acute respiratory distress syndrome (ARDS)-induced lung injury complicated with sepsis in rat mainly through suppressing inflammation, oxidative stress and reactive oxygen species [17]. Besides, this study attempted to test the hypothesis that PreD-SCP and human ESC-derived exosomes (hESCExo) therapies could effectively reduce the brain infarct area (BIA) and preserve neurological function in rodent following acute IS.
Materials and Methods
▪ Animals and ethics
The Institute of Animal Care and Use Committee at Kaohsiung Chang Gung Memorial Hospital approved all animal experimental procedures (Affidavit of Approval of Animal Use Protocol No. 2015092204) which were performed in compliance with the Guide for the Care and Use of Laboratory Animals, 8th edition (NIH publication No. 85-23, National Academy Press, Washington, DC, USA, revised 2011). All animals were accommodated in an animal facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) in our hospital with controlled temperature and light cycles (24°C and 12/12 light cycle).
▪ Animal surgical procedure
The protocol and procedures for the induction of acute IS in a rodent model have been described in our previous studies [6,18]. In details, adult male Sprague-Dawley rats weighing 350-375 g (Charles River Technology, BioLASCO Taiwan Co., Ltd., Taiwan) were used in the current study. Each animal was anesthetized in a supine position with 2% inhalational isoflurane on a warming pad maintained at 37 ºC. Following exposure of the left common carotid artery (LCCA) through a transverse neck incision, a small incision was made on the LCCA. A 0.28 mm diameter nylon monofilament was then carefully advanced through the incision into the distal left internal carotid artery to occlude the left middle cerebral artery, thereby causing brain ischemia and infarction. After occlusion for 90 minutes, the nylon monofilament was removed and the muscle and skin were closed in layers. The rats were allowed to recover in a portable animal intensive care unit (ThermoCare®) for 24 hours with free access to food and water.
▪ Animal grouping and treatment protocol and specimen preparation
Twenty-four Sprague-Dawley rats were equally divided into sham controls (SC) (neck skin incision and LCCA dissection only), IS, IS + PreD-SCP (intravenous administration of 3.0 x 108 cells for three consecutive doses at 2/6/24 hours after IS procedure for each animal) and IS + hESC-Exo (intravenous administration of 100 μg for three consecutive doses at 2/6/24 hours after IS procedure for each animal). All animals were euthanized by post-IS day 28. The brain tissue of each rat was harvested, immersed in cold saline, and snap-frozen in liquid nitrogen before being stored at -80ºC for later studies.
▪ Corner test for assessment of neurological function
Each rat underwent sensorimotor functional test (corner test) at baseline and on days 1, 3, 7, 14 and 28 following induction of acute IS as previously described [6,18]. In details, the rat walked through a tunnel to face diversion 60 degrees apart. The rat then had to turn either left or right to exit the tunnel. The test was repeated 10 to 15 times with a 30-second interval. All results were recorded by a technician blinded to the animal grouping. For each animal, the number of right and left turns from 10 successful trials was used for statistical analysis.
▪ Measurement of brain infarct area (BIA)
The procedures and protocol for assessment of BIA were described in details in our previous studies [6,18]. To evaluate the impacts of PreDSCP and hESC-Exo treatments on BIA, coronal sections of the brain were acquired from 24 extra animals (n=6 for each group). In details, all brain sections were put on a tray attached to a scaled vertical bar with a digital camera. After all the coronal sections were obtained, each brain cross-section (i.e., 2 mm per each slice) was stained with 2% 3,5-Triphenyl-2HTetra- zolium chloride (TTC) (Alfa Aesar). For statistical analysis, three consecutive sections were utilized for BIA analysis (i.e., summation of the three areas divided by 3 to obtain the mean area). Photographs of the sections were taken directly from above at a fixed height. The images were then analyzed with Image Tool 3 (IT3) analysis software (University of Texas, Health Science Center, San Antonio, UTHSCSA; Image Tool for Windows, Version 3.0, USA). The BIA was characterized by its whitish or pale yellowish discoloration. The infarcted area was further confirmed by microscopic examination. The percentage of BIA was then calculated by dividing the area with the total left-side crosssectional area of the brain.
▪ Immunofluorescent (IF) and immunohistochemical (IHC) staining
The procedures of IF and IHC staining have been depicted in our previous reports [6,18]. In details, serial cryosections (7 μm thick) with an average thickness of 5 μm from the BIA were obtained. After fixation of the sections in acetone for 15 minutes at -20°C, 200 μL of signal enhancer was used for blocking non-specific signals at room temperature for 30 minutes to reduce the background signals. Primary antibodies specifically against aquaporin4 [(APQ4) 1:200, Abcam], CD14 (1:200, BioSS), CD31 (1:200, MCA), CD68 (1:100, Abcam), CXCR4 (1:100, Abcam), glial fibrillary acid protein [(GFAP) 1:500, DAKO], NeuN (1:100, Millipore), stromal cell-derived factor-1 [(SDF-1α) 1: 100, Santa Cruz], γ-H2AX (1:100, Abcam), and von Willebrand factor [(vWF), 1:200, Millipore] at 4°C overnight. Alexa Fluor488 FITC and Alexa Fluor594 Rodamin-conjugated goat anti-mouse or rabbit IgG (Molecular Probes, 1:200) were adopted to locate signals. The sections, which were counterstained with 4’, 6-Diamid- ino-2- phenylindole [(DAPI) 1: 500, Sigma-Aldrich] to identify cellular nuclei for cell counting, were examined with a fluorescent microscope equipped with epifluorescence (Olympus IX- 40). Three brain sections were analyzed for each animal. For quantification, three randomly selected high-power fields (HPFs; 400x for IF staining; 200x for IHC staining) were analyzed in each section. The process was repeated in two other sections. The average number of positive -stained cells per HPF for each animal was then decided by summation of all numbers divided by nine.
▪ Western blot analysis of brain specimens
Equal amounts (40-60 μg) of protein extracts from infract core were loaded and separated by SDS-PAGE with 12 – 13% acrylamide gradients. After electrophoresis, the separated proteins were electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane (Amersham Biosciences). Nonspecific proteins were blocked by incubating the membrane in blocking buffer (5% nonfat dry milk in T-TBS containing 0.05% Tween 20) overnight. The membranes were incubated with the appropriate primary antibodies against Caspase 3 (1:1000, Cell Signaling), CD31 (1:1000, Abcam), cyclooxygenase-2 [Cox-2, 1:500, Abcam], endothelial nitric oxide synthase [(eNOS), 1:1000, Cell Signaling], inducible nitric oxide synthase [(iNOS), 1:200 Abcam], poly (ADPribose) polymerase [(PARP), 1:1000, Cell Signaling], NADPH oxidase 1 [(NOX-1), 1:2000, Sigma-Aldrich], NOX-2 (1:500, Sigma- Aldrich), matrix metalloproteinase 9 [(MMP-9), 1:1000, Abcam], mitochondrial Bax (1:1000, Abcam), SDF-1α (1:1000, Cell Signaling), tumor necrosis factor alpha [(TNF-α), 1:1000, Cell Signaling], vascular endothelial growth factor [(VEGF), 1:1000, Abcam] and vWF (1:1000, Abcam) for one hour at room temperature. Horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin IgG (1:2000, BioLegend) for one hour at room temperature. Horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin IgG (1:2000, Cell Signaling) was used as a second antibody for one hour at room temperature.
Statistical Analysis
Data are shown as means ± SEM. Statistical analysis was performed with ANOVA and Bonferroni multiple-comparison post hoc test. SAS software for Windows version 8.2 (SAS Institute, Cary, NC) was utilized. A probability value less than 0.05 was considered statistically significant.
Results
Effects of PreD-SCP and hESC-Exo on BIA and neurological function by day 28 after acute IS (Figure 1).
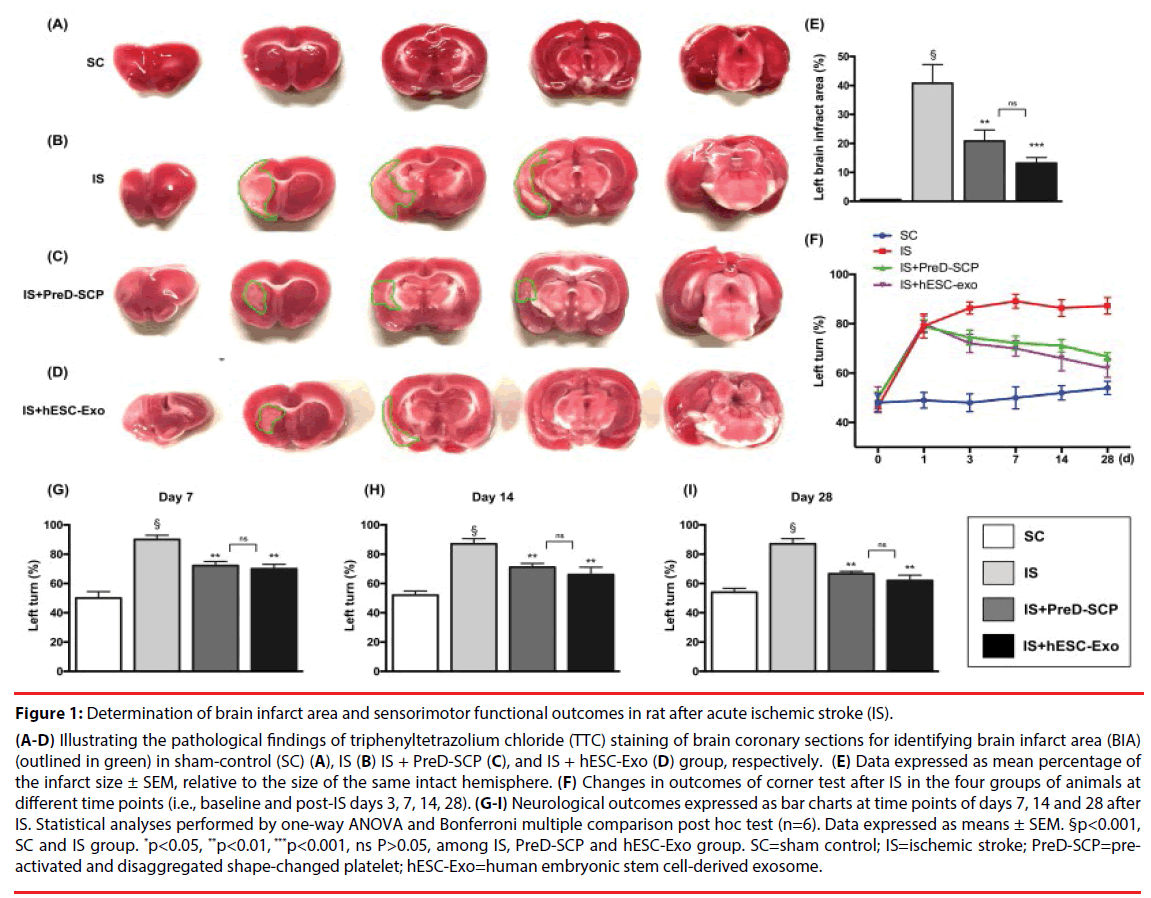
Figure 1: Determination of brain infarct area and sensorimotor functional outcomes in rat after acute ischemic stroke (IS).
(A-D) Illustrating the pathological findings of triphenyltetrazolium chloride (TTC) staining of brain coronary sections for identifying brain infarct area (BIA)(outlined in green) in sham-control (SC) (A), IS (B) IS + PreD-SCP (C), and IS + hESC-Exo (D) group, respectively. (E) Data expressed as mean percentage of the infarct size ± SEM, relative to the size of the same intact hemisphere. (F) Changes in outcomes of corner test after IS in the four groups of animals at different time points (i.e., baseline and post-IS days 3, 7, 14, 28). (G-I) Neurological outcomes expressed as bar charts at time points of days 7, 14 and 28 after IS. Statistical analyses performed by one-way ANOVA and Bonferroni multiple comparison post hoc test (n=6). Data expressed as means ± SEM. §p<0.001, SC and IS group. *p<0.05, **p<0.01, ***p<0.001, ns P>0.05, among IS, PreD-SCP and hESC-Exo group. SC=sham control; IS=ischemic stroke; PreD-SCP=preactivated and disaggregated shape-changed platelet; hESC-Exo=human embryonic stem cell-derived exosome.
The ratio of left infarct area to total left-brain area was significantly higher in group 2 (IS) than that in group 1 (SC), 3 (IS + PreD-SCP) and 4 (IS + hESC-Exo), significantly higher in group 3 and 4 than that in group 1, but there was no significant difference between group 3 and 4. Corner test (i.e., neurological function test) demonstrated consistent neurological functional impairment from post-IS days 1 to 3 among group 2 to 4. In addition, notable improvement in neurological function was evident in group 3 and 4 but not in group 2 on days 7 and 14. Compared to group 2, further substantial improvements in group 3 and 4 were observed on day 28 after IS.
Effects of PreD-SCP and hESC-Exo on attenuating the inflammatory reaction by day 28 following acute IS (Figure 2).
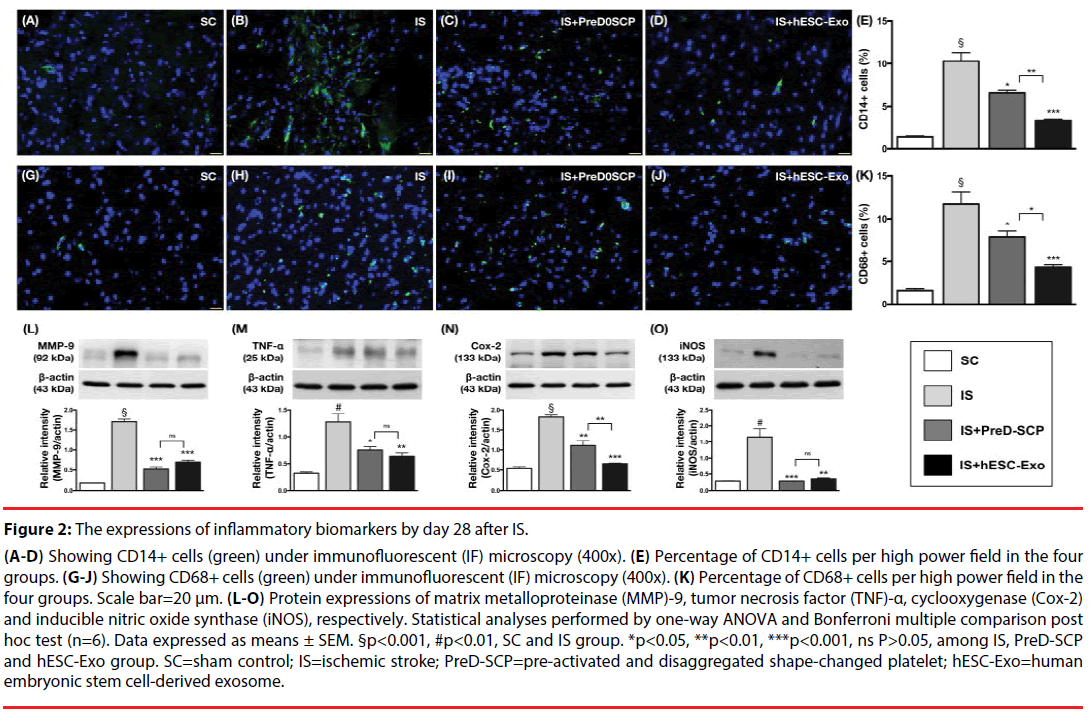
Figure 2: The expressions of inflammatory biomarkers by day 28 after IS.
(A-D) Showing CD14+ cells (green) under immunofluorescent (IF) microscopy (400x). (E) Percentage of CD14+ cells per high power field in the four groups. (G-J) Showing CD68+ cells (green) under immunofluorescent (IF) microscopy (400x). (K) Percentage of CD68+ cells per high power field in the four groups. Scale bar=20 μm. (L-O) Protein expressions of matrix metalloproteinase (MMP)-9, tumor necrosis factor (TNF)-α, cyclooxygenase (Cox-2) and inducible nitric oxide synthase (iNOS), respectively. Statistical analyses performed by one-way ANOVA and Bonferroni multiple comparison post hoc test (n=6). Data expressed as means ± SEM. §p<0.001, #p<0.01, SC and IS group. *p<0.05, **p<0.01, ***p<0.001, ns P>0.05, among IS, PreD-SCP and hESC-Exo group. SC=sham control; IS=ischemic stroke; PreD-SCP=pre-activated and disaggregated shape-changed platelet; hESC-Exo=human embryonic stem cell-derived exosome.
IF microscopy showed that the numbers of CD14+ and CD68+ cells, two inflammation indices, were lowest in group 1, highest in group 2, and significantly higher in group 3 than those in group 4. The protein expressions of MMP-9, TNF-α, Cox-2 and iNOS, four indicators of inflammation, were significantly higher in group 2 than those in other groups, and significantly higher in group 3 and 4 than those in group 1. There was statistical significant difference between group 3 and 4 in the protein expression of TNF-α and Cox-2 but not in other parameters Effects of PreD-SCP and hESC-Exo on oxidative-stress, apoptosis and DNA damage biomarkers by post-IS day 28 (Figure 3).
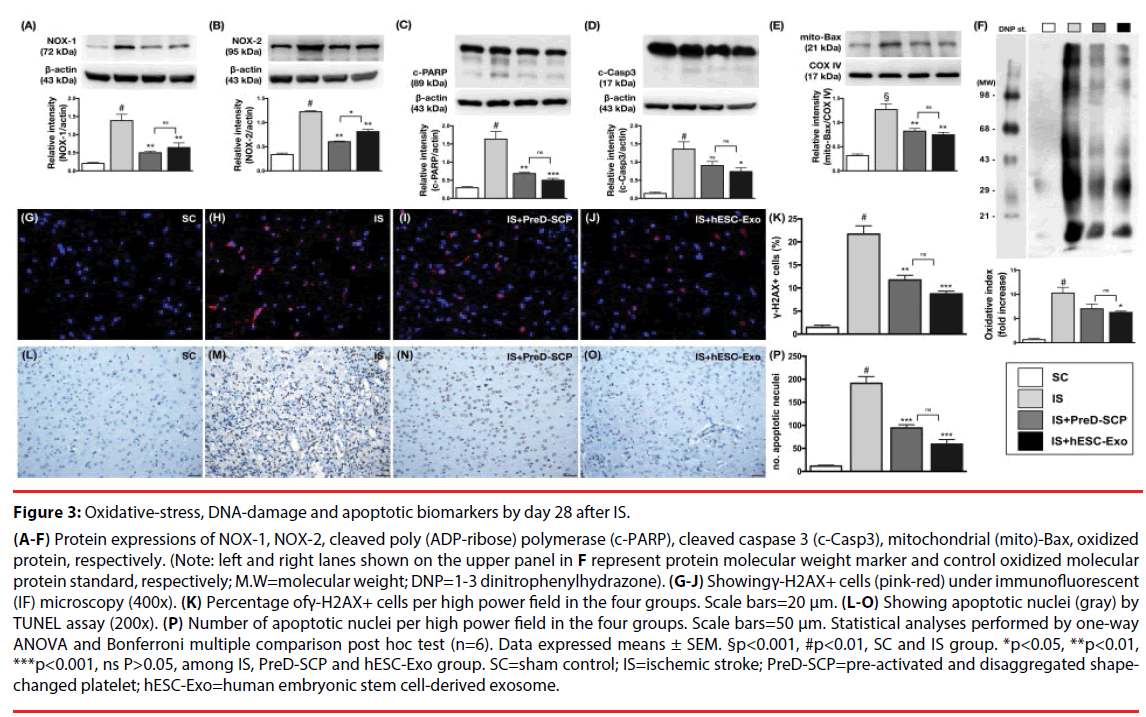
Figure 3: Oxidative-stress, DNA-damage and apoptotic biomarkers by day 28 after IS.
(A-F) Protein expressions of NOX-1, NOX-2, cleaved poly (ADP-ribose) polymerase (c-PARP), cleaved caspase 3 (c-Casp3), mitochondrial (mito)-Bax, oxidized protein, respectively. (Note: left and right lanes shown on the upper panel in F represent protein molecular weight marker and control oxidized molecular protein standard, respectively; M.W=molecular weight; DNP=1-3 dinitrophenylhydrazone). (G-J) Showingγ-H2AX+ cells (pink-red) under immunofluorescent (IF) microscopy (400x). (K) Percentage ofγ-H2AX+ cells per high power field in the four groups. Scale bars=20 μm. (L-O) Showing apoptotic nuclei (gray) by TUNEL assay (200x). (P) Number of apoptotic nuclei per high power field in the four groups. Scale bars=50 μm. Statistical analyses performed by one-way ANOVA and Bonferroni multiple comparison post hoc test (n=6). Data expressed means ± SEM. §p<0.001, #p<0.01, SC and IS group. *p<0.05, **p<0.01, ***p<0.001, ns P>0.05, among IS, PreD-SCP and hESC-Exo group. SC=sham control; IS=ischemic stroke; PreD-SCP=pre-activated and disaggregated shapechanged platelet; hESC-Exo=human embryonic stem cell-derived exosome.
The protein expressions of three indicators of oxidative stress, NOX-1, NOX-2 and oxidized protein, were significantly higher in group 2 than those in other groups, significantly higher in group 3 and 4 than those in group 1. However, there was no statistically significant difference in the expressions of these parameters between group 3 and 4. Moreover, the protein expressions of cleaved-caspase 3, cleaved-PARP and mitochondrial-Bax, three indices of apoptosis, displayed a pattern similar to those of oxidative stress among the four groups. IF microscopy demonstrated that the change in the number of γ-H2AX+ cells, an indicator of DNA damage, exhibited an identical pattern compared to those of oxidative stress among the four groups. In addition, TUNEL assay demonstrated that the number of apoptotic nuclei was substantially increased in group 2 than that in other groups, notably higher in group 3 and 4 than that in group 1, and significantly higher in group 4 than that in group 1.
Effects of PreD-SCP and hESC-Exo on brain edema/expressions of inflammation biomarkers and integrity of neuron/myelin sheath by post- IS day 28 (Figure 4).
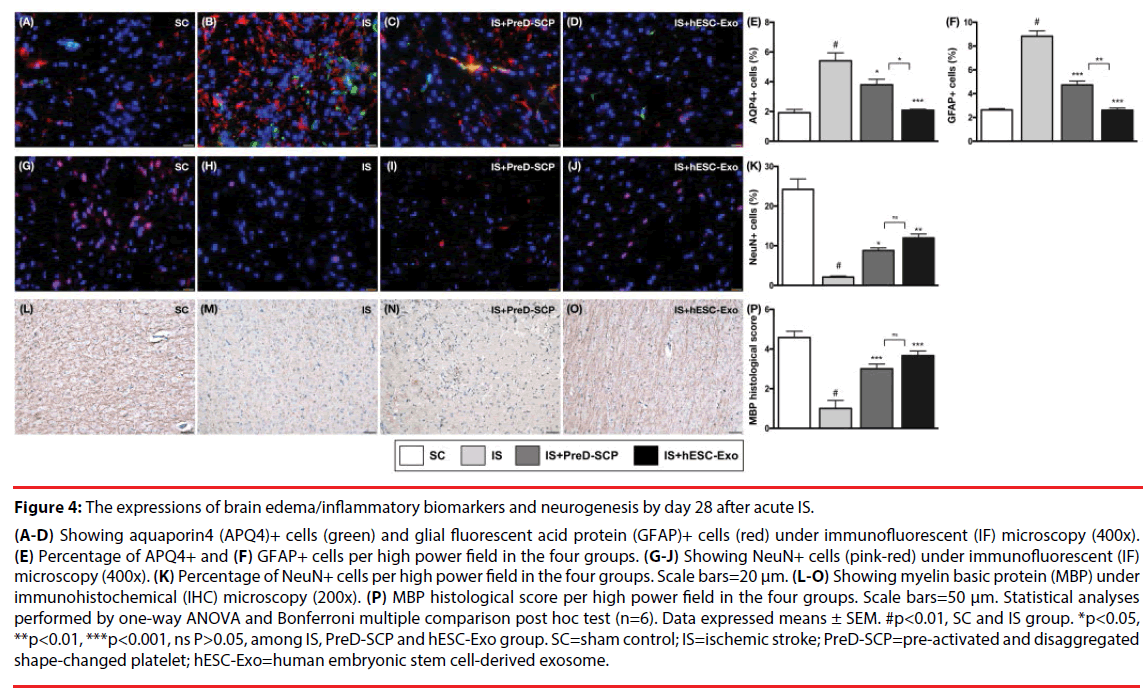
Figure 4: The expressions of brain edema/inflammatory biomarkers and neurogenesis by day 28 after acute IS.
(A-D) Showing aquaporin4 (APQ4)+ cells (green) and glial fluorescent acid protein (GFAP)+ cells (red) under immunofluorescent (IF) microscopy (400x). (E) Percentage of APQ4+ and (F) GFAP+ cells per high power field in the four groups. (G-J) Showing NeuN+ cells (pink-red) under immunofluorescent (IF) microscopy (400x). (K) Percentage of NeuN+ cells per high power field in the four groups. Scale bars=20 μm. (L-O) Showing myelin basic protein (MBP) under immunohistochemical (IHC) microscopy (200x). (P) MBP histological score per high power field in the four groups. Scale bars=50 μm. Statistical analyses performed by one-way ANOVA and Bonferroni multiple comparison post hoc test (n=6). Data expressed means ± SEM. #p<0.01, SC and IS group. *p<0.05, **p<0.01, ***p<0.001, ns P>0.05, among IS, PreD-SCP and hESC-Exo group. SC=sham control; IS=ischemic stroke; PreD-SCP=pre-activated and disaggregated shape-changed platelet; hESC-Exo=human embryonic stem cell-derived exosome.
IF microscopy revealed that the number of AQP4+ cells, a marker of brain edema, was highest in group 2, lowest in group 1, and significantly higher in group 3 than that in group 4. Additionally, the number of GFAP+ cells, an indicator of inflammatory reaction, showed a pattern identical to that of AQP4+ cells among the four groups. On the other hand, the number of NeuN+ cells, an indicator of neuron integrity in the brain, was significantly higher in group 1 than that in other groups, and significantly higher in group 3 and 4 than that in group 2, but it did not show statistical significant difference between group 3 and 4. Additionally, the intensity of myelin-binding protein (MBP), an indicator of the integrity of myelin sheath that connected to neurons in the brain, displayed an identical pattern compared to that of NeuN+ cells among the four groups.
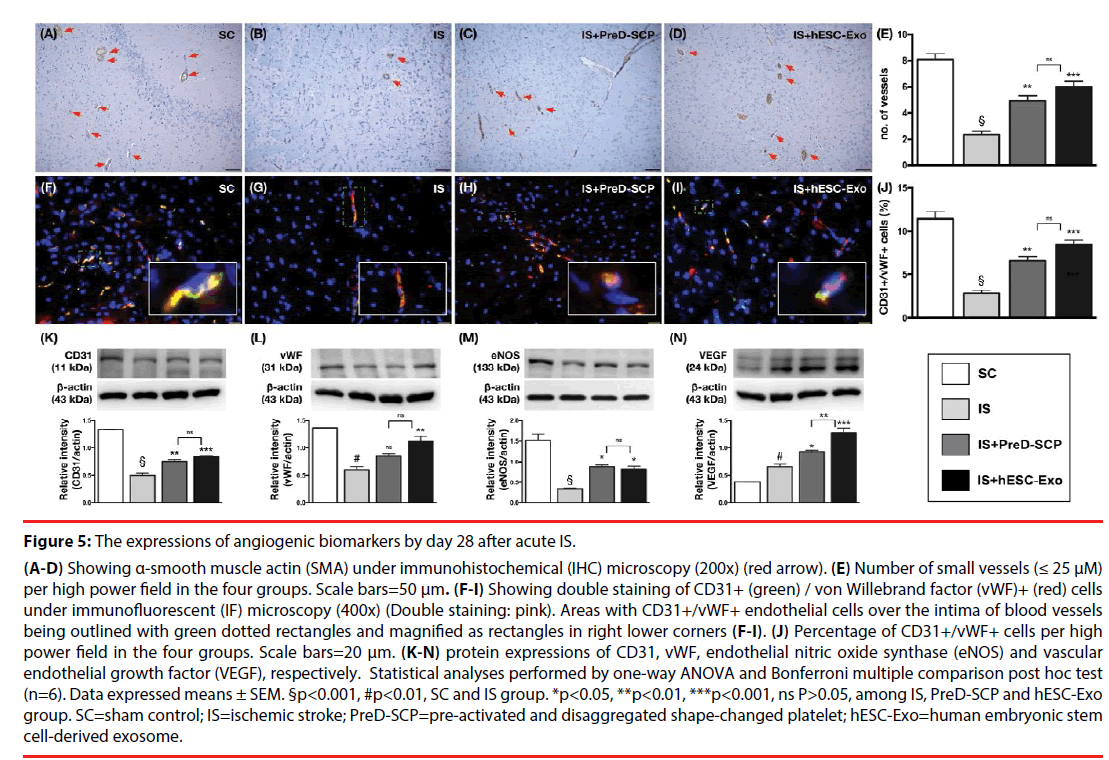
Effects of PreD-SCP and hESC-Exo on enhancing the angiogenesis by post-IS day 28 (Figure 5).
Figure 5: The expressions of angiogenic biomarkers by day 28 after acute IS.
(A-D) Showing α-smooth muscle actin (SMA) under immunohistochemical (IHC) microscopy (200x) (red arrow). (E) Number of small vessels (≤ 25 μM) per high power field in the four groups. Scale bars=50 μm. (F-I) Showing double staining of CD31+ (green) / von Willebrand factor (vWF)+ (red) cells under immunofluorescent (IF) microscopy (400x) (Double staining: pink). Areas with CD31+/vWF+ endothelial cells over the intima of blood vessels being outlined with green dotted rectangles and magnified as rectangles in right lower corners (F-I). (J) Percentage of CD31+/vWF+ cells per high power field in the four groups. Scale bars=20 μm. (K-N) protein expressions of CD31, vWF, endothelial nitric oxide synthase (eNOS) and vascular endothelial growth factor (VEGF), respectively. Statistical analyses performed by one-way ANOVA and Bonferroni multiple comparison post hoc test (n=6). Data expressed means ± SEM. §p<0.001, #p<0.01, SC and IS group. *p<0.05, **p<0.01, ***p<0.001, ns P>0.05, among IS, PreD-SCP and hESC-Exo group. SC=sham control; IS=ischemic stroke; PreD-SCP=pre-activated and disaggregated shape-changed platelet; hESC-Exo=human embryonic stem cell-derived exosome.
IHC (i.e., α-SMA) staining showed that the number of small vessels (i.e., ≤ 25 μM) was significantly higher in group 1 than that in other groups, and significantly higher in group 3 and 4 than that in group 1, but there was no significant difference between group 3 and 4. Besides, IF microscopy demonstrated that the number of CD31+/vWF+ cells, double staining for the identification of intact endothelial cells, expressed a pattern identical to that of the number of small vessels among the four groups. The protein expressions of CD31, eNOS and vWF, three biomarkers of angiogenesis, displayed a similar pattern compared to those of CD31+/vWF+ cells among the four groups. Furthermore, the protein expression of VEGF, another indicator of angiogenesis, significantly and progressively increased from group 1 to 4, indicating an intrinsic response to ischemic stimulation and enhanced by PreD-SCP and hESC-Exo treatments.
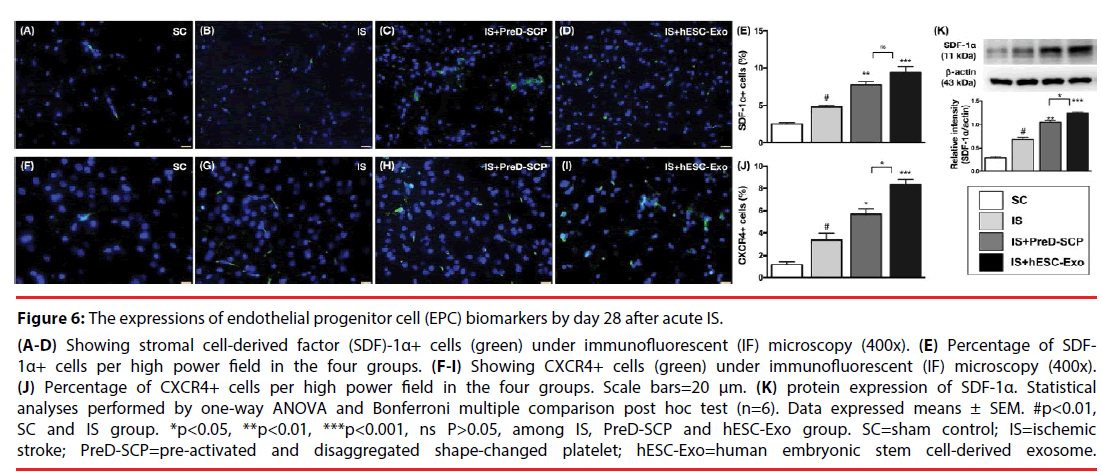
Effects of PreD-SCP and hESC-Exo on enhancing the expression of endothelial progenitor cell (EPC) biomarkers by post-IS day 28 (Figure 6).
Figure 6: The expressions of endothelial progenitor cell (EPC) biomarkers by day 28 after acute IS.
(A-D) Showing stromal cell-derived factor (SDF)-1α+ cells (green) under immunofluorescent (IF) microscopy (400x). (E) Percentage of SDF- 1α+ cells per high power field in the four groups. (F-I) Showing CXCR4+ cells (green) under immunofluorescent (IF) microscopy (400x). (J) Percentage of CXCR4+ cells per high power field in the four groups. Scale bars=20 μm. (K) protein expression of SDF-1α. Statistical analyses performed by one-way ANOVA and Bonferroni multiple comparison post hoc test (n=6). Data expressed means ± SEM. #p<0.01, SC and IS group. *p<0.05, **p<0.01, ***p<0.001, ns P>0.05, among IS, PreD-SCP and hESC-Exo group. SC=sham control; IS=ischemic stroke; PreD-SCP=pre-activated and disaggregated shape-changed platelet; hESC-Exo=human embryonic stem cell-derived exosome.
IF microscopy demonstrated that the number of CXCR4+ cells, a biomarker of EPCs, was highest in group 4, lowest in group 1, and significantly higher in group 3 than that in group 2. In addition, the protein and cellular expressions of SDF-1α, two indices of EPCs, were significantly higher in group 3 and 4 than those in group 1 and 2, and significantly higher in group 2 than those in group 1, and there were statistically significant differences between group 3 and 4.
Discussion
This study, which investigated the therapeutic potential of PreD-SCP and hESC-Exo against acute IS in rats, yielded several striking implications. First, to the best of our knowledge, the present report is the first to demonstrate that administration of PreD-SCP and hESC-Exo reduced BIA and attenuated neurological deficits in rats after acute IS. Second, the results of the current study elucidated that the underlying mechanisms of therapeutic improvement of neurological outcomes were mainly through inhibiting inflammation, oxidative stress, and cellular apoptosis as well as enhancing aangiogenesis. Third, preservation of the integrity of neurons/myelin sheath by PreD-SCP and hESC-Exo treatments was another fundamental finding that may account for improvement in neurological function in rats after IS.
The most important finding in the present study is that, as compared with animals after IS without treatment, the neurological function was notably improved by day 3 and further improved afterwards in animals with IS after receiving either PreD-SCP or hESC-Exo treatment. Interestingly, our previous investigations have demonstrated that adipose-derived mesenchymal stem cell (ADMSC) [13,18] therapy markedly improved the neurological function in rodent after acute IS. Accordingly, our findings, in addition to supporting our previous studies [13,18], highlights that MSC/MSC-derived Exo may be of therapeutic potential for IS patients who were refractory to conventional therapy. Another interesting finding from our recent work is that PreD-SCP treatment significantly protected the rat lung against ARDS [17]. In the present study, we showed that PreD-SCP treatment enhanced the recovery of the neurological defect in rat after IS. Our findings, therefore, in addition to reinforcing those of our previous study [17], extended the scope of PreD-SCP application in protecting the brain from IS. An essential finding of the present study is the reduction of BIA (i.e., anatomical structure) and preservation of neuron (i.e., NeuN staining)/myelin sheath (i.e., MBP staining) (i.e., molecular-cellular level) integrity in hESC-Exo/PreD-SCP-treated animals as compared with those in animals after IS without treatment. Intriguingly, we have also previously shown that both autologous, allogenic or exogenous MSC therapy ameliorated brain infarct size in rats after IS [13,18,19]. In this way, our findings were comparable to those of our previous studies [13,18,19].
An association between the degree of organ damage/apoptosis and inflammatory reaction and oxidative stress has been investigated in depth in previous studies [9,10,18,20]. One important finding in the present study is the marked increase in inflammatory reaction, oxidative stress and expressions of apoptotic biomarkers in the IS group without treatment compared to those in the sham-operated controls. Therefore, our findings, in addition to being consistent with those of the previous studies [9,10,18,20], could partially explain the notably increased BIA and deteriorated neurological function in IS animals than those in the sham-operated animals. Of importance is that these parameters were markedly suppressed in IS animals after receiving hESC-Exo/PreD-SCP treatments.
Augmentation of angiogenesis has been identified in ischemic organs after receiving cell therapy [21-24]. The distinctive importance is that angiogenesis has been established to play a critical role in improving ischemia-related organ dysfunction [21-24]. A principal finding of the current study is notable increases in the expressions of angiogenesis biomarkers (i.e., at molecularcellular levels) in IS animals after receiving hESCExo/ PreD-SCP treatments compared to those in IS only animals. In this way, our findings, in addition to being consistent with the results of previous studies [13,15,19,25], partially explain the significant preservation of the number of neurons and reduction of BIA in animals receiving hESCExo/ PreD-SCP treatments.
Study Limitations
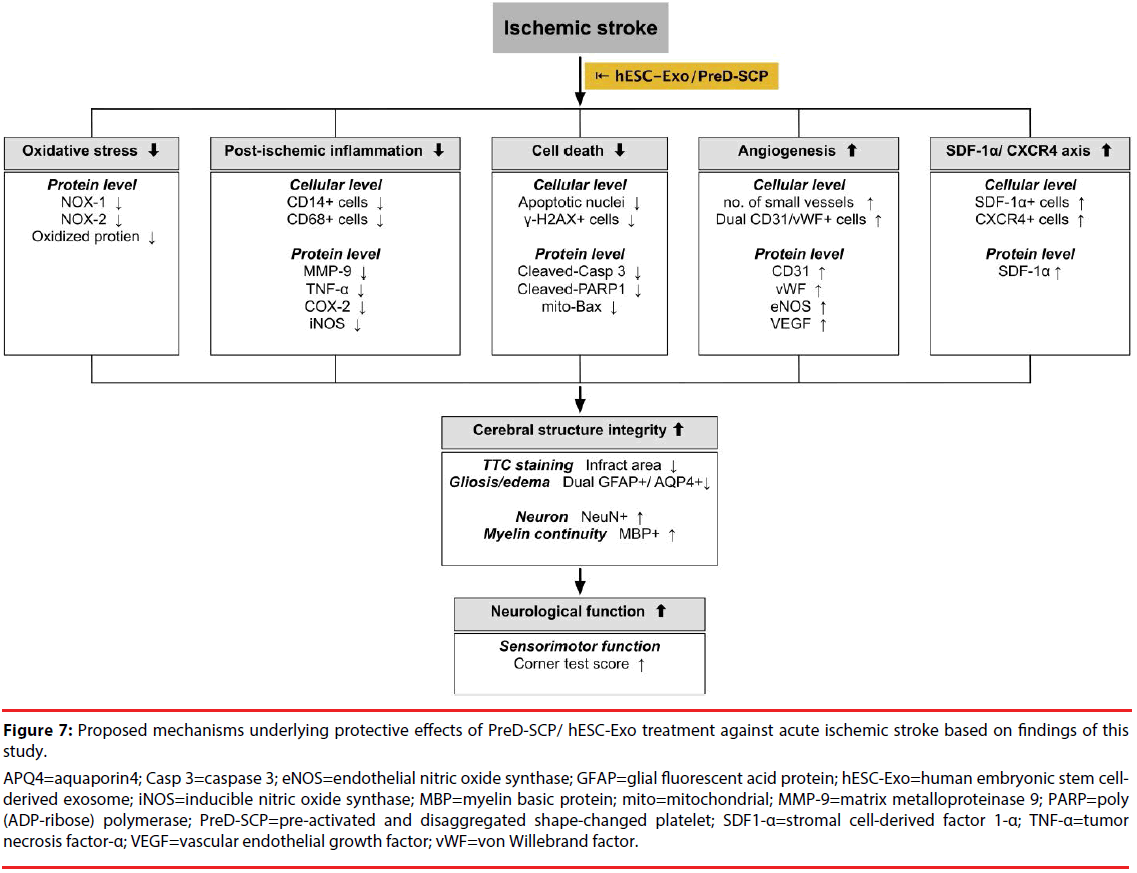
This study has limitations. Firstly, this study did not explore the optimal dosage of PreD-SCP and hESC-Exo for protecting the brain against acute IS. Therefore, whether the therapeutic effect of PreD-SCP is superior to hESC-Exo or vice versa remains unknown. Secondly, although extensive work had been done in the present study, the exact mechanism underlying the alleviation of brain damage after acute IS through PreD-SCP/hESC-Exo treatments is still unclear. The proposed mechanisms by which administration of PreD-SCP and hESC-Exo improved the outcome of animals after acute IS are summarized in Figure 7. Thirdly, this study did not investigate the safety of PreD-SCP and hESC-Exo in sham-operative controls so that the side-effect of PreD-SCP and hESC-Exo treatments remains unclear. Finally, lowerpower panoramic image for more neuron’s marker detection did not provided in the present study.
Figure 7: Proposed mechanisms underlying protective effects of PreD-SCP/ hESC-Exo treatment against acute ischemic stroke based on findings of thisstudy.
APQ4=aquaporin4; Casp 3=caspase 3; eNOS=endothelial nitric oxide synthase; GFAP=glial fluorescent acid protein; hESC-Exo=human embryonic stem cellderived exosome; iNOS=inducible nitric oxide synthase; MBP=myelin basic protein; mito=mitochondrial; MMP-9=matrix metalloproteinase 9; PARP=poly (ADP-ribose) polymerase; PreD-SCP=pre-activated and disaggregated shape-changed platelet; SDF1-α=stromal cell-derived factor 1-α; TNF-α=tumor necrosis factor-α; VEGF=vascular endothelial growth factor; vWF=von Willebrand factor.
Conclusion
In conclusion, the results of current study highlighted that molecular-cellular perturbations, including acute inflammatory reaction, oxidative damage and apoptosis in brain ischemia/ infarction zones were quickly upregulated after acute IS. However, PreD-SCP and hESC-Exo treatments significantly protected the brain from acute IS damage and improved neurological outcome mainly through suppressing these molecular-cellular perturbations.
Acknowledgements
This study was supported by a program grant from Chang Gung Memorial Hospital, Chang Gung University (Grant no. CMRPG8E1341).
Conflict of interest statement
The authors report no conflict of interest.
References
- Kawle AP, Nayak AR, Lande NH, et al. Comparative evaluation of risk factors, outcome and biomarker levels in young and old acute ischemic stroke patients. Ann. Neurosci 22(2), 70-77 (2015).
- Hsieh FI, Chiou HY. Stroke: morbidity, risk factors, and care in taiwan. J. Stroke 16(2), 59-64 (2014).
- Liang J, Qi Z, Liu W, et al. Normobaric hyperoxia slows blood-brain barrier damage and expands the therapeutic time window for tissue-type plasminogen activator treatment in cerebral ischemia. Stroke 46(5), 1344-1351 (2015).
- Albers GW, Clark WM, Madden KP, et al. ATLANTIS trial: results for patients treated within 3 hours of stroke onset. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. Stroke 33(2), 493-495 (2002).
- Yepes M. Tissue-type plasminogen activator is a neuroprotectant in the central nervous system. Front. Cell. Neurosci 9(1), 304 (2015).
- Chen YL, Tsai TH, Wallace CG, et al. Intra-carotid arterial administration of autologous peripheral blood-derived endothelial progenitor cells improves acute ischemic stroke neurological outcomes in rats. Int. J. Cardiol 201(1), 668-683 (2015).
- Khoshnam SE, Winlow W, Farzaneh M, et al. Pathogenic mechanisms following ischemic stroke. Neurol. Sci 38(7), 1167-1186 (2017).
- Yip HK, Liou CW, Chang HW, et al. Link between platelet activity and outcomes after an ischemic stroke. Cerebrovasc. Dis 20(2), 120-128 (2005).
- Yuen CM, Chung SY, Tsai TH, et al. Extracorporeal shock wave effectively attenuates brain infarct volume and improves neurological function in rat after acute ischemic stroke. Am. J. Transl. Res 7(6), 976-994 (2015).
- Yuen CM, Sun CK, Lin YC, et al. Combination of cyclosporine and erythropoietin improves brain infarct size and neurological function in rats after ischemic stroke. J. Transl. Med 9(1), 141 (2011).
- Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J. Leukoc. Biol 87(5), 779-789 (2010).
- McCombe PA, Read SJ. Immune and inflammatory responses to stroke: good or bad? Int. J. Stroke 3(4), 254-265 (2008).
- Chen KH, Chen CH, Wallace CG, et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 7(46), 74537-74556 (2016).
- Ko SF, Yip HK, Zhen YY, et al. Adipose-Derived Mesenchymal Stem Cell Exosomes Suppress Hepatocellular Carcinoma Growth in a Rat Model: Apparent Diffusion Coefficient, Natural Killer T-Cell Responses, and Histopathological Features. Stem. Cells. Int 2015(1), 853506 (2015).
- Lin KC, Yip HK, Shao PL, et al. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int. J. Cardiol 216(1), 173-185 (2016).
- Liu XY, Wang CP, Liu M, et al. Transplantation of human embryonic neural stem cells protects rats against cerebral ischemic injury. Sheng. Li. Xue. Bao 66(6), 691-701 (2014).
- Day YJ, Chen KH, Chen YL, et al. Preactivated and Disaggregated Shape-Changed Platelets Protected Against Acute Respiratory Distress Syndrome Complicated by Sepsis Through Inflammation Suppression. Shock 46(5), 575-586 (2016).
- Leu S, Lin YC, Yuen CM, et al. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J. Transl. Med 8(1), 63 (2010).
- Lee FY, Chen KH, Glenn Wallace C, et al. Xenogeneic human umbilical cord-derived mesenchymal stem cells reduce mortality in rats with acute respiratory distress syndrome complicated by sepsis. Oncotarget (2017).
- Yip HK, Yuen CM, Chen KH, et al. Tissue plasminogen activator deficiency preserves neurological function and protects against murine acute ischemic stroke. Int. J. Cardiol 205(1), 133-141 (2016).
- Fu M, Sun CK, Lin YC, et al. Extracorporeal shock wave therapy reverses ischemia-related left ventricular dysfunction and remodeling: molecular-cellular and functional assessment. PLoS. One 6(9), e24342 (2011).
- Lee FY, Chen YL, Sung PH, et al. Intracoronary Transfusion of Circulation-Derived CD34+ Cells Improves Left Ventricular Function in Patients With End-Stage Diffuse Coronary Artery Disease Unsuitable for Coronary Intervention. Crit. Care. Med 43(10), 2117-2132 (2015).
- Sheu JJ, Ali HEE, Cheng BC, et al. Extracorporeal shock wave treatment attenuated left ventricular dysfunction and remodeling in mini-pig with cardiorenal syndrome. Oncotarget (2017).
- Yeh KH, Sheu JJ, Lin YC, et al. Benefit of combined extracorporeal shock wave and bone marrow-derived endothelial progenitor cells in protection against critical limb ischemia in rats. Crit. Care. Med 40(1), 169-177 (2012).
- Lee FY, Zhen YY, Yuen CM, et al. The mTOR-FAK mechanotransduction signaling axis for focal adhesion maturation and cell proliferation. Am. J. Transl. Res 9(4), 1603-1617 (2017).