Research Article - Neuropsychiatry (2018) Volume 8, Issue 2
Progressive Brain Changes in the Early Stage of Schizophrenia: A Combined Structural MRI and DTI Study
- *Corresponding Author:
- Dengtang Liu
Shanghai Mental Health Center, Shanghai Jiao Tong University, School of Medicine, 600 Wan Ping Nan Road, Shanghai 200030, China
Tel: +86-21-34289888
Fax: +86-21-64387986
Abstract
Abstract
Purpose: The developmental trajectory of brain structure in the early stage of schizophrenia has not been well defined. In this study, structural MRI and diffusion tensor imaging (DTI) were used to explore the brain gray matter (GM) and white matter (WM) changes in patients with schizophreniform disorder or early schizophrenia.
Methods: Sixteen schizophreniform patients, 19 early schizophrenia patients (duration of illness < 5 years) and 25 healthy controls were enrolled in this study. Structural MRI and DTI images were obtained. GM volumes were examined using voxel-based morphometry (VBM), and fractional anisotropy (FA) was used to measure WM integrity.
Results: Compared with healthy controls, GM volume was smaller in the right hippocampus and larger in the left middle frontal gyrus and right temporal gyrus in schizophreniform patients; there were no significant differences in FA values between the two groups. GM volumes were reduced in the right hippocampus, bilateral parahippocampal gyri, right middle temporal gyrus and precuneus, and left inferior occipital lobe, and increased in the left temporal lobe in schizophrenia patients compared with healthy controls; furthermore, FA values were reduced in the bilateral cingulate, right insular cortex, precuneus and superior frontal gyrus, and increased in the bilateral fusiform gyri, right superior temporal gyrus, right middle frontal lobe of schizophrenia patients compared with healthy controls.
Conclusion: Our study suggests that brain GM and WM abnormalities occur in early stage schizophrenia, with the occurrence of GM changes, particularly those in the right hippocampus, likely preceding changes in WM.
Keywords
Schizophrenia; Early stage; Magnetic resonance imaging (MRI); Diffusion tensor imaging (DTI)
Introduction
Schizophrenia is a chronic and disabling disorder characterized by extensive functional impairment [1]. However, the etiology and pathophysiology of schizophrenia remain elusive.
Previous studies have shown grey matter (GM) volume reduction and compromised white matter (WM) integrity in patients with schizophrenia [2,3]. Furthermore, cortical thinning has been reported in corresponding brain areas in individuals at high risk for psychosis and those with schizophrenia, particularly in the frontal and medial temporal cortices, insular and cerebellar, anterior cingulate cortex (ACC), superior temporal gyrus (STG) and hippocampal regions [4-6]. Studies in first-episode psychosis patients have shown reduced whole brain volume and hippocampal volumes, as well as increased lateral ventricular volume [7,8]. Finally, a meta-analysis that included individuals with chronic schizophrenia found that patients also had smaller mean cerebral volumes, greater total ventricular volumes, smaller hippocampus and parahippocampus, amygdala, frontal lobes, and temporal lobes; these changes were largely bilateral [8].
Although previous studies have shed substantial light on the pathophysiological features of the brain associated with schizophrenia, the extent to which factors such as duration of illness, clinical symptom severity, and exposure to antipsychotic treatment have influenced their findings is currently unknown. Importantly, recent studies have indicated that treatment with antipsychotics might give rise to some of the neuroanatomical changes that are commonly attributed to the illness process [9,10].
The majority of studies detailed above have focused on macrostructural indices of volume changes in GM and WM. [9-11]. However, diffusion tensor imaging (DTI) provides a useful method to assess WM integrity and connectivity in vivo [12]. Fractional anisotropy (FA), a DTI index, is thought to be a marker of the structural integrity of fibers, the degree of myelination, coherence of fiber tracts, and fiber diameter and packing density [13-15]; changes in FA may indicate changes in any of these characteristics of the WM microstructure or a combination of them. Meta-analyses have reported significant differences in DTI measures in the brains of chronic schizophrenia patients in the fornix, corpus callosum, cingulum bundle, internal capsule, external capsule, superior and inferior occipito-frontal fasciculus, arcuate fasciculus, and uncinate fasciculus [16]. Combined volumetric MRI and DTI analyses suggest that FA abnormalities precede WM volume loss in schizophrenia [17].
In the present study, we sought to examine brain GM and WM abnormalities by comparing groups of patients with schizophreniform disorder and early schizophrenia relative to healthy controls. We hypothesized that both GM and WM abnormalities evolve over time as the illness progresses.
Methods
▪ Participants
This study was conducted at Shanghai Mental Health Center (SMHC), China. Thirtyfive patients with a clinical diagnosis of schizophreniform disorder (16 patients) or schizophrenia (19 patients) were recruited for participation. The upper limit for illness duration in the clinical diagnosis of schizophreniform disorder is 6 months, after which a diagnosis of schizophrenia is made if symptoms persist; we confined the disease duration for patients with schizophrenia to 5 years (early schizophrenia). The diagnosis of each patient was confirmed by a research psychiatrist (Y.W.) using the MINI plus v 5.0 [18]. All schizophreniform patients were eventually diagnosed with schizophrenia at post- 6 month follow-up. To minimize the potential confounding effects of antipsychotic medication, all schizophreniform patients were drug-naïve, and of the 19 schizophrenia patients, 9 of them were drug-naïve, others had discontinued antipsychotic medications from 8 months to 18 months prior to study participation. All subjects had the capacity to provide informed consent, were in relatively stable clinical condition and were deemed competent for study participation by their treating psychiatrists.
Exclusion criteria for the study included: (1) inability to provide informed consent, (2) acute psychosis or unstable clinical condition (e.g., aggression or uncooperative behavior), (3) current substance abuse, (4) any other psychiatric diagnosis, (5) significant medical conditions including neurological disease, severe cardiovascular, hepatic, or renal diseases, (6) pregnancy or breastfeeding.
Twenty-five healthy controls (HC) were recruited from the local community through advertising. All of them completed a structured clinical interview conducted by a research psychiatrist using MINI plus v 5.0 [18]. Those with any psychiatric disease, neurological disease, or a positive family history of psychiatric disease were excluded.
Written informed consent was obtained from each participant. The study protocol was approved by the SMHC Ethics Committee in compliance with the Helsinki Declaration. Clinical symptoms were assessed using the Scale for Assessment of Negative Syndrome (SANS) [19] and Scale for Assessment of Positive Syndrome (SAPS) [19]. The severity of illness was also assessed by the Clinical Global Impressions-severity scale (CGI) [20].
Image Acquisition
▪ Structural imaging
Imaging scans were performed using a SIEMENS Verio 3.0 T scanner at the Shanghai Mental Health Center (SMHC). Scan sequences were identical for all subjects. Head movement was minimized by foam padding across the forehead and chin. A 3-dimensional volumetric spoiled gradient recalled echo in the steady state sequence generated 124 contiguous, 1 mm thick coronal slices. Imaging parameters were: timeto- echo, 2.96msec; time-to-repetition, 2300 msec; flip angle, 90; matrix size, 240×256; field of view, 16 ×16 cm matrix; voxel dimensions, 1mm×1mm×1mm.
▪ Diffusion tensor imaging
3 mm thick coronal slices were obtained. Imaging parameters were: time-to-echo, 87 msec; time-torepetition, 8500 msec; diff directions 32; matrix size, 64×64; field of view, 230; voxel dimensions, 1 mm×1 mm×1 mm.
The scanner was calibrated fortnightly using the same proprietary phantom to ensure stability and accuracy of measurements. A subject code was used to ensure patient confidentiality and blindness of data. A neuroradiologist reviewed all MRI scans.
Image Processing
▪ Structural images
We quantitatively characterized GM changes using VBM. All anatomical images were processed in SPM8 and VBM8 toolboxes (http://dbm.neuro.uni-jena.de/vbm/) with default parameters under MATLAB 7.0. VBM is a whole-brain, unbiased, semi-automated technique for characterizing regional cerebral differences in structural magnetic resonance images. First, structural images were segmented to extract GM and then normalized to an asymmetric T1-weighted template in Montreal Neurological Institute (MNI) stereotactic space, in a recursive fashion. Image segmentation incorporated an intensity nonuniformity correction to account for smooth intensity variations caused by gradient distortions and different positions of cranial structures within the MRI coil. The resultant GM images were smoothed with a FWHM kernel of 6 mm in SPM8 (http://www.fil.ion.ucl.ac.uk/spm). Smoothing is required to compensate for the inexact nature of spatial normalization and to maximize the chance that regional effects are expressed at a spatial scale where homologies in structural anatomy exist over subjects. After smoothing, each voxel represents the local average amount of GM in the region, the size of which is defined by the smoothing kernel.
▪ Diffusion tensor images
The diffusion-tensor images were first corrected for eddy current distortion using a mutual information based registration scheme, and then masked using locally written software plus the Brain Extraction Tool (BET) in the Functional Software Library package (http://www.fmrib. ox.ac.uk/fsl). Fractional anisotropy (an index of WM microstructural organisation) was calculated at each voxel to produce a multislice fractional anisotropy image in FLRIT. We thus generated a transformation matrix by normalizing each FA image to the Montreal Neurological Institute (MNI)-152 FA brain template [21,22].
▪ Statistical analyses
We performed independent samples t-tests in SPM8 to compare GM volumes and FA values between schizophreniform patients and healthy controls (HC) schizophrenia patients and HCs, schizophreniform and schizophrenia patients. Imaging results were considered significant against AlphaSim correction for completeness (p<0.001). The relationships between imaging measures and clinical measures in patients, including hallucinations、bizarre behavior, delusions, positive forms of thought disorder, poverty of thought, emotion dull, lack of will, lack of interest, attention disorder,age, age at onset, duration of illness and education level, were assessed by Spearman rank-order correlation .
Results
▪ Demographic characteristics and clinical variables
Table 1 shows the demographic and clinical characteristics of all the participant groups. One- Way ANOVA showed no significant differences among the three groups in gender and age. However, education level differed among the three groups. Group comparisons between the schizophreniform group and the early schizophrenia group revealed no differences in age of illness onset, SAPS score, SANS score and CGI score. As expected, the illness duration in the early schizophrenia group was significantly longer than in the schizophreniform group (t=−5.976, p <0.001), and the number of episodes in the early schizophrenia group was significantly higher than in the schizophreniform group (t=−2.247, p=0.031).
| Patients with schizophreniform disorder (n=16) | Patients with Schizophrenia (n=19) | Healthy controls (n=25) | Group comparisons | ||
|---|---|---|---|---|---|
| Mean(SD) | Mean (SD) | Mean (SD) | F or t | p | |
| Age (years) | 27.19(7.43) | 29.26(4.34) | 27.08(4.86) | 0.979 | 0.382 |
| Education (years) | 13.63(2.06) | 15.00(2.08) | 16.24(1.05) | 11.40 | <0.001 |
| Age of onset (years) | 27.56(7.25) | 26.68(4.15) | 0.449 | 0.657 | |
| Duration of illness (months) | 2.38(1.98) | 32.11(19.77) | -5.976 | <0.001 | |
| Episode | 1.0(0.00) | 1.47(0.84) | -2.247 | 0.031 | |
| SAPS | 41.00(13.43) | 40.84(12.58) | 0.036 | 0.972 | |
| SANS | 33.88(21.39) | 37.16(18.56) | -0.486 | 0.630 | |
| CGI-severity | 5.33(0.49) | 5.21(0.63) | / | 0.642 | 0.525 |
| N% | N (%) | N (%) | x2 | p | |
| Gender | 0.059 | 0.971 | |||
| Male | 7(43.8) | 8(42.1) | 10(40) | ||
| Female | 9(56.3) | 11(57.9) | 15(60) | ||
Notes: SANS: Scale for Assessment of Negative Syndrome , SAPS: Scale for Assessment of Positive Syndrome, CGI-S: Clinical Global Impression-Servity.
Table 1: Demographic characteristics and clinical variables of the study groups.
▪ Patients with schizophreniform disorder compared to healthy controls
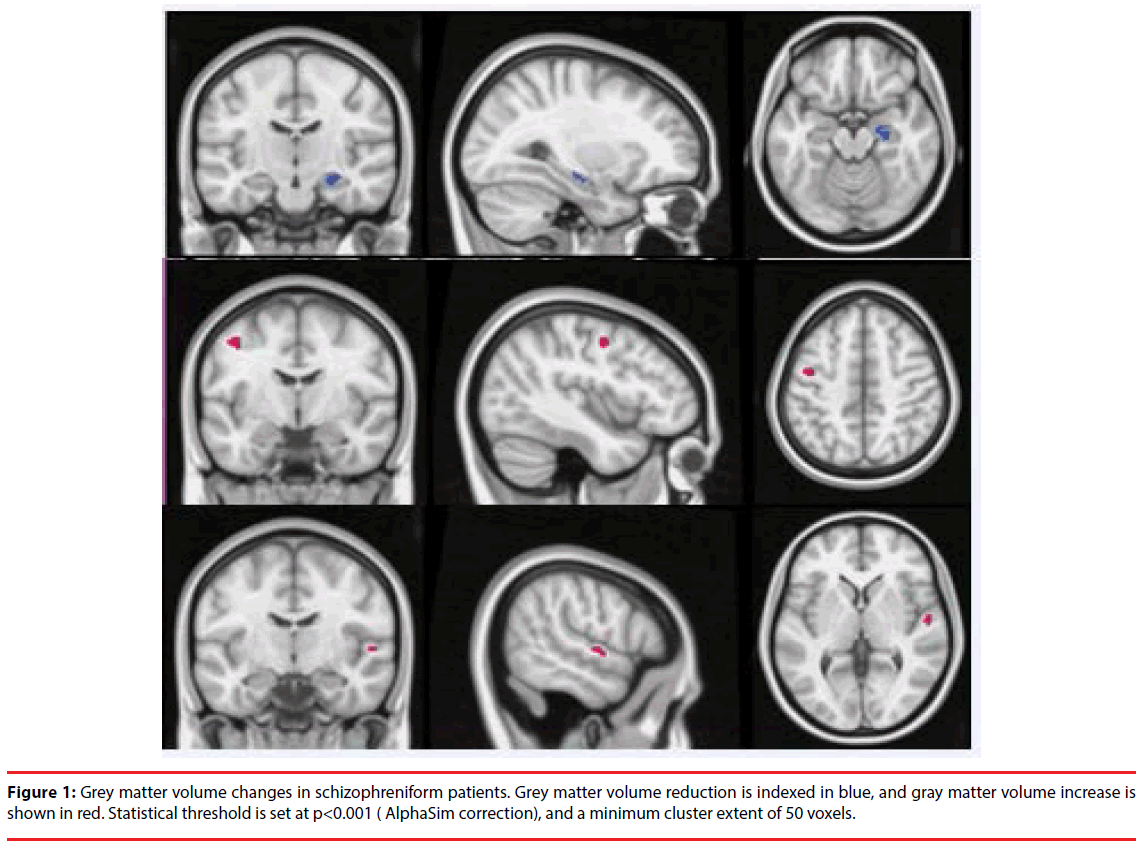
Compared to HCs, the schizophreniform group exhibited minor variations of brain GM: smaller volume of the right hippocampus, and larger GM volume in the left precentral and right superior temporal gyrus . However, no significant differences were found for any brain WM FA values between the schizophreniform group and HCs (Figure 1).
▪ Patients with Early Schizophrenia Compared to Healthy Controls
Both GM and WM abnormalities were found in patients with schizophrenia compared to HCs. The results are shown in Table 2.
| MNI Coordinates | ||||||
|---|---|---|---|---|---|---|
| Cerebrum | Anatomical region | Cluster size | x | y | z | Peak intensity |
| Grey matter | ||||||
| Schizophreniapatients < Healthy controls | ||||||
| L | parahippocampal gyrus | 118 | -25.5 | -19.5 | -12 | -4.5431 |
| L | Inferior occipital gyrus | 399 | -36 | -90 | -13.5 | -4.3192 |
| R | parahippocampal gyrus | 82 | 33 | -28.5 | -18 | -4.1679 |
| R | Hippocampus | 59 | 27 | -16.5 | -13.5 | -4.5419 |
| R | Middle temporal gyrus | 56 | 64.5 | -30 | -6 | -4.0477 |
| R | Precuneus | 135 | 4.5 | -66 | 31.5 | -3.9406 |
| Schizophrenia patients > Healthy controls | ||||||
| L | Inferior temporal gyrus | 59 | -48 | -9 | -42 | 4.1878 |
| L | Inferior temporal gyrus | 55 | -40.5 | -31.5 | -18 | 3.7725 |
| White matter | ||||||
| Schizophreniapatients < Healthy controls | ||||||
| L | Cingulate gyrus | 656 | -4 | -26 | 43 | -4.4675 |
| R | Cingulate gyrus | 390 | 12 | -29 | 41 | -4.127 |
| R | precuneus | 631 | 7 | -67 | 31 | -5.6586 |
| R | Insula | 374 | 43 | -21 | 14 | -4.4877 |
| R | Superior frontal gyrus | 408 | 22 | 51 | -19 | -3.82 |
| Schizophrenia patients > Healthy controls | ||||||
| L | Fusiform | 2260 | -21 | 4 | -43 | 5.1562 |
| R | Fusiform | 848 | 26 | 3 | -46 | 3.9692 |
| R | Superior temporal gyrus | 1128 | 52 | -23 | 2 | 5.094 |
| R | Medial frontal gyrus | 299 | 6 | -1 | 66 | 4.8427 |
Notes: MNI=Montreal Neurological Institute, R/L=right/left.
Table 2: The changes of regional gray matter volume and white matter FA value in patients with Schizophrenia.
GM volumes of the right hippocampus, bilateral parahippocampal gyrus, left inferior occipital gyrus, right middle temporal gyrus and precuneus were decreased, and left inferior temporal gyrus were increased in patients. With regard to WM, the FA values of bilateral cingulate gyrus, right precuneus, insula and superior frontal gyrus were decreased, and bilateral fusiform, right superior temporal gyrus, right medial frontal gyrus were increased in patients.
▪ Group differences between the two patient groups
Further comparisons were performed between the two patient groups, and the results are displayed in Table 3. Compared to schizophreniform patients, those with schizophrenia showed decreased GM volume of the bilateral middle temporal gyri, right precuneus, left cingulate gyrus and precentral gyrus, and increased GM volume of the posterior cerebellum; FA values of the right superior thalamic radiation were decreased, and those of the bilateral fusiform and left posterior cerebellum were increased (Table 3).
| MNI Coordinates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cerebrum | Anatomical region | Cluster size | x | y | z | Peak intensity | ||
| Grey matter | ||||||||
| Schizophreniapatients < schizophreniform patients | ||||||||
| R | Middle temporal gyrus | 159 | 63 | -28.5 | -7.5 | -4.9056 | ||
| R | Precuneus | 391 | 4.5 | -69 | 33 | -6.391 | ||
| L | Middle temporal gyrus | 86 | -49.5 | -64.5 | 19.5 | -3.942 | ||
| L | Cingulate gyrus | 116 | -1.5 | -43.5 | 37.5 | -4.2024 | ||
| L | Precentral gyrus | 139 | -42 | -15 | 51 | -4.4863 | ||
| Schizophrenia patients > schizophreniform patients | ||||||||
| L | Posterior cerebellum lobe | 69 | -18 | -66 | -31.5 | 4.4649 | ||
| White matter | ||||||||
| Schizophreniapatients < schizophreniform patients | ||||||||
| R | Superior thalamic radiation | 401 | 22 | -18 | 30 | -4.2767 | ||
| Schizophrenia patients > schizophreniform patients | ||||||||
| L | Fusiform | 2723 | -27 | -2 | -23 | 5.988 | ||
| R | Fusiform | 2340 | 21 | 6 | -45 | 4.6838 | ||
| L | Posterior cerebellum lobe | 3114 | -2 | -80 | -24 | 4.7008 | ||
Notes: MNI=Montreal Neurological Institute, R/L=right/left.
Table 3: Comparison of regional grey matter volume and white matter FA value between two patients groups.
▪ Associations between regional gm volumes, fa values and clinical symptoms
In order to further explore associations between regional GM or WM abnormalities with demographic characteristics and clinical variables, schizophreniform patients and schizophrenia patients were combined into one group for correlation analyses. The mean GM volume of the right parahippocampal gyrus correlated negatively with SAPS total score (r=-0.355,p=0.039), bizarre behavior score (r=- 0.369, p=0.032), and SANS avolition apathy score (r=-0.418, p=0.014). The mean WM FA value of the right superior temporal gyrus correlated positively with age at illness onset (r=0.388,p=0.023), and negatively with alogia score in SANS (r=-0.347,p=0.044).
Discussion
To our knowledge, this is the first study to investigate differences in brain structure among individuals diagnosed with schizophrenia, those with schizophreniform disorder, and healthy controls. Our main findings show that GM and WM abnormalities in schizophrenia appear to advance as the stage of illness progresses. The right hippocampus was the only structure showing volume reduction in schizophreniform patients, indicating the potential utility of this finding as a biomarker for the early identification of schizophrenia. As the disease progresses, changes in terms of volume reduction of the bilateral parahippocampus, right middle temporal gyrus and precuneus may gradually emerge. In addition, schizophreniform patients showed no significant abnormalities in WM microstructure. However, our results indicate that FA values of some brain regions decrease, while those of other brain regions increase at later stages of the disease. All of the above findings are consistent with the notion that GM abnormalities precede WM changes in schizophrenia.
The majority of previous studies comparing first-episode patients to those with chronic schizophrenia do not explicitly report illness duration or stage [23-25]. An advantage of the present study is the clear distinction between patients with schizophreniform disorder and early schizophrenia at defined stages of illness. More widespread brain changes in early schizophrenia patients than in schizophreniform disorder may imply the progression of brain anatomical changes after illness onset. Meanwhile such abnormalities can be used as biomarkers. Continuing changes may evolve as illness duration increases [26].
Past studies have found reductions in hippocampal volume in first episode patients with schizophrenia [27,28]. This indicates with relative certainty that a loss of tissue in the hippocampus occurs in the period near disease onset. In addition, studies in high risk individuals who later developed schizophrenia found decreased hippocampal volume compared with healthy controls [27,29].The first VBM study to examine GM volumes in high-risk individuals found reductions in right hippocampal and parahippocampal areas. Our finding of GM volume reductions of right hippocampus in schizophreniform patients is in line with these past findings.
Temporolimbic structures including the hippocampus, parahippocampus and amygdala, are part of a network that integrates external and internal information [30]. Volume reduction in these brain regions has been reported in previous VBM investigations of schizophrenia [26,31,32]. Here, we did not find abnormalities of the amygdala. This discrepancy might be due to different imaging methods used here and in other studies [28]. Moreover, it could be related to different spatial normalization and image processing procedures [33,34]. Furthermore, previous studies either did not consider sex differences, or included a preponderance of patients. This is particularly relevant since divergent abnormalities of the amygdala have been shown in men and women with schizophrenia. Specifically, men show decreased volume while women show increased volume [35]. Investigations in patients with treatmentnaïve, first-episode schizophrenia have revealed significant GM volume abnormalities in bilateral hippocampal gyri, right middle temporal gyrus, left fusiform gyrus and left orbital inferior frontal gyrus, shown to be related to cognitive deficits [36]. In cortical regions, the superior temporal gyrus (STG) of schizophrenia patients is often reported to be smaller than that of HCs [37,38]. The superior temporal gyrus is involved in auditory processing as well as language functions and auditory memory; GM abnormalities of the superior temporal gyrus in patients with schizophrenia may be related to auditory and higher cognitive functioning deficits that often manifest in this disorder. However, we did not find a generalized reduction in STG volume, possibly because most schizophreniform patients included here were experiencing auditory hallucinations at illness onset. STG volume may have increased as an early compensatory effect.
The present study shows that GM abnormalities, including those in the middle temporal gyrus, parahippocampal gyrus, hippocampus, and precuneus, are more extensive in early schizophrenia. Activity in the middle temporal gyrus is related to semantic memory processing and language processes, which suggest that structural abnormalities in this region may play a role in the cognitive deficits in semantic function that have been found among Han Chinese family members with schizophrenia. And the above results consistent with a systematic meta-review reported that the GM reductions of temporal lobe and hippocampus were magnified over time in psychotic phases.
The precuneus is a brain area that occupies the posterior region of the medial parietal cortex, and is involved in numerous complex functions, including recollection and memory, integration of information relating to perception of the environment, cue reactivity, mental imagery strategies, episodic memory retrieval, and affective responses to pain [39]. Schizophrenia patients typically display cognitive deficits, which may be related to GM reduction in the precuneus. Our finding of extensive GM abnormalities in early schizophrenia patients is consistent with the results of previous studies comparing groups of first-episode and chronic schizophrenia patients [11,26,31,32,]. Our findings are also in agreement with a metaanalysis that found extensive GM deficits involving the frontal cortices, cingulated cortex, temporolimbic region and temporal gyrus in schizophrenia [32].
WM is usually assessed using the FA index of water diffusion, which is calculated from diffusion tensor imaging data [40]. Schizophrenia was one of the first psychiatric disorders in which FA methods were applied. Reduced FA at the onset and chronic states of schizophrenia has been reported in a number of studies [3,16,41]. However, the regions in which abnormalities have been detected are not consistent between them. Commonly reported regions of significant FA reduction in schizophrenia are the frontal and temporal WM tracks, including the uncinate fasciculus, cingulum bundle, arcuate fasciculus, corpus callosum that connect the bilateral frontal lobes. Our results showed no differences between schizophreniform patients and HCs. In patients with early schizophrenia, reduced FA was found in the cingulate gyrus, WM regions in occipital lobe, precuneus, WM regions in insula, and WM regions in the superior frontal gyrus, compared to HCs. Higher FA values were also found in a number of regions. The fusiform area, known as the (discontinuous) occipitotemporal gyrus, is part of the temporal lobe and occipital lobe in Brodmann area 37. [42]. Anatomically, the fusiform gyrus is the largest macro-anatomical structure within the ventral temporal cortex, and mainly includes structures involved in high-level vision [42,43]. Though the functionality of the fusiform gyrus is not fully understood, it has been linked with various neural pathways related to recognition, such as face hallucinations, dyslexia, and synaesthesia [44]. We found that FA values in WM regions of the fusiform are increased in schizophrenia compared to HCs, which may be related to the regularly experienced hallucinations of most patients in this study. We think that is because the compensatory increase in these regions in schizophrenia patients.
The neurodevelopmental and neurodegenerative theories of schizophrenia are two competing hypotheses on the etiology and clinical course of this disease [45]. The neurodevelopment model proposes that schizophrenia is caused by environmental or genetic insults that occur during prenatal, perinatal, or early childhood, leading to changes of brain structure and function in schizophrenia [46-48]. This model is supported by studies reporting significant changes in cerebral structures undergone prior to the onset of psychosis in individuals at high risk for developing schizophrenia [49,50]. However, it fails to address the heterogeneous and sometimes deteriorating clinical course of schizophrenia after illness onset. The role of progressive neurodegeneration in schizophrenia was first proposed by Kraepelin who observed mental decline in schizophrenia patients [51,52]. Patients with schizophrenia often follow a progressive, neurodegenerative clinical course, an observation that has been repeatedly been upheld [51]. However, studies focused on this model have yielded controversial results, with some researchers finding no changes in brain volume over time [53]. Neither of these two models can independently or accurately explain the etiology and clinical course of schizophrenia. Our findings can be taken to lend partial support to each of these two models, indicating that the schizophrenic brain begins to change at disease onset and progressively worsens throughout the course of the disease.
Several limitations of the present study should be taken into account. First, the patient sample size is relatively small. Second, groups were not matched for education level, and education level was therefore controlled for in the following analyses. Third, the data here is cross-sectional rather than longitudinal, and longitudinal researches are needed to further corroborate the present findings. Last, since many studies had mentioned that antipsychotics had an effect on the brain volumes, and the drugs ever taken by schizophrenia patients in the present study might be a potential confounding factor.
Conclusion
Abnormalities of brain GM and WM in schizophrenia seem to evolve as the stage of illness progresses, lending support to the neurodegenerative hypothesis of schizophrenia. Decreased volume in the right hippocampus may be a potential biomarker for early diagnosis of schizophrenia.
Funding
This work was supported by National Natural Science Foundation of China (81371479), Shanghai Science and Technology Committee Foundations (15411964400, 16ZR1430500), Shanghai Municipal Health and Family Planning Commission Foundations (20144Y0054, 2014ZYJB0002), Shanghai Municipal Hospital Appropriate Technology Program (SHDC12014214). It was also supported by Shanghai Key Laboratory of Psychotic Disorders (14K03), Early Psychosis Program of Shanghai Mental Health Center (2013-YJTSZK-05) and International Cooperation Foundation of Shanghai Mental Health Center (2015-YJGJ-02). These funding agents had no role in the study design, collection, analysis and interpretation of the data, writing of the manuscript, or decision to submit the paper for publication.
References
- Jung WH, Kim JS, Jang JH, et al. Cortical thickness reduction in individuals at ultra-high-risk for psychosis. Schizophr. Bull 37(1), 839-849 (2011).
- Bora E, Fornito A, Radua J, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr. Res 127(1), 46-57 (2011).
- Cookey J, Bernier D, Tibbo PG. White matter changes in early phase schizophrenia and cannabis use: an update and systematic review of diffusion tensor imaging studies. Schizophr. Res 156(1), 137-142 (2014).
- Wood SJ, Pantelis C, Velakoulis D, et al. Progressive changes in the development toward schizophrenia: studies in subjects at increased symptomatic risk. Schizophr. Bull 34(1), 322-329 (2008).
- Pantelis C, Yucel M, Bora E, et al. Neurobiological markers of illness onset in psychosis and schizophrenia: The search for a moving target. Neuropsychol. Rev 19(1), 385-98 (2009).
- Smieskova R, Fusar-Poli P, Allen P, et al. Neuroimaging predictors of transition to psychosis--a systematic review and meta-analysis. Neurosci. Biobehav. Rev 34(1), 1207-1222 (2010).
- Konrad A, Winterer G. Disturbed structural connectivity in schizophrenia primary factor in pathology or epiphenomenon? Schizophr. Bull 34(1), 72-92 (2008).
- Ian Cw, Psych MRC, Sophia RH, et al. Meta-Analysis of Regional Brain Volumes in Schizophrenia. Am. J. Psychiatry 157(1), 16–25 (2000).
- Ho BC, Andreasen NC, Ziebell S, et al. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry 68(1), 128-137 (2011).
- Ulysses ST, Stefan B, Geraldo FB. Structural brain changes associated with antipsychotic treatment in schizophrenia as revealed by voxel-based morphometric MRI: an activation likelihood estimation meta-analysis. BMC Psychiatry 13 (2013).
- Olabi B, Ellison-Wright I, Mcintosh AM, et al. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol. Psychiatry 70(1), 88-96 (2011).
- Ardekani BA, Nierenberg J, Hoptman MJ, et al. MRI study of white matter diffusion anisotropy in schizophrenia. Neuroreport 14(1), 2025-2029 (2003).
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomedicine 15(1), 435-455 (2002).
- Gulani V, Webb AG, Duncan ID, et al. Apparent diffusion tensor measurements in myelin-deficient rat spinal cords. Magn. Reson. Med 45(1), 191-195 (2001).
- Jiro OAF, Koushi HB, Masaya TC, et al. Differentiation between dysmyelination and demyelination using magnetic resonance diffusional anisotropy. Brain Res 671(1), 141-148 (1995).
- Kubicki M, Park H, Westin CF, et al. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage 26(1), 1109-1118 (2005).
- Hugenschmidt CE, Peiffer AM, Kraft RA, et al. Relating Imaging Indices of White Matter Integrity and Volume in Healthy Older Adults. Cereb. Cortex 18(1), 433-442 (2007).
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59(20), 22-33 (1998).
- Andreasen Nc. The Scale for the Assessment of Negative Symptoms(SANS). lowa City:University of lowa (1984).
- Guy W, Bonato RR. CGI: Clinical Global Impressions: Manual for the EDCEU Assessment Battery. Maryland: National Institute of Mental Health (1976).
- Mazziotta JC, Evans A, Fox P, et al. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM). Neuroimage 2(1), 89-101 (1995).
- Garcia-Marti G, Aguilar EJ, Lull JJ, et al. Schizophrenia with auditory hallucinations: a voxel-based morphometry study. Prog. Neuropsychopharmacol Biol. Psychiatry 32(1), 72-80 (2008).
- Honea RA, Meyer-Lindenberg A, Hobbs KB, et al. Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol. Psychiatry 63(1), 465-474 (2008).
- Meda SA, Giuliani NR, Calhoun VD, et al. A large scale (N=400) investigation of gray matter differences in schizophrenia using optimized voxel-based morphometry. Schizophr. Res 101(1), 95-105 (2008).
- Radulescu E, Ganeshan B, Shergill SS, et al. Grey-matter texture abnormalities and reduced hippocampal volume are distinguishing features of schizophrenia. Psychiatry Res 223(1), 179-186 (2014).
- Ellison-Wright I, Glahn, DC, Laird AR, et al. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am. J. Psychiatry 165(1), 1015-1023 (2008).
- Ebdrup B. Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J. Psychiatry Neurosci 35(1), 95-104 (2010).
- Vita A, De PL, Silenzi C. Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies. Schizophr. Res 82(1), 75-88 (2006).
- Phillips LJ, VelakouliS D, Pantelis C, et al. Non-reduction in hippocampal volume is associated with higher risk of psychosis. Schizophr. Res 58(1), 145–158 (2002).
- White T, Cullen K, Rohrer LM, et al. Limbic structures and networks in children and adolescents with schizophrenia. Schizophr. Bull 34(1), 18-29 (2008).
- Robyn Honea, Dick P, Clare EM. Regional Deficits in Brain Volume in Schizophrenia : A Meta-Analysis of Voxel-Based Morphometry Studies. Am. J. Psychiatry 162(1), 2233-2245 (2005).
- Chan RC, Di X, Mcalonan GM, et al. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr. Bull 37(1), 177-188 (2011).
- Shen S, Sterr A. Is DARTEL-based voxel-based morphometry affected by width of smoothing kernel and group size? A study using simulated atrophy. J. Magn. Reson. Imaging 37(1), 1468-1475 (2013).
- Radua J, Canales-Rodriguez EJ, Pomarol-Clotet E, et al. Validity of modulation and optimal settings for advanced voxel-based morphometry. Neuroimage 86(1), 81-90 (2014).
- Raquel EG, Bruce IT, Patricia EC, et al. Temporolimbic Volume Reductions in Schizophrenia. Arch Gen. Psychiatry 57(1), 769-775 (2000).
- Guo X, Li J, Wang J, et al. Hippocampal and orbital inferior frontal gray matter volume abnormalities and cognitive deficit in treatment-naive, first-episode patients with schizophrenia. Schizophr. Res 152(1), 339-343 (2014).
- Narayanaswamy JC, Kalmady SV, Venkatasubramanian G, et al. Clinical correlates of superior temporal gyrus volume abnormalities in antipsychotic-naive schizophrenia. J. Neuropsychiatry Clin. Neurosci 27, e128-33 (2015).
- Tang J, Liao Y, Zhou B, et al. Decrease in temporal gyrus gray matter volume in first-episode, early onset schizophrenia: an MRI study. PLoS One 7(1), e40247 (2012).
- Borsook D, Maleki N, Burstein R. Chapter 42 - Migraine A2 - Zigmond, Michael J. In: ROWLAND, L. P. & COYLE, J. T. (eds.) Neurobiology of Brain Disorders. San Diego: Academic Press (2015).
- Lee SH, Kubicki M, Asami T, et al. Extensive white matter abnormalities in patients with first-episode schizophrenia: a Diffusion Tensor Iimaging (DTI) study. Schizophr. Res 143(1), 231-238 (2013).
- Rigucci S, Santi G, Corigliano V, et al. White matter microstructure in ultra-high risk and first episode schizophrenia: A prospective study. Psychiatry Res 247(1), 42-48 (2016).
- Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci 7(1), 555-562 (2004).
- Grill-Spector K, Weiner KS. The functional architecture of the ventral temporal cortex and its role in categorization. Nat. Rev. Neurosci 15(1), 536-548 (2014).
- Weiner KS, Zilles K. The anatomical and functional specialization of the fusiform gyrus. Neuropsychologia 83(1), 48-62 (2016).
- Kochunov P, Hong LE. Neurodevelopmental and neurodegenerative models of schizophrenia: white matter at the center stage. Schizophr. Bull 40(1), 721-728 (2014).
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci, 25, 409-432.
- Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol. Psychiatry 17(1), 1228-1238 (2012).
- Rapoport JL, Addington AM, Frangou S, et al. The neurodevelopmental model of schizophrenia: update 2005. Mol. Psychiatry 10(1), 434-449 (2005b).
- Sun D, Phillips L, Velakoulis D, et al. Progressive brain structural changes mapped as psychosis develops in 'at risk' individuals. Schizophr. Res 108(1), 85-92 (2009).
- Rapoport JL, Addington A, Frangou S. The neurodevelopmental model of schizophrenia: what can very early onset cases tell us? Curr. Psychiatry Rep 7(1), 81-82 (2005a).
- Anderson JE, O'donnell BF, Mccarley RW, et al. Progressive changes in schizophrenia: do they exist and what do they mean? Restor. Neurol. Neurosci 12(1), 175-184 (1998).
- Knoll JLT, Garver DL, Ramberg, JE, et al. Heterogeneity of the psychoses: is there a neurodegenerative psychosis? Schizophr. Bull 24(1), 365-379 (1998).
- Whitworth AB, Kemmler G, Honeder M, et al. Longitudinal volumetric MRI study in first- and multiple-episode male schizophrenia patients. Psychiatry Res 140(1), 225-237 (2005).