Research Article - (2019) Volume 9, Issue 2
Post-Stroke Delirium Course in Prospective Observational Polish Study (PROPOLIS)
- *Corresponding Author:
- Aleksandra Klimkowicz-Mrowiec
Department of Neurology Jagiellonian University, School of Medicine 31-503 Krakow, Botaniczna 3, Poland
Tel: +48 12 424 86 00
Fax: +48 12 424 86 26
Abstract
Delirium is a very common neurobehavioral complication after a stroke. Several studies have investigated this issue with conflicting results; therefore the course, severity and fluctuations of cognitive and motor features still need to be elucidated on a large group of patients with delirium post-stroke. As such, the aim of this prospective study was to assess the natural history of post-stroke delirium in a large cohort of patients with stroke. Consecutive stroke patients were screened for delirium using the Confusion Assessment Method. The delirium was then diagnosed according to DSM-5 criteria. Subtypes of delirium were classified with Delirium Motor Subtype Scale-4, and the Delirium Motor Checklist was applied to assess the number of hyper- and hypoactive symptoms. Finally, the severity of delirium symptoms was assessed by Delirium Rating Scale-Revised-98 and Cognitive Test for Delirium. Out of 203 patients, 144 were diagnosed with delirium on the first day of their hospital stay. Delirium resolved in 42 patients within 3 days of hospitalization, with median delirium duration of 3 days. The seventy seven patients were still delirious on the seventh day of their hospitalization. With time, the tendency towards an increase in hypoactive features was observed in both mixed and hypoactive delirium subtypes. The severity of delirium symptoms was stable during individual episodes. Delirium usually develops at the beginning of hospitalization. The number of delirium cases declines gradually but many patients are still delirious at the time of discharge. The severity of delirium symptoms is generally stable, but there is a tendency for an increase in the number of hypoactive signs and a decrease in the number of hyperactive signs during an episode.
Keywords
Post-stroke delirium, Delirium subtype, Delirium severity, Motor activity, Delirium course
Introduction
Delirium is a very common neurobehavioral complication in acute hospital admissions of the elderly [1]. It is characterized by disturbances in attention and awareness, changes in cognition that develop over a short period of time, and by a fluctuating course [2]. It is also a frequent complication of stroke, with the incidence estimated to be between 10 to 48% [3].
Delirium is associated with a poor outcome in terms of short- and long-term prognoses [4]. Therefore knowledge of its risk factors, frequency, and clinical presentation is a very important aspect of in-hospital care of patients with stroke. Data on the natural course of poststroke delirium is scarce. Clinical observation suggests that the presentation of delirium varies among patients, but there is limited data on the longitudinal observation of motor and cognitive profiles. It is therefore of great clinical relevance to further knowledge in the best time to screen for delirium in order to not only identify cases and indications for evaluating patients at discharge, but also to raise awareness of its clinical cognitive and motor manifestations. This information should prove useful for clinical practice and for better treatment of patients, especially in busy stroke units.
For this reason the aim of PRospective Observational POLIsh Study on post-stroke delirium (PROPOLIS) was to assess the natural history of delirium; that is, the prevalence, duration and severity of motor and cognitive fluctuations in the Polish stroke population within 7 days of hospital stay.
Material and Methods
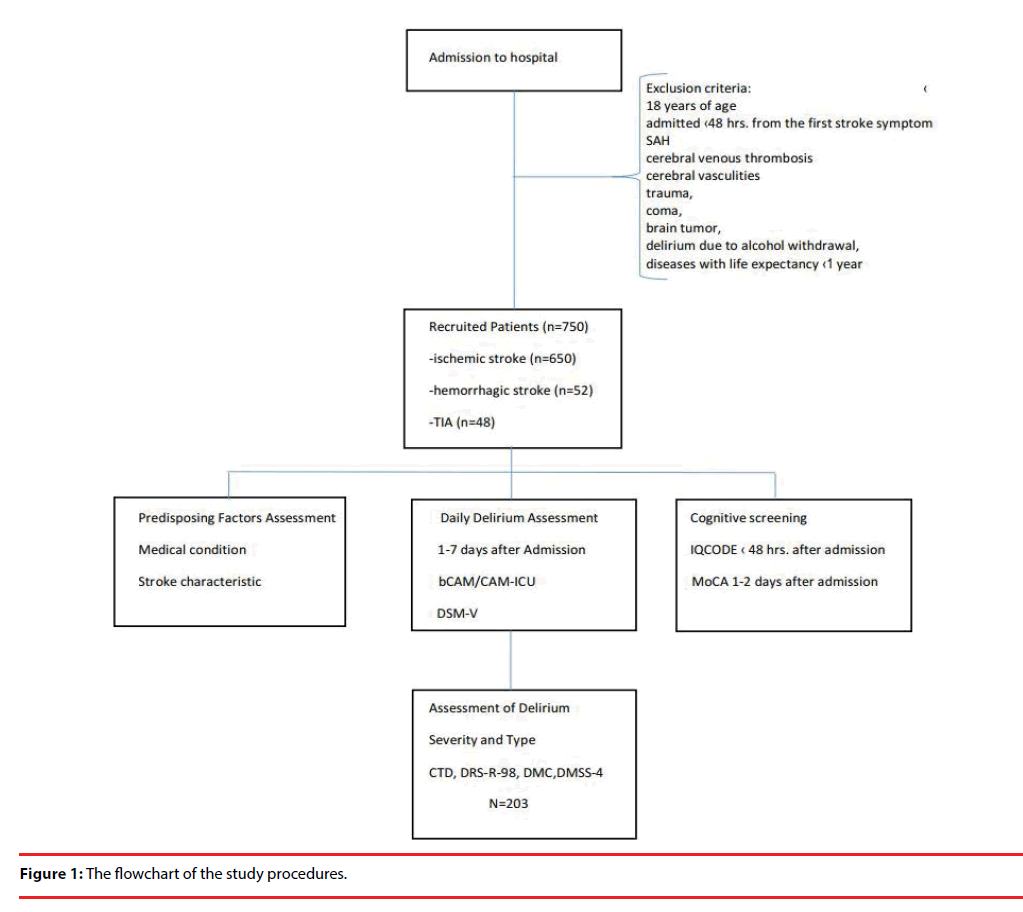
750 consecutive patients with stroke (ischemic/ hemorrhagic) or transient ischemic attack that were admitted to the Stroke Unit at the University Hospital in Krakow and met the inclusion criteria for this study were investigated for the presence of delirium. Stroke was defined as a sudden onset of neurological deficit lasting longer than 24 hours. All patients were treated according to the standard protocols of international guidelines [5]. Inclusion and exclusion criteria for this study and study design have been previously described in detail elsewhere [6]. Briefly, the patients that were excluded from the study were those under 18 years of age, admitted over 48 hours from the first stroke symptoms, with subarachnoid hemorrhage, cerebral venous thrombosis, cerebral vasculities, trauma, coma, brain tumor, delirium due to alcohol withdrawal, and/ or diseases with life expectancy shorter than one year. At the time of hospital admission, all patients had neuroimaging (CT/MRI). The flowchart of the study is shown in Figure 1.
All patients were screened for delirium every day at the same time (3-6 p.m.). First assessments were performed within 24 hours after admission, and the last assessments were held on the 7th day of hospitalization. An abbreviated version of the Confusion Assessment Method (bCAM) was used for delirium screening, whereas the Intensive Care Units version (CAM-ICU) was used for those with speech output problems [7,8]. bCAM has been validated in emergency units and demonstrated sensitivity of 84% and specificity of 95.8% [7]. CAM-ICU was adapted for nonverbal patients and can be used in stroke patients with severe motor aphasia [8]. This method has been validated in the acute phase of stroke and demonstrated sensitivity of 76%, specificity of 96%, and an overall accuracy of 94% [9]. Diagnosis of delirium was concluded by clinical observation and structural assessment. Delirium was diagnosed according to the DSM-5 criteria [10]. For those patients who were not able to undergo cognitive evaluation, the diagnosis was based on clinical observation and DSM-5 criteria for delirium. Patients diagnosed with delirium had further evaluation. Delirium Motor Subtype Scale 4 was used to classify delirium motor subtype presentation: hyperactive, hypoactive, mixed or no-motor subtype [11,12]. Severity of delirium symptoms was assessed by Delirium Rating Scale Revised 98 (DRS-R–98)[13] and Cognitive Test for Delirium (CTD)[2]. Both scales were constructed to measure the severity of delirium and although the correlation between the two scales was found to be strong (r=-0.63, P0.001 for DRS-R-98 severity) [13], there are some noticeable differences between them. The DRS-R-98 measures symptoms more broadly than CTD, which assesses cognition. The CTD rates cognitive symptoms at the time of administration, while DRS-R–98 rates the cognition and behavior in the preceding hours. In this study, we chose a 24-hour time frame for DRS-R-98 severity scale assessment. The Delirium Motor Checklist (DMC) [14] was used to assess the number of hyper- and hypoactive symptoms. To control for possible delirious symptoms during all 24 hours, ward nurses completed a short questionnaire regarding patients’ behavior and cognitive fluctuations for each patient.
To screen for prestroke dementia, a Polish version of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) was used [15]. Auditory and visual impairments, stroke-related factors, laboratory tests results, pneumonia, and urinary tract infections during hospitalization were recorded. For cognitive assessment, the Montreal Cognitive Assessment (MoCA) [16], and Cognitive Test for Delirium were used between the first and second day of admission, and again on the seventh day after admission.
A resident neurologist specially trained in delirium diagnosis was responsible for screening for delirium, and a trained psychologist was responsible for cognitive assessment. The senior neurologist/ neuropsychologist was responsible for evaluating all data. The physicians rating the patients did not change during the study.
This study was approved by the medical ethics committee at the Jagiellonian University and all methods were performed in accordance with the relevant guidelines and regulations. Informed consent was given by the patient after the procedures were fully explained. If a patient was unable to fully understand the procedures, their caregiver was asked for informed assent; then, when the patient’s condition improved, he or she was asked to provide informed consent.
▪ Statistics
All of the statistical analyses were calculated using STATISTICA for Windows version 12 (StatSoft Inc., Tulsa, Oklahoma). First, descriptive statistics were performed to obtain information about delirium – number of cases, distribution, histograms of the beginning and the end of delirium, and delirium duration. Then, the medians of CTD and DRS-R-98 and the scores for DMC hyper- and hypoactive signs for each delirium day were calculated and relevant graphs were prepared. ANOVA Friedman test for each subtype of delirium was used to examine delirium variability by the differences of DRS-R-98 scores between delirious days. The value of alpha=0.05 was considered as a threshold for statistical significance. Casewise deletion was used to handle missing data.
Results
Of the 750 patients (mean age of 71.75 ± 13.13 years) that were included in this study (398 women, mean age 74.72 ± 13.20; 352 men, mean age 68.40 ± 12.23), 650 patients had ischemic stroke, 52 hemorrhagic stroke and 48 had TIA. The NIHSS for the whole cohort was 8.52 ± 7.31 (8.85 ± 7.23; 11.15 ± 7.35; and 1.17 ± 2.19 for patients with ischemic stroke, hemorrhagic stroke and TIA, respectively).
203 patients (27.07%) had delirium [119 women (29.90%) and 84 men (23.86%)]. Hyperactive delirium was identified in 31 (15.27%), hypoactive in 85 (41.87%), mixed type in 77 (39.93%), and unspecified in 10 (4.93%) of the included patients. Delirious patients had significantly worse IQCODE score than patients without delirium (3.34 ± 0.60 vs. 3.15 ± 0.35 respectively OR (95% CI 2.46 (1.66-3.65), p <0.001). Out of the 203 patients with delirium, 183 patients had a consecutive course of delirium, while in 20 patients delirium resolved for at least 24 hours and then reemerged again. Thirty-nine patients with delirium died during the observation period.
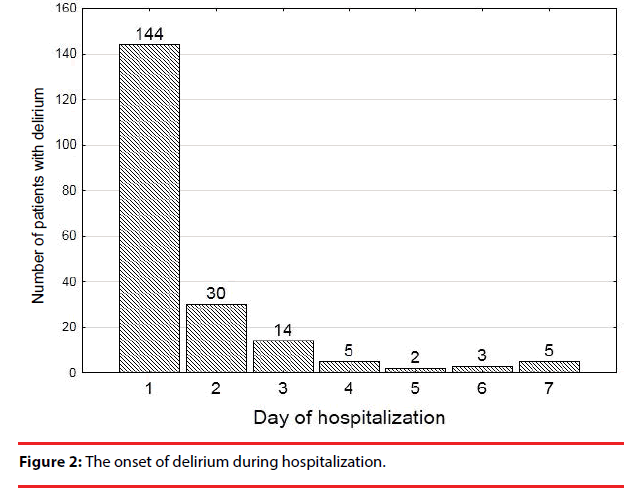
Delirium began within the first 24 hours of hospital stay in 71% of patients (Figure 2).
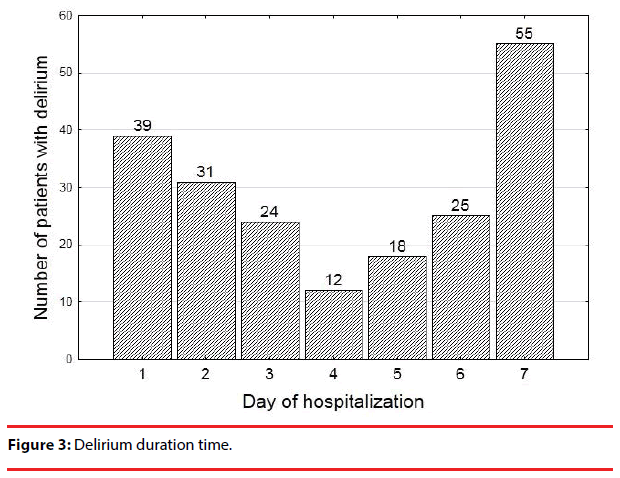
The median time of delirium episodes was 3 days. Most cases of delirium resolved within the first 3 days, but almost 40% of patients were still delirious at the end of the observation period (Figure 3, Table 1).
| Day of hospitalization | Delirium N=203 |
|
|---|---|---|
| The onset | The end | |
| 1 | 144 | 20 |
| 2 | 30 | 20 |
| 3 | 14 | 31 |
| 4 | 5 | 12 |
| 5 | 2 | 13 |
| 6 | 3 | 11 |
| 7 | 5 | |
Table 1: Delirium in post-stroke patients.
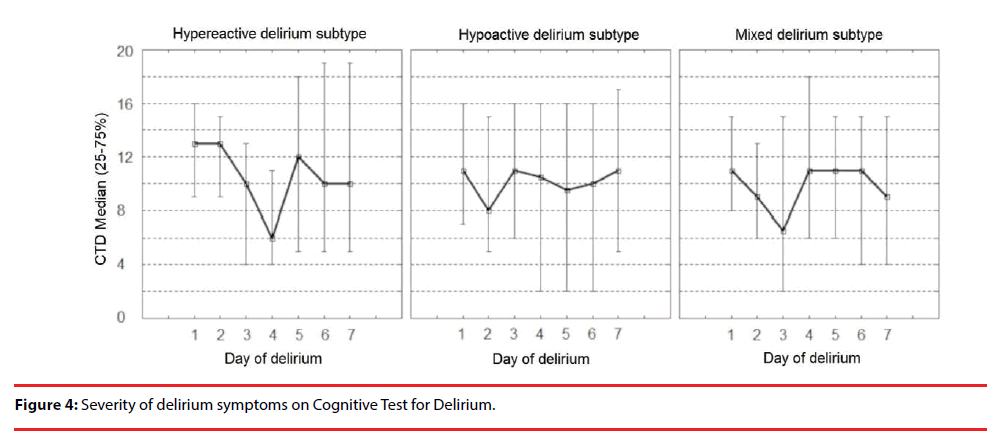
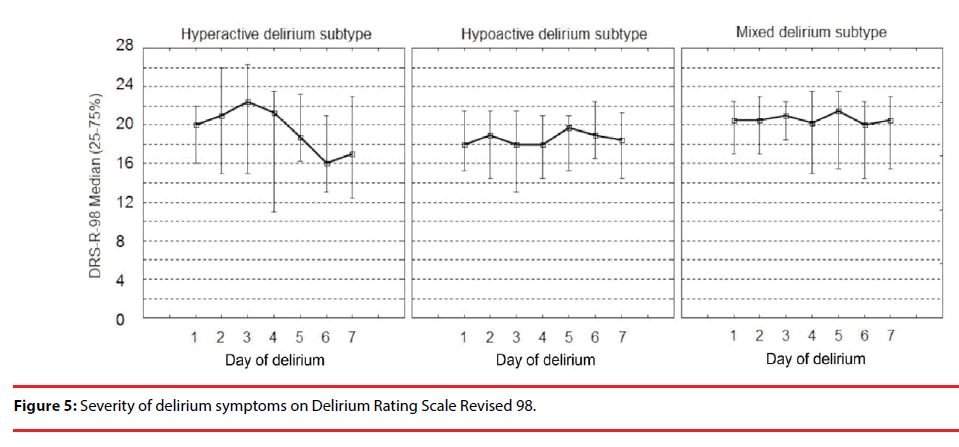
The severity of symptoms in different delirium sub-types on CTD and DRS-R-98 are shown in Figure 4 and Figure 5, respectively.
The severity of signs for all delirium sub-types was significantly stable on DRS-R-98 during the episode, except for the hypoactive delirium of five days duration time (the Friedman ANOVA p=0.023). The severity of symptoms on CTD differed significantly during consecutive days of delirium.
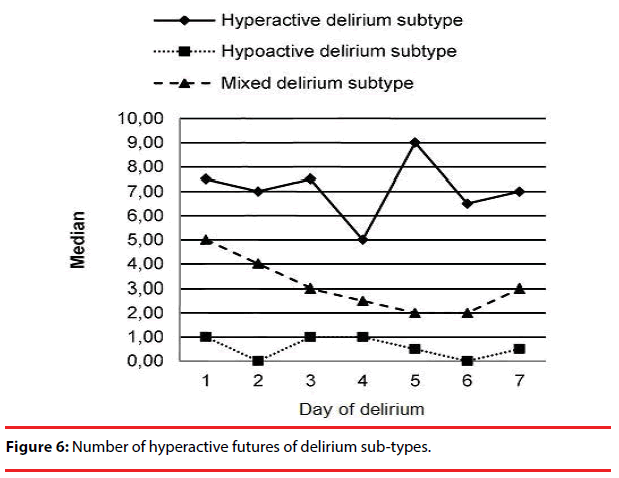
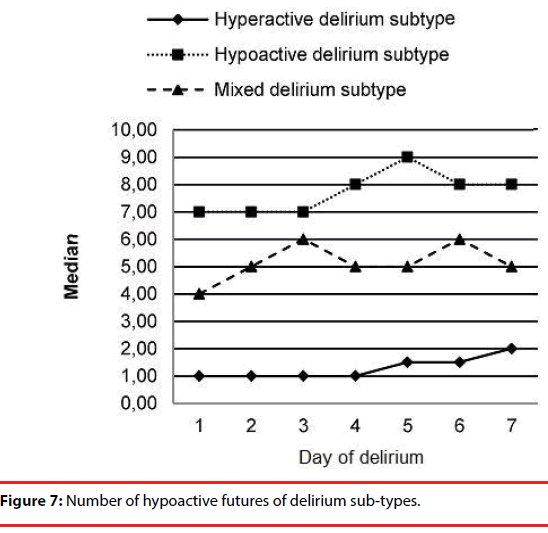
The number of hyper and hypoactive features in the 3 delirium sub-types are shown in Figure 6 and Figure 7, respectively. We observed an increase in the number of hypoactive features and a decrease in the number of hyperactive features with time.
Discussion
Stroke is a syndrome that often causes cognitive impairment and psychiatric disturbances. One of these is delirium, a heterogeneous condition. Diagnosis of delirium in the stroke population is difficult and time-consuming because one has to differentiate post-stroke neurocognitive and behavioral complications with delirium. To distinguish between these conditions, information was gathered about cognitive and behavioral functioning pre-stroke and evaluation of cognitive functioning and neurological deficit was performed after admission in addition to screening for delirium. Apart from systematic assessment, all included patients had longitudinal observation by medical personnel over 24 hours.
We identified most delirium cases within the first 24 hours of hospitalization. Delirium was then observed during the following days in most patients, and in only 20 patients the delirium resolved for at least 24 hours before reemerging again. The mean duration of delirium was 3 days.
There is no study addressing the severity of delirium symptoms during an episode in the post-stroke population. In this study, both CTD and DRS-98-R severity scales were used to assess delirium severity. The symptoms of delirium severity, when measured with DRS-R-98 scale, were stable during an episode (only in the hypoactive delirium sub-type did the severity of delirium of 5 days duration differ significantly from other sub-types of delirium. The cognitive symptoms showed more disparity when measured by the CTD scale. The DRS-R-98 score was based on a 24-hour observation period complete with both cognitive and behavioral assessments instead of relying solely on direct cognitive evaluation as this value may fluctuate greatly during an episode. Although both scales highly correlate, they do not measure the same aspects of delirium severity.
The number of hypo- and hyperactive items on DMC significantly differed among the various delirium subtypes. With time, the number of hypoactive features increased significantly in mixed and hypoactive delirium subtypes. In the hyperactive subtype, there was a decrease in hyperactive features while hypoactive features remained stable during the course of the delirium.
Various other studies also noted that in most cases delirium is diagnosed in the first days of hospital stay. McManus et al. [17] followed 82 patients with stroke and found delirium in 23 (28%) of them, 21 of which were diagnosed within the first 4 days of hospital stay. Mitasowa et al. [9] found delirium in 55 (42.6%) patients out of 129; in 37 (67.3%) within the first day of hospital-stay and in 100% within 5 days of stroke onset. Median duration of delirium was 4 days and it lasted less than 24 hours in 25.5 % of delirium-positive cases. Oldenbeuving et al. [18] screened for delirium in stroke patients between 2-4 and 5-7 day after admission to the hospital. Out of 527 patients, 52 (9.7%) were diagnosed with delirium at the first screening, and an additional 10 patients (1.9%) were diagnosed during the second screening. 33 (64%) patients out of the 52 with delirium diagnosed during the first screening did not have delirium at the second screening. The mean duration of delirium was 4.8 days.
The main strength of this study is investigation of different aspects of delirium post-stroke. A very large number of consecutive patients with stroke were examined and a very careful and repeated cognitive and neuropsychiatric assessment was performed. Diagnosis of delirium may sometimes be a challenge, especially in stroke patients, because of prevalent language disorders, visuo-spatial dysfunctions, mood disturbances, and cognitive impairment that can be mixed up with delirium and may make proper assessment impossible. Only systematic assessment and longitudinal observation by medical personnel can give reliable answers to questions regarding disturbance of a patient’s awareness. In this study, structural assessment was conducted every day and the final diagnosis included observation charts provided by medical personnel for each stroke patient to make it as complete as possible.
For delirium screening, we used bCAM for verbal patients or CAM-ICU for patients who could not speak but were able to communicate in a non-verbal way. Both methods have a high sensitivity and specificity. The same assessor administered the scale from day 1 to day 7, thus making the bias of interobserver variation minimal [7,8].
This study included patients of a wide age range. Enrollment of younger patients might decrease the percentage of cases with post-stroke delirium. However, the mean age of our cohort was high, similar to other studies; therefore it is unlikely that including younger patients caused a bias.
The incidence of post-stroke delirium in our sample might be underestimated due to the restricted 7-day observation period. This is the average duration of a hospital-stay in Krakow’s stroke unit, therefore this time was chosen as the observational period for everybody. Not all cases of delirium recovered during this period, therefore we can only speculate that the trend of a decrease in delirium incidence and prevalence would continue to decline.
Our finding that the number of hypoactive features increases with time may have practical application as hypoactive signs are usually more difficult to notice and can be easily overlooked when one does not look for them actively. Moreover, when the number of hyperactive features decreases and the number of hypoactive features increases, a patient’s condition can be interpreted incorrectly; one can have the impression that the symptoms have resolved while the patient is actually presenting with another aspect of the very same disorder. Delirium has a broad spectrum of presentations, all of which are clinically important.
Another practical observation is that the best time to screen for delirium is at the beginning of hospitalization. Clinicians should also be aware that at the end of a hospital-stay there is still a large number of patients that may be overlooked and discharged with delirium.
Conclusions
Delirium has a fluctuating presentation and therefore only frequent and repetitive screening may show the real prevalence of delirium.
Most delirium cases develop in the beginning of the hospital stay, however a lot of patients are still delirious at the time of hospital dismissal.
The number of hypoactive signs of delirium increases with time, therefore one should pay close attention in order to prevent discharge of unrecognized patients with delirium from the hospital.
Sources of funding
The Leading National Research Centre of Medical Faculty of Jagiellonian University funded the collection of data for the study.
Acknowledgments
We thank Justyna Kacarow and Malgorzata Mazurek for editing assistance, Elzbieta Klimiec for data acquisition and Tomasz Dziedzic for co-supervision of the research group.
Conflict of Interest
The authors declare no conflict of interest.
References
- Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: A systematic literature review. Age. Ageing 35 (‑4), 350-364 (2006).
- Hart RP, Levenson JL, Sessler CN, et al. Validation of a cognitive test for delirium in medical ICU patients. Psychosomatics 37(6), 533-546 (1996).
- McManus J, Pathansali R, Stewart R, et al. Delirium post-stroke. Age. Ageing 36(6), 613-618 (2007).
- Oldenbeuving AW, deKort PLM, Jansen BPW, et al. Delirium in acute stroke: a review. Int. J. Stroke 2(4), 270-275 (2007).
- Intercollegiate Stroke Working Party. National clinical guideline for stroke:4th edtn. London: Royal College of Physicians (2012).
- Pasinska P, Kowalska K, Klimiec E, et al. A. Frequency and predictors of post-stroke delirium in PRospective Observational POLIsh Study (PROPOLIS). J. Neurol 265(4), 863-870 (2018).
- Han JH, Wilson A, Vasilevskis EE, et al. Diagnosing delirium in older emergency department patients: validity and reliability of the delirium triage screen and the brief confusion assessment method. Ann. Emerg. Med 62(5), 457-465 (2013),
- Ely EWE, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 286(21), 2703-2710 (2001).
- Mitasova A, Kostalova M, Bednarik J, et al. Poststroke delirium incidence and outcomes: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit. Care. Med 40(2), 484-490 (2012).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: 5th edtn. Arlington, VA: American Psychiatric Association (2013).
- Boettger S, Nuñez DG, Meyer R, et al. Brief assessment of delirium subtypes: Psychometric evaluation of the Delirium Motor Subtype Scale (DMSS)-4 in the intensive care setting. Palliat. Support. Care 15(5), 535-543 (2017).
- Meagher D, Adamis D, Leonard M, et al. Development of an abbreviated version of the delirium motor subtyping scale (DMSS-4). Int. Psychogeriatr 26(4), 693-702 (2014).
- Trzepacz PT, Mittal D, Torres R, et al. Validation of the delirium rating scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J. Neuropsych. Clin. Neurosci 13(2), 229-242 (2001).
- Meagher DJ, Moran M, Raju B, et al. Motor symptoms in 100 patients with delirium versus control subjects: comparison of subtyping methods. Psychosomatics 49(4), 300-308 (2008).
- Klimkowicz A, Dziedzic T, Slowik A, et al. Incidence of pre- and poststroke dementia: Cracowe Stroke Registry. Dement. Geriatr. Cogn. Disord 14(3), 137-140 (2002).
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc 53(4), 695-699 (2005).
- McManus J, Pathansali R, Hassan H, et al. The course of delirium in acute stroke. Age. Ageing 38(4), 385-389 (2009).
- Oldenbeuving AW, de Kort PL, Jansen BP, et al. Delirium in the acute phase after stroke: incidence, risk factors, and outcome. Neurology 76(11), 993-999 (2011).