Research Article - Neuropsychiatry (2017) Volume 7, Issue 6
Plasma Pregnancy-Associated Plasma Protein A and CD40 are Potential Biomarkers of Vulnerable Plaque Associated with Middle Cerebral Artery Stenosis
- Corresponding Authors:
- Dr. Yingqi Xing
Department of Neurology
The First Hospital of Jilin University
Xinmin Street 71#, 130021, Changchun, China
Tel: +86 431 88782378
Fax: +86 431 88782378
- Prof. Kangding Liu
Department of Neurology
The First Hospital of Jilin University
Xinmin Street 71#, 130021, Changchun, China
Abstract
Intracranial artery stenosis, especially middle cerebral artery stenosis (MCAS), is prevalent in the Chinese population. Currently, few approaches target evaluating MCAS-associated plaque vulnerability. Herein we aimed to explore potential plasma biomarkers for vulnerable plaque in patients with MCAS. A total of 81 subjects who received microembolic signal (MES) detection were enrolled between September 2012 and September 2014. Of them, 19 patients did not have either acute ischemic infraction or cranial artery stenosis, 19 were patients with acute ischemic infarction whereas without any cranial artery stenosis, and the remaining 43 were patients with acute ischemic infraction caused by MCAS. Acute ischemic infraction (mean volume, 3.8 ± 2.81 cm3) was demonstrated by diffusion-weighted imaging in 30 patients.The acute infarction volume didn’t correlated with plasma concentrations of pregnancy associated plasma protein A (PAPP-A), insulin-like growth factor 1, inducible nitric oxidesynthase, lipoprotein-associated phospholipase A2, CD40, and CD40L (P > 0.05). The plasma PAPP-A (odds ratio [OR], 0.983; P = 0.024; 95% confidence interval [CI], 0.968-0.998), CD40 (OR,1.063; P = 0.015; 95% CI, 1.012–1.117), and CD40L (OR, 0.867; P = 0.015; 95% CI, 0.773–0.972) concentrations severed as predictors for moderate to severe MCAS by multivariate logistic regression analysis. In addition, plasma PAPP-A (OR, 0.982; P = 0.016; 95% CI, 0.967–0.997) and CD40 (OR, 1.040; P = 0.025; 95% CI, 1.005–1.007) concentrations were also identified to be predictive for MES. In summary, plasma PAPP-A and CD40 might be potential biomarkers for
vulnerable plaque associated with MCAS.
Keywords
Microembolic signal, Middle cerebral artery, Pregnancy-associated plasma protein A, CD40
Introduction
Stroke, one of the primary causes of morbidity and mortality worldwide, is a heterogeneous diseases caused by a variety of pathophysiological mechanisms. Cranial artery stenosis is an important risk factor for ischemic stroke. There are marked differences in the distribution of arterial lesions among different populations [1,2]. For example, intracranial artery stenosis is the predominant vascular lesion found in patients of Asian, African, or Hispanic ancestry; while extracranial artery stenosis is prevalent in white individuals. Artery-to-artery embolism due to the vulnerable plaque is one of the predominant mechanisms of ischemic infarction in patients with cranial artery stenosis. The major criteria for vulnerable plaque include active inflammation, thin cap with large lipid core, endothelial denudation with superficial platelet aggregation, fissured plaque, stenosis > 90% [3]. Since patients with vulnerable plaque are at high risk of recurrent events of stroke and usually have poor outcomes [4], the evaluation of plaque vulnerability in patients with extracranial/intracranial artery stenosis is of great importance. Different approaches targeting different plaque compositions in patients with carotid artery stenosis, such as carotid ultrasound [5-7], contrast-enhanced ultrasonography [8-11], high-resolution magnetic resonance imaging (HR-MRI) [12-16], and 18F-fluorodexyglucose positron emission tomography [17-19] are able to assess plaque vulnerability. However, the characterization of vulnerable plaques associated with the intracranial artery remains incompletely clear and there are limited approaches to their evaluation. HR-MRI can illustrate intraplaque hemorrhage and compensatory middle cerebral artery stenosis (MCAS) remodeling, both of which might be associated with vulnerable plaque [20-21]. In addition, the microembolic signal (MES) detected by transcranial Doppler sonography (TCD) is another sensitive and real-time approach in detecting vulnerable plaques [22], which is frequently involved in patients with acute ischemic infraction caused by cranial artery stenosis. We previously found that MES frequency differed between patients with symptomatic and asymptomatic patients and it was significantly higher in symptomatic patients with moderate to severe MCAS [23]. It is of note that both HR-MRI and MES detection have their limitations. Thus, searching for a simple and feasible method for screening vulnerable plaques of MCAS is urgently needed, especially for the Chinese population. Many plasma molecules, such as pregnancy-associated plasma protein A (PAPP-A), insulin-like growth factor 1 (IGF- 1), inducible nitric oxide synthase (iNOS), lipoprotein-associated phospholipase A2 (Lp- PLA2) have been found increased in patients with acute coronary syndrome (ACS) or vulnerable carotid plaques. However, whether those molecules could sever as potential biomarkers for vulnerable plaques of MCAS is unknown. In this study, MES was considered to be a hallmark of vulnerable plaque of MCAS, and we explored the potential plasma biomarkers for MES.
Subjects and Methods
▪ Subjects
This study was approved by the ethics committee of The First Hospital of Jilin University. The experiment was conducted with the understanding and written informed consent was obtained from all patients. A total of 81 subjects who received MES detection were enrolled between September 2012 and September 2014. Of them, 19 patients did not have acute ischemic infraction or cranial artery stenosis, 19 were patients with acute ischemic infarction whereas without any cranial artery stenosis, and the remaining 43 were patients with acute ischemic infraction caused by MCAS. The acute ischemic infraction of the enrolled patients was in the MCA territory that occurred within 1 week. Of note was that patients with MCAS did not have any other intra- or extracranial artery stenosis. Patients were excluded if they met one of the following criteria: (1) presence of a poor temporal acoustic window; (2) could not consent to participate in the study (e.g. in an agitated or confused state); (3) had other potential sources of embolism, such as cardiogenic emboli and bloodborne emboli as well as any other artery borne embolic source; or (4) had an inflammatory or infectious disease or an autoimmune disorder. All of the enrolled patients with acute ischemic infraction did not receive heparin treatment or thrombolytic therapy.
All of the recruited patients underwent examinations including TCD, carotid duplex, and MES detection. The ischemic infraction was demonstrated by cranial MRI or computed tomography. The volume of the acute ischemic infraction of each diffusion-weighted imaging (DWI) lesion was calculated by two independent neuroradiologists who were blinded to the clinical data using Image J. All axial DWI lesion volumes were computed by multiplying the area of each slice by the section thickness. At baseline, demographic data including sex, age, and vascular risk factors including previous history of hypertension, diabetes mellitus (DM), ischemic heart disease, and smoking history were recorded for all patients. Hypertension, DM, and ischemic heart disease were diagnosed by cardiologists and diabetologists.
▪ Diagnostic criteria of MCAS
The intracranial arteries were assessed by TCD (EME-TC8080; Nicolet, Germany) and the extracranial arteries were assessed by carotid duplex (iU22; Philips, USA). Through the temporal window, the MCA-Ml, anterior cerebral artery (ACA)-Al, posterior cerebral artery (PCA), and intracranial segment of the internal carotid artery (mainly the siphon and terminal segments) were detected by TCD using a 2 MHz probe; through the pillow window, the intracranial segment of the vertebral artery and the basilar artery were detected. The ophthalmic artery and the siphon segment of internal carotid artery were detected through the eye window. The criteria for the diagnosis of MCAS by TCD were based on the previously published criteria as follows [24-26]: mild stenosis was defined as a systolic peak velocity of 140–209 cm/s, moderate stenosis was 210–280 cm/s, and severe stenosis was >280 cm/s.
▪ MES detection
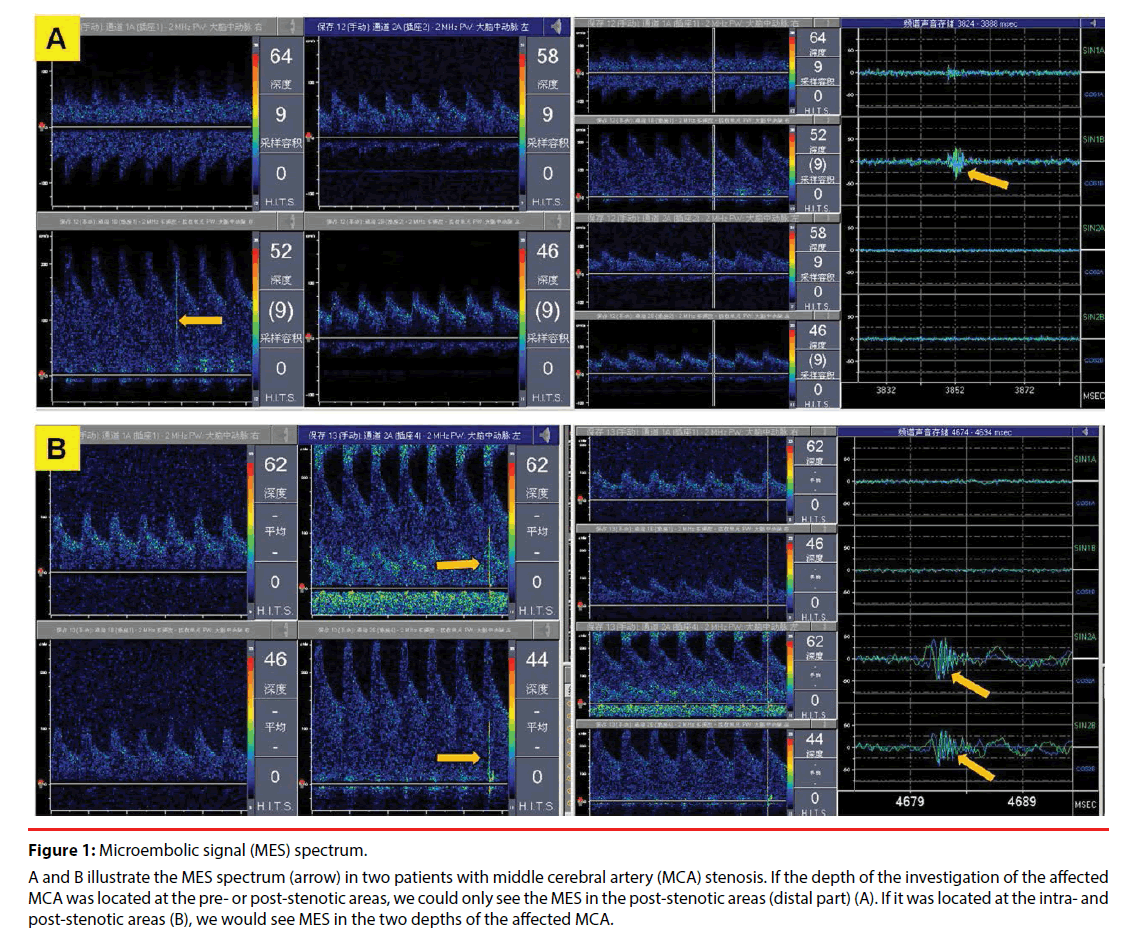
MES detection was performed by using two 2 MHz probes which were fixed on bilateral temporal window. The investigation depth of the affected MCA was located in the pre- and post-stenotic areas or the intra- and post-stenotic areas. The sample volumes were chosen to be as low as possible to avoid overlap, usually 8mm. In addition, the distance of the two investigation depths of the same vessel was greater than the sample volume. Emboli originating proximal to the MCAS passed through the different sample volumes, while emboli exiting from the MCAS would only pass the second channel located distal to the stenosis and produce a typical signal but no signal in the pre-stenotic sample volume. Therefore, an embolic signal would be recorded in the distal but not proximal sample volume. Artifacts simultaneously produce a typical pattern in both segments. The probe on the healthy side adopted the same settings as the affected side; gain and power were turned down as far as possible. In addition, all data were continuously recorded onto a four-channel digital audio tape recorder at normal speed. The recorded data were then analyzed by two experienced observers who were blinded to the clinical data. The number of MES during the 30-minute recording was noted. The following definitions for embolic signals were used: typical visible and audible (click, chirp, whistle) short-duration, high-intensity signal within the Doppler flow spectrum with its occurrence at random in the cardiac cycle, and an intensity increase of ≥5 dB above the background signal, and MES recorded from MCA stenoses has the special characteristic of multiple frequencies [27,28]. The spectrum of MES in patients with MCAS is illustrated in Figure 1.
Figure 1: Microembolic signal (MES) spectrum.
A and B illustrate the MES spectrum (arrow) in two patients with middle cerebral artery (MCA) stenosis. If the depth of the investigation of the affected MCA was located at the pre- or post-stenotic areas, we could only see the MES in the post-stenotic areas (distal part) (A). If it was located at the intra- and post-stenotic areas (B), we would see MES in the two depths of the affected MCA.
▪ Plasma sample collection and enzyme-linked immunosorbent assay measurements
Plasma samples were collected by venous puncture at 7:00 a.m and then aliquoted and stored at -80°C until further enzyme-linked immunosorbent assay (ELISA) analysis. ELISA kits for the detection of PAPP-A (R&D Systems, Minneapolis, USA), IGF-1 (R&D Systems), iNOS (Elabscience Biotechnology, Wuhan, China), Lp-PLA2 (R&D Systems), CD40 (Abnova, Taibei, Taiwan), and CD40L (Abnova, Taibei, Taiwan) were purchased from their respective manufacturers. Each kit employed the quantitative sandwich enzyme immunoassay technique. The kits were used according to the manufacturers’ instructions.
▪ Statistical Analysis
The statistical analysis was performed with SPSS version 17.0 software (IBM, West Grove, PA, USA). Categorical data are presented as proportions, while continuous data are presented as means and standard deviations or medians and interquartile ranges depending on the data distribution. Independent predictors were determined using multivariate logistic regression analysis using the variables with statistically significant contributions obtained on univariate analysis. For all statistical tests, values of P < 0.05 were considered significant.
Results
▪ Demographic features of enrolled patients
Among the 81 enrolled subjects, there were 19 subjects without ischemic infraction or cranial artery stenosis, 19 patients with acute ischemic infraction but without cranial artery stenosis, and 43 patients with symptomatic MCAS. The baseline demographics of all recruited patients are illustrated in Table 1. It was of note that the frequency of MES in healthy subjects and patients without cranial artery stenosis was both 0%; while it was 24/43 (55.8%) in patients with MCAS.
| Variables | Group 1 (N=19) | Group 2 (N=19) | Group 3 (N=43) |
|---|---|---|---|
| Age (year-old) | 56.62 ± 11.83 | 55.16 ± 11.53 | 57.58 ± 8.57 |
| Male to female ratio | 16:3 | 16:3 | 36:7 |
| Hypertension | 12/19 (63.2%) | 11/19 (57.9%) | 27/43 (62.8%) |
| Diabetes mellitus | 4/19 (21.1%) | 2/19 (10.6%) | 8/43 (18.6%) |
| Ischemic heart disease | 4/19 (21.1%) | 0 (0%) | 5/43 (11.6%) |
| Smoking | 11/19 (57.9%) | 4/19 (21.1%) | 20/43 (46.5%) |
| MES (%) | 0 | 0 | 24/43 (55.8%) |
| PAPP-A (pg/ml) | 130.1 ± 40.0 | 156.6 ± 43.9 | 118.5 ± 32.3 |
| IGF-1 (ug/ml) | 433.8 ± 243.6 | 372.1 ± 215.3 | 461.1 ± 252.6 |
| iNOS (pg/ml) | 521.7 ± 475.6 | 562.9 ± 510.6 | 503.5 ± 464.4 |
| Lp-PLA2 (ug/ml) | 18.9 ± 12.8 | 16.3 ± 12.4 | 13.7 ± 9.0 |
| CD40 (ug/ml) | 4.5 ± 12.9 | 11.4 ± 12.8 | 23.0 ± 42.9 |
| CD40L (ug/ml) | 12.7 ± 6.6 | 11.5 ± 4.6 | 9.1 ± 4.1 |
Group 2: acute ischemic infraction group without artery stenosis;
Group 3: symptomatic middle cerebral artery stenosis (MCAS) group regardless of microembolic signal (MES); PAPP-A: pregnancy-associated plasma protein A; IGF-1: insulin-like growth factor 1; iNOS: inducible nitric oxide synthase; Lp-PLA2: lipoprotein-associated phospholipase A2
Table 1: Demographic and clinical characteristic of the enrolled patients.
▪ Acute ischemic infarction volume was negatively associated with plasma concentrations of different molecules
We further investigated the correlation between the acute ischemic infarction volume as demonstrated by DWI on MRI and the plasma concentrations of PAPP-A, IGF-1, iNOS, Lp- PLA2, CD40, and CD40L. The acute ischemic infraction demonstrated by DWI was available for 30 patients. The mean volume of the acute ischemic infraction was 3.8 ± 2.81 cm3. The mean plasma concentrations of PAPP-A, IGF-1, iNOS, Lp-PLA2, CD40, and CD40L of these 30 patients was 132.9 ± 34.59 pg/mL, 401.4 ± 219.93 μg/mL, 523.5 ± 506.0 pg/mL, 15.2 ± 11.6 μg/mL, 25.6 ± 19.1 μg/mL, and 9.7 ± 0.6 μg/mL, respectively. Correlation analysis revealed negative results between the acute ischemic infarction volume and the plasma concentrations of different molecules including PAPP-A, IGF-1, iNOS, Lp-PLA2, CD40, and CD40L (P > 0.05).
▪ Predictors for moderate to severe MCAS
As artery stenosis is one of the major criteria for vulnerable plaque [3], we first explored the predictor for MCAS. Univariate analysis suggested that plasma PAPP-A (odds ratio [OR], 0.982; P = 0.004; 95% confidence interval [CI], 0.969– 0.994), CD40 (OR, 1.055; P = 0.016; 95% CI, 1.010–1.102) and CD40L (OR, 0.881; P = 0.012; 95% CI, 0.799–0.973) were statistically significant variables, while the concentrations of IGF-1, iNOS, and Lp-PLA2 (all P > 0.05) were insignificantly different. Further, these statistically significant variables were confirmed by the multivariate analysis (Table 2). Thus, plasma PAPP-A, CD40, and CD40L were predictive for moderate to severe MCAS. The plasma concentrations of PAPP-A and CD40L were protective factors, while that of CD40 was a risk factor for MCAS.
| Variable | Regression coefficient (95% CI) | p-value | Exp (B) |
|---|---|---|---|
| PAPP-A | -0.017 (0.968-0.998) | 0.024 | 0.983 |
| CD40 | 0.061 (1.012-1.117) | 0.015 | 1.063 |
| CD40L | -0.143 (0.773-0.972) | 0.015 | 0.867 |
Table 2: Plasma molecules related to moderate to severe middle cerebral artery stenosis.
▪ Plasma concentrations of PAPP-A and CD40 were predictors for presence of MES
Since MES was predominantly found in patients with acute ischemic infraction, especially those caused by moderate to severe MCAS [21], we further investigated the predictors for MES by using multivariate logistic regression analysis. Univariate analysis found that PAPP-A (OR, 0.985; P = 0.029; 95% CI, 0.972–0.999), Lp- PLA2 (OR, 0.933; P = 0.04; 95% CI, 0.874– 0.997), and CD40 (OR, 1.042; P = 0.035; 95% CI, 1.003–1.083) were significant variables, while plasma concentrations of IGF-1, iNOS, and CD40L (P > 0.05) were insignificant variables. Based on the above findings, the final model for multivariate analysis identified that plasma concentrations of PAPP-A and CD40 were predictive for the presence of MES (Table 3). The plasma concentration of PAPP-A was a protective factor, while that of CD40 was a risk factor for MES.
| Variable | Regression coefficient (95% CI) | p-value | Exp (B) |
|---|---|---|---|
| PAPP-A | -0.018 (0.967-0.997) | 0.016 | 0.982 |
| CD40 | 0.039 (1.005-1.077) | 0.025 | 1.040 |
Table 3: Plasma molecules associated with microembolic signal.
Discussion
In this study, we found that the acute ischemic infarction volume was not related to the plasma concentrations of PAPP-A, IGF-1, iNOS, Lp- PLA2, CD40, and CD40L. However, plasma PAPP-A, CD40, and CD40L were predictors for moderate to severe MCAS, while PAPP-A and CD40 were predictive for presence of MES.
The concept of vulnerable plaque has been proposed as a paradigm to improve cardiovascular disease prevention and outcome, and the currently proposed definition criteria of vulnerable plaque includes active inflammation, a thin cap with a large lipid core, fissured cap, endothelial denudation, severe stenosis, or a combination thereof [3,29]. However, approaches for assessing the plaque vulnerability associated with intracranial artery stenosis were insufficient due to its location. Searching for the simple approach to vulnerable plaques evaluation in patients with MCAS is essential in the Chinese population. MES was mainly found in symptomatic patients with moderate to severe MCAS [23]. Moreover, patients with MES could predict the recurrent cerebral ischemic events in patients with MCAS, implying that MES detection is a potential technique for evaluating plaque vulnerability [30]. However, MES detection has some limitations, and searching for the plasma biomarkers for vulnerable plaques is urgently needed. In the present study, we found that PAPP-A and CD40 were predictive for MES. However, due to the small sample size, the ability of plasma PAPP-A and CD40 to serve as plasma biomarkers for vulnerable MCASassociated plaques requires validation in further studies with larger sample sizes.
PAPP-A, a high-molecular-weight, zinc-binding matrix metalloproteinase with an incompletely clear biological function, was first identified in the sera of pregnant women. To date, the clinical significance of PAPP-A has predominantly focused on gynecology and obstetrics. In 2001, PAPP-A was first evaluated in patients with ACS and proposed to be caused by vulnerable plaques [31]. Similar results were found in the study conducted by Heeschen and colleagues [32]. However, Heider and colleagues found that PAPP-A concentrations were slightly higher in asymptomatic than in symptomatic patients with carotid stenosis [33]. Other studies also demonstrated that patients with hyper- or isoechoic carotid plaques exhibit significantly higher PAPP-A levels than those with hypoechoic early carotid lesions [34]. These studies on the correlation between PAPP-A and carotid plaque indicated the possible protective role of PAPP-A in the development of vulnerable carotid plaque [33,34]. Collectively, the correlation between plasma PAPP-A concentration and vulnerable plaque from different studies remains controversial. In addition, PAPP-A could cleave IGF-binding proteins 4 and 5 and lower their affinity to IGF, after which free IGF interacts with its receptors. The binding of IGF to its receptors plays a role in atherosclerosis by increasing cholesterol uptake into plaques and increasing cytokine release [35]. However, IGF- 1 also had favorable effects as its metabolic ability in coupling vasodilation has been suggested to play a key role in its anti-atherogenic function [36]. These contrasting effects keep the role of PAPP-A in atherosclerosis unclear. Here we found that plasma PAPP-A concentration but not IGF-1 was related to MES and moderate to severe MCAS. However, the mechanisms of the protective role of PAPP-A could not ascribe to the IGF-1 and remains unclear, which warrants further validation studies.
Plasma CD40, i.e. soluble CD40, is proteolytically cleaved from membrane-bound CD40, which could bind to CD154 and inhibit CD40-CD154-mediated immune responses. To our knowledge, the CD40–CD154 interaction plays a critical role in immunity and inflammatory regulation as well results in the production of numerous chemokines and cytokines, upregulation of adhesion molecules, secretion of matrix metalloproteinases, and induction of apoptosis [37]. Plasma CD40, serving as a CD40 antagonist, could bind to CD154, thereby inhibiting the CD40–CD154- mediated immune responses. Theoretically, plasma CD40 should be protective for vulnerable plaques by inhibiting active inflammation. In our study, however, we found that plasma CD40 was a risk factor for MES and moderate to severe MCAS, although its mechanism was unclear. Of note was that inflammatory markers differed between carotid and MCA atherosclerosis, and MCA atherosclerosis–associated plaques might be more stable than those associated with carotid atherosclerosis [38]. Thus, it is reasonable to speculate that the characterization of vulnerable MCAS-associated plaques might be also different from that related to carotid stenosis. However, this finding requires further study.
This study has some limitations. First, its sample size was small, and the normal concentration of PAPP-A and CD40 in the plasma of healthy individuals is mandatory when considering the protein as a biomarker, which still needs further study with a large sample size. Second, subjects with carotid artery stenosis were not included in the current study. Further studies to investigate whether the PPAP-A and CD40 would serve as predictors for vulnerable carotid plaque are warranted. Moreover, whether the plasma biomarkers for intracranial vulnerable plaques differ from those of carotid plaques remains to be elucidated.
In summary, PAPP-A and CD40 might sever as biomarkers for vulnerable plaque of MCAS. This preliminary finding requires validation in future studies.
Competing interests
The authors declare no competing interests.
Acknowledgements
The work was supported by grants from the technology development project of Jilin Province (No. 20170623092TC).
References
- Sacco RL, Kargman DE, Gu Q, et al. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stoke Study. Stroke 26(1), 14-20 (1995).
- Wityk RJ, Lehman D, Klag M, et al. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke 27(11), 1974-1980 (1996).
- Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation 108(14), 1664-1672 (2003).
- Zhang C, Qu S, Li H, et al. Microembolic signals and carotid plaque characteristics in patients with asymptomatic carotid stenosis. Scand. Cardiovasc. J 43(5), 345-351 (2009).
- Tegos TJ, Sabetai MM, Nicolaides AN, et al. Patterns of brain computed tomography infarction and carotid plaque echogenicity. J. Vasc. Surg 33(2), 334-339 (2001).
- Sabetai MM, Tegos TJ, Clifford C, et al. Carotid plaque echogenicity and types of silent CT-brain infarcts. Is there an association in patients with asymptomatic carotid stenosis? Int. Angiol 20(1), 51-57 (2001).
- Mayor I, ComelliM, Vassileva E, et al. Microembolic signals and carotid plaque morphology: a study of 71 patients with moderate or high grade carotid stenosis. Acta. Neurol. Scand 108(2), 114-117 (2003).
- Zhou Y, Xing Y, Li Y, et al. An assessment of the vulnerability of carotid plaques: a comparative study between intraplaque neovascularization and plaque echogenicity. BMC. Med. Imaging 13(13) (2013).
- Faggioli GL, Pini R, Mauro R, et al. Identification of carotid 'vulnerable plaque' by contrast-enhanced ultrasonography: correlation with plaque histology, symptoms and cerebral computed tomography. Eur. J. Vasc. Endovasc. Surg 41(2), 238-248 (2011).
- Giannoni MF, Vicenzini E, Citone M, et al. Contrast carotid ultrasound for the detection of unstable plaques with neoangiogenesis: a pilot study. Eur. J. Vasc. Endovasc. Surg 37(6), 722-727 (2009).
- Xiong L, Deng YB, Zhu Y, et al. Correlation of carotid plaque neovascularization detected by using contrast-enhanced US with clinical symptoms. Radiology 251(2), 583-589 (2009).
- Qiao Y, Etesami M, Astor BC, et al. Carotid Plaque Neovascularization and Hemorrhage Detected by MR Imaging are Associated with Recent Cerebrovascular Ischemic Events. AJNR. Am. J. Neuroradiol 33(4), 755-760 (2012).
- Virmani R, Kolodgie FD, Burke AP, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler. Thromb. Vasc. Biol 25(10), 2054-2061 (2005).
- Kurosaki Y, Yoshida K, Endo H, et al. Association between carotid atherosclerosis plaque with high signal intensity on T1-weighted imaging and subsequent ipsilateral ischemic events. Neurosurgery 68(1), 62-67 (2011).
- Sadat U, Teng Z, Young VE, et al. Association between biomechanical structural stresses of atherosclerotic carotid plaques and subsequent ischaemic cerebrovascular events: A longitudinal in vivo magnetic resonance imaging-based finite element study. Eur. J. Vasc. Endovasc. Surg 40(4), 485-4991 (2010).
- Sadat U, Teng Z, Young VE, et al. Utility of Magnetic Resonance Imaging-Based Finite Element Analysis for the Biomechanical Stress Analysis of Hemorrhagic and Non-Hemorrhagic Carotid Plaques. Circ. J 75(4), 884-889 (2011).
- Paulmier B, Duet M, Khayat R, et al. Arterial wall uptake of fluorodeoxyglucose on PET imaging in stable cancer disease patients indicates higher risk for cardiovascular events. J. Nucl. Cardiol 15(2), 209-217 (2008).
- Rominger A, Saam T, Wolpers S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J. Nucl. Med 50(10), 1611-1620 (2009).
- Marnane M, Merwick A, Sheehan OC, et al. Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission comography predicts early stroke recurrence. Ann. Neurol 71(5), 709-718 (2012).
- Xu WH, Li ML, Gao S, et al. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann. Neurol 71(2), 195-198 (2012).
- Shi MC, Wang SC, Zhou HW, et al. Compensatory remodeling in symptomatic middle cerebral artery atherosclerotic stenosis: a high-resolution MRI and microemboli monitoring study. Neurol. Res 34(2), 153-158 (2012).
- Wu X, Zhang H, Liu H, et al. Microembolic signals detected with transcranial Doppler sonography differ between symptomatic and asymptomatic middle cerebral artery stenoses in northeast China. PLoS. One 9(1), e88986 (2014).
- Wu XJ, Xing YQ, Wang J, et al. Clinical utilization of microembolus detection by transcranial Doppler sonography in intracranial stenosis-occlusive disease. Chin. Med. J (Engl) 126(7), 1355-1359 (2013).
- Wong KS, Li H, Chan YL, et al. Use of transcranial Doppler ultrasound to predict outcome in patients with intracranial large-artery occlusive disease. Stroke 31(11), 2641-2647 (2000).
- Wang L, Xing Y, Li, Y, et al. Evaluation of flow velocity in unilateral middle cerebral artery stenosis by Transcranial Doppler. Cell. Biochem. Biophys 70(2), 823-830 (2014).
- Chen J, Wang L, Bai J, et al. The optimal velocity criterion in the diagnosis of unilateral middle cerebral artery stenosis by transcranial Doppler. Cell. Biochem. Biophys 69(1), 81-87 (2014).
- Spencer MP, Thomas GI, Nicholls SC, et al. Detection of middle cerebral artery emboli during carotid endarterectomy using transcranial Doppler ultrasonography. Stroke 21(3), 415-423 (1990).
- Gao S, Wong KS. Characteristics of microembolic signals detected near their origins in middle cerebral artery stenoses. J. Neuroimaging 13(2), 124-132 (2003).
- Alshikh-Ali AA, Kitsios, GD, Balk EM, et al. The Vulnerable Atherosclerotic Plaque: Scope of the Literature. Ann. Intern. Med 153(6), 387-395 (2010).
- Gao S, Wong KS, Hansberg T, et al. Microembolic signal predicts recurrent cerebral ischemic events in acute stroke patients with middle cerebral artery stenosis. Stroke 35(12), 2832-2836 (2004).
- Antoni BG, Cheryl A, Conover CA, et al. Pregnancy-associated Plasma protein A as a marker of acute coronary syndromes. N. Engl. J. Med 345(14), 1022-1029 (2001).
- Heeschen C, Dimmeler S, Hamm CW, et al. Pregnancy-associated plasma protein-A levels in patients with acute coronary syndrome. J. Am. Coll. Cardiol 45(2), 229-237 (2005).
- Heider P, Pfäffle N, Pelisek J, et al. Is serum pregnancyassociated plasma protein A really a potential marker of atherosclerotic carotid plaque stability? Eur. J. Vasc. Endovasc. Surg 39(1), 668-675 (2010).
- Beaudeux JL, Burc L, Imbert-Bismut F, et al. Serum plasma pregnancy-associated protein A: a potential marker of echogenic carotid atherosclerotic plaques in asymptomatic hyperlipidemic subjects at high cardiovascular risk. Arterioscler. Thromb. Vasc. Biol 23(1), e7-10 (2003).
- Bayes-Genis A, Conover CA, Schwartz RS. The insulin-like growth factor axis: a review of atherosclerosis and restenosis. Circ. Res 86(2), 125-130 (2000).
- Conti E, Musumeci MB, De Giusti M, et al. IGF-1 and atherothrombosis: relevance to pathophysiology and therapy. Clin. Sci 120(9), 377-402 (2000).
- Suttles J, Stout RD. Macrophage CD40 signaling: a pivotal regulator of disease protection and pathogenesis. Semin. Immunol 21(5), 257-264 (2009).
- Bang OY, Lee PH, Yoon SR, et al. Inflammatory markers, rather than conventional risk factors, are different between carotid and MCA atherosclerosis. J. Neurol. Neurosurg. Psychiatr 76(8), 1128-1134 (2005).