Research Article - Neuropsychiatry (2017) Volume 7, Issue 1
Plasma ammonia levels in patients treated with valproic acid
- Corresponding Author:
- Prof. Reiji Yoshimura
Department of Psychiatry
University of Occupational and Environmental Health
1-1 Iseigaoka, Yahatanishi-ku, Kitakyushu, Fukuoka 807-8555, Japan
Tel: +81-93-691-7253
Fax: +81-93-692-4894
E-mail: [email protected]
Abstract
Objective:
Valproic acid (VPA) is used as a treatment for individuals with epilepsy, migraine, and psychiatric disorders such as bipolar disorder. Hyperammonemia has been reported to be associated with patients treated with VPA. Here we investigated the relationships among plasma ammonia concentrations, VPA dosages and plasma VPA concentrations, and we attempted to identify the factors associated with VPA-induced hyperammonemia.
Methods:
This was a retrospective chart-review investigating inpatients at The University of Occupational and Environmental Health, Japan (UOEH) Hospital from September 2003 to March 2014. We examined the cases of 430 inpatients. The patients’ diagnoses of schizophrenia, mood disorder and other psychiatric disorders were based on the DSM-IV-TR criteria. The upper normal limit of plasma ammonia is 54 μg/dl according to our hospital’s reference; we defined hyperammonemia as a plasma ammonia level above this value. We examined the correlations among the VPA dose, plasma VPA concentrations and plasma ammonia concentrations. We used Pearson’s correlation coefficient to examine the relationship between pairs of variables and performed a multiple regression analysis to identify variables that were associated with the plasma VPA concentrations and with the plasma ammonia concentrations.
Results:
Of the 430 hospitalized patients, 139 patients were eligible. We observed that 84 of these patients (60.4%) had hyperammonemia. Sex (female) and VPA dosage were associated with the plasma VPA concentrations. Concomitant antidepressant dosage and plasma VPA concentrations were associated with the plasma ammonia concentrations.
Conclusion:
We identified a high frequency of hyperammonemia among asymptomatic patients receiving VPA. Because asymptomatic hyperammonemia can potentially induce ammonia neurotoxicity, patients being treated with VPA should be monitored closely, especially if they have clinical symptoms and risk factors.
https://bluecruiseturkey.co
https://bestbluecruises.com
https://marmarisboatcharter.com
https://bodrumboatcharter.com
https://fethiyeboatcharter.com
https://gocekboatcharter.com
https://ssplusyachting.com
Keywords
Valproic acid, Hyperammonemia, Psychiatric disorder, Risk factor, Retrospective, Inpatient
Introduction
Valproic acid (VPA) is broadly used in the treatment of neuropsychiatric disorders, and it is also prescribed for epilepsy, migraine, and mood disorders. The commonly adverse events associated with VPA treatment are fatigue, gastrointestinal disturbances, weight gain, tremor, hair loss, thrombocytopenia, and an increase in hepatic enzymes. Hyperammonemia has also been reported to be associated with VPA treatment [1]. Hyperammonemia induced by VPA may be an important clinical consideration, especially when associated with liver disease or encephalopathy. The reported frequency of hyperammonemia induced by VPA ranges from 16.2% to 83.3% [2-4].
Most patients with hyperammonemia induced by VPA are asymptomatic. VPA may however rarely lead to hyperammonemic encephalopathy, which results in significant morbidity and central nervous system damage. The plasma ammonia levels of patients being treated with VPA should thus be carefully monitored. VPA-evoked hyperammonemic encephalopathy may occur even in the presence of therapeutic plasma VPA levels and normal liver function test results [5].
Several studies have reported a correlation between the plasma ammonia level or the plasma VPA concentration and the possible risk factors associated with VPA-induced hyperammonemia in patients with epilepsy or other disorders [6-8]. Monitoring the plasma VPA concentration to avoid adverse effects when VPA is prescribed is therefore a good clinical practice. It would also be useful to know the factors that contribute to plasma VPA concentrations so that clinicians can prescribe VAP safely. Thus, in the present study, we examined the relationships among hyperammonemia, VPA dose, and plasma VPA concentrations, and we attempted to elucidate the factors correlated with hyperammonemia in patients who are being treated with VPA.
▪ Patients and Methods
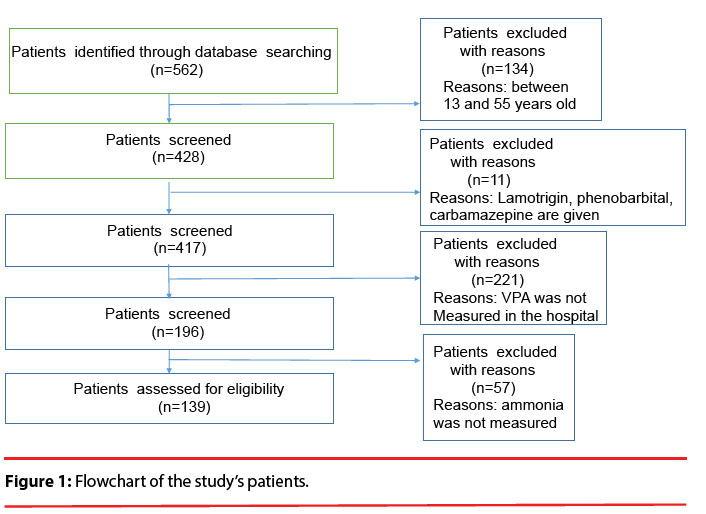
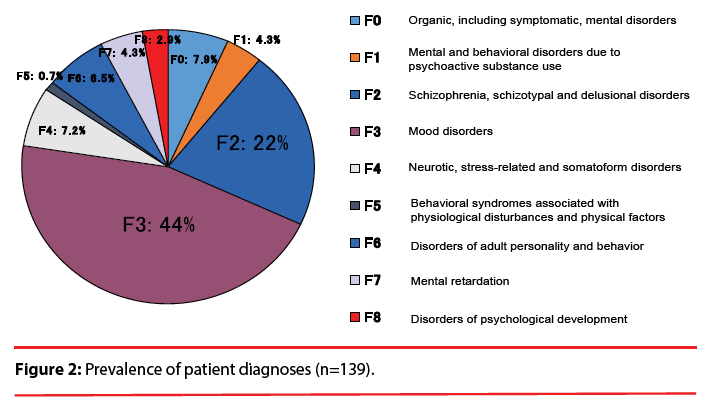
This study was a retrospective investigation of the cases of the 430 in-patients treated with VPA for a psychiatric disorder at The University of Occupational and Environmental Health, Japan (UOEH) Hospital in the 10.5-year period from September 2003 to March 2014. According to the following criteria, 139 inpatients were eligible for the present analysis (Figure 1): i.e., they were 13–55 years old; they were prescribed VPA; and their plasma VPA concentration and plasma ammonia concentration were monitored. Patients co-administered lamotrigine, phenobarbital, carbamazepine, and topiramate because of altering plasma VPA concentration were not selected for the study. The study protocol was approved by the Ethics Committee of The University of Occupational and Environmental Health. The patients had been diagnosed with schizophrenia, mood disorder and other psychiatric disorders based on the DSM-IV-TR (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision) criteria (Figure 2).
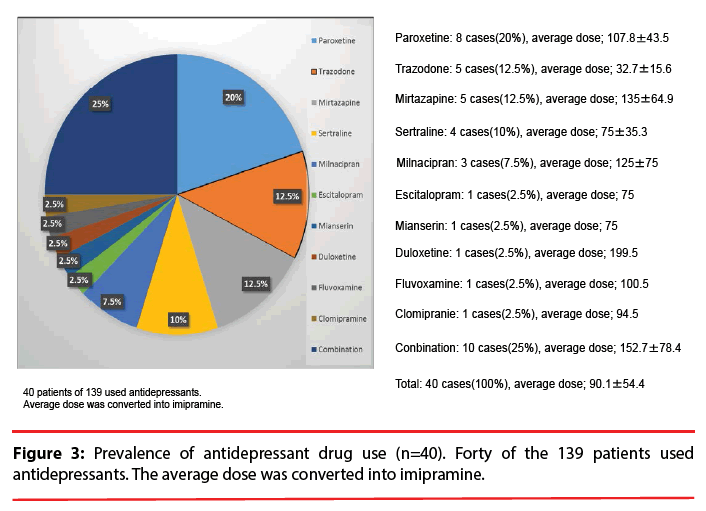
The VPA and ammonia concentration in each patient’s blood was measured at UOEH Hospital. Plasma VPA concentration was measured by using high-performance liquid chromatography according to Zhang et al. [9]. In brief, the method was based on derivatization of VPA using 2-bromo-2′-acetonaphthone as a new derivatization reagent. Caprylic acid was used as an internal standard. Under the optimized extraction and derivatization conditions, the method showed good linearity in the range of 0.05–200 μg/mL and the limit of detection was as low as 0.01 μg/mL. The normal range of plasma ammonia is 15–54 μg/dl according to the hospital’s standard. We defined hyperammonemia as a plasma ammonia level above 54 μg/dl. The coadministered drugs are summarized in Figure 3. We calculated the antipsychotics dose using the chlorpromazine equivalent scale, the antidepressant dose using the imipramine equivalent scale, and the dose of concomitant anxiolytics and hypnotics using the diazepam equivalent scale [10].
▪ Statistical analysis
The data were analyzed using Microsoft Excel software and STATA ver. 13.1 software (Stata Corp., TX, USA). We performed a Pearson’s correlation coefficient analysis to determine the correlations between the VPA dose and the plasma VPA concentration, the VPA dose and the plasma ammonia concentration, and the plasma VPA concentration and plasma ammonia concentration. We conducted a multiple linear regression analysis to identify variables associated with the VPA concentration in blood and with ammonia concentrations. The independent variables that showed p-values ≥0.20 in the bivariate analysis were not considered in the further analysis, whereas all variables with p-values ≤0.20 such as antipsychotic use, gender, VPA dose, albumin (alb), and Creatinine (Cre) as dependent variables for VPA concentrations were included in the multivariate analysis. A p-value <0.05 was considered significant.
Results
Of the 430 hospitalized patients, 139 patients were eligible (Figure 1). There were 90 females (64.7%) and 49 males (35.3%). The average age was 36.4±11.8 years (Table 1). We identified 84 (60.4%) of the 139 patients as hyperammonemic. None of these 84 patients were symptomatic, and no hepatotoxicity was found. As illustrated in Figure 2, the psychiatric diagnoses distribution was as follows: 44% (61 cases) were mood disorders, 22% (31 cases) were schizophrenia, schizotypal or delusional disorders, and the remaining third of the cases were various psychiatric disorders including epilepsy.
| The normal range | ||
|---|---|---|
| Years | 36.4 ̱̉ 11.9 | |
| Gender(Male/female) | 49/90 | |
| BMI | 24.1 ̱̉ 5.4 | |
| VPA dose | 678.4 ̱̉ 273.9 | |
| VPA concentrations | 56.2 ̱̉ 26.2 | 30-100̵̉g/ml |
| ammonia concentration | 62.3 ̱̉ 26.0 | 15-54̵̉g/dl |
| Alb | 4.1 ̱̉ 0.5 | 4.0-5.0g/dl |
| AST | 25.2 ̱̉ 32.0 | 13-33U/l |
| ALT | 30.0 ̱̉ 57.6 | 8-42U/l |
| BUN | 11.5 ̱̉ 4.9 | 8-22mg/dl |
| Cre | 0.74 ̱̉ 0.70 | M:0.6-1.1mg/dl,F:04-0.7mg/dl |
| TG | 156.8 ̱̉ 127.6 | M:38-207mg/dl,F:30-137mg/dl |
| T-cho | 186.7 ̱̉ 46.6 | 128-256mg/dl |
| Combination use ratio (%) | Average usage of concurrent users | |
| Antipsychotics | 59.0 | 441.3 ̱̉ 407.7 |
| Antidepressant | 29.5 | 121.3 ̱̉ 44.2 |
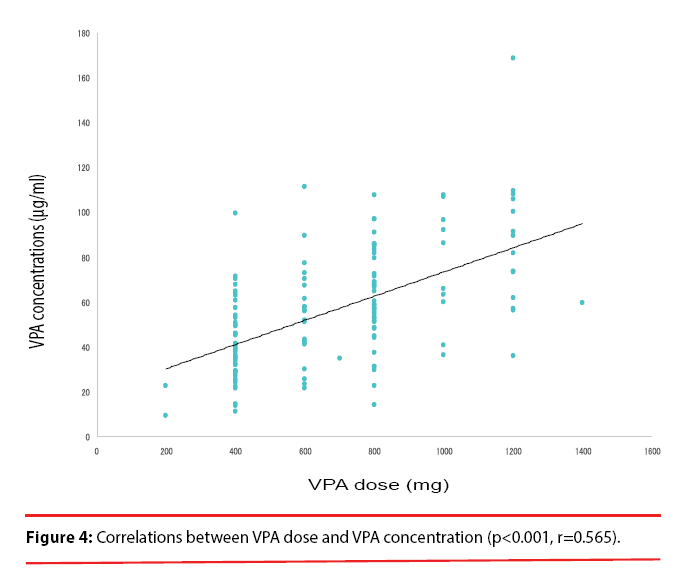
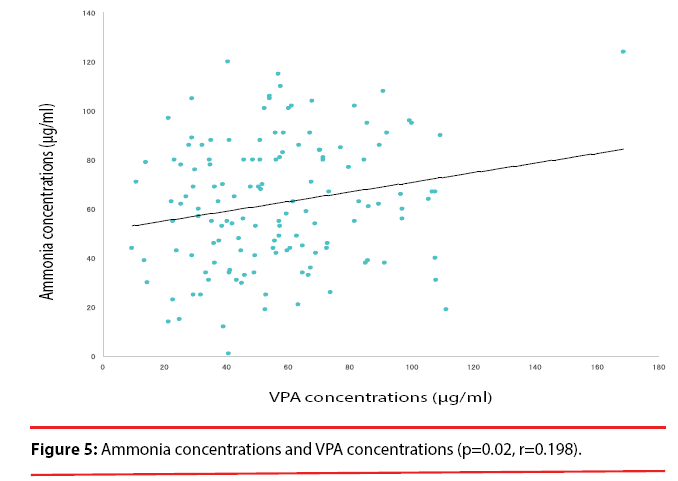
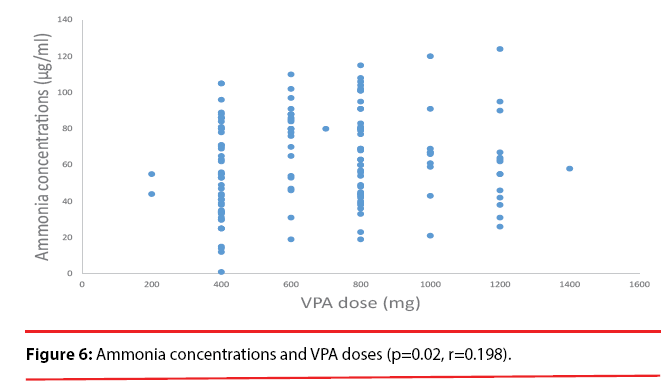
A significant correlation was observed between the VPA dose and the plasma VPA concentrations (p<0.001, r=0.565) (Figure 4). A significant correlation also was found between the plasma VPA concentrations and the plasma ammonia concentrations (p=0.02, r=0.198) (Figure 5). No correlation was found between the VPA dose and the plasma ammonia concentrations (p=0.137, r=0.013) (Figure 6).
The results of the regression statistical analysis are shown in Table 1. The independent variables were as follows: age, antipsychotic dose, antidepressant dose, benzodiazepine dose, gender, VPA dose, VPA concentration, albumin (Alb), aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine (Cre), triglyceride (TG), total cholesterol (T-cho), and body mass index (BMI). According to the multiple regression analysis, the variables that were associated with the plasma VPA concentration were female and the VPA dose (Table 2). The variables that were associated with the plasma ammonia concentration were the antidepressant dose and the plasma VPA concentration (Table 3).
| Independent variables | univariate | multiple | ||||
|---|---|---|---|---|---|---|
| β | SE | p value | β | SE | p value | |
| Age | -0.092 | 0.155 | 0.554 | |||
| Antipsychotics | 1.117 | 0.448 | 0.014 | 0.075 | 0.484 | 0.878 |
| Antidepressant | -1.538 | 3.673 | 0.676 | |||
| Benzodiazepine | 0.646 | 0.661 | 0.330 | |||
| Gender | 14.309 | 3.557 | 0.000 | 13.264 | 4.077 | 0.002 |
| VPA dose | 0.045 | 0.005 | 0.000 | 0.055 | 0.007 | 0.000 |
| Alb | -6.144 | 3.622 | 0.092 | -7.469 | 4.560 | 0.105 |
| AST | 0.029 | 0.066 | 0.660 | |||
| ALT | -0.014 | 0.036 | 0.693 | |||
| BUN | -0.152 | 0.393 | 0.699 | |||
| Cre | -4.362 | 3.059 | 0.156 | -4.800 | 2.533 | 0.061 |
| TG | -0.013 | 0.017 | 0.434 | |||
| T-cho | -0.013 | 0.048 | 0.788 | |||
| BMI | 0.230 | 0.352 | 0.513 | |||
Table 2: Association with VPA concentrations.
| Independent variables | univariate | multiple | ||||
|---|---|---|---|---|---|---|
| β | SE | p value | β | SE | p value | |
| Age | 0.272 | 0.185 | 0.144 | -0.047 | 0.223 | 0.833 |
| Antipsychotics | -0.007 | 0.582 | 0.991 | |||
| Antidepressant | 12.657 | 4.437 | 0.005 | 13.231 | 4.571 | 0.005 |
| Benzodiazepine | -0.832 | 0.803 | 0.302 | |||
| Gender | -1.635 | 4.624 | 0.724 | |||
| VPA dose | 0.013 | 0.008 | 0.107 | -0.003 | 0.011 | 0.794 |
| VPA concentrations | 0.196 | 0.083 | 0.020 | 0.308 | 0.115 | 0.009 |
| Alb | -11.273 | 4.273 | 0.009 | -4.553 | 5.894 | 0.442 |
| AST | -0.014 | 0.072 | 0.849 | |||
| ALT | -0.003 | 0.040 | 0.945 | |||
| BUN | 0.727 | 0.466 | 0.121 | -0.218 | 0.668 | 0.745 |
| Cre | 4.826 | 3.248 | 0.140 | 7.958 | 4.319 | 0.068 |
| TG | -0.005 | 0.020 | 0.791 | |||
| T-cho | -0.019 | 0.058 | 0.748 | |||
| BMI | 0.353 | 0.419 | 0.400 | |||
Table 3: Association with ammonia concentrations.
Discussion
The main finding in the present study was the significant correlation between the plasma VPA concentrations and the plasma ammonia concentrations. In contrast, the VPA dose was not related to the plasma ammonia concentration.
The question of whether or not the plasma ammonia concentration is associated with encephalopathy remains controversial. We could not elucidate this point in the present study. One investigation indicated that plasma ammonia concentrations were associated with plasma VPA concentrations [11], but another study by Murphy and Marquardt did not identify a correlation between plasma ammonia concentrations and plasma VPA concentrations [12]. It is thus not confirmed whether an individual’s plasma VPA level is associated with his or her plasma ammonia concentration.
In the present investigation, we expected that there would be high relativity among the ammonia concentrations, VPA doses and VPA concentrations in patients in good compliance with their VPA treatment regimen (as hospitalized inpatients). We observed a correlation between the VPA doses and plasma VPA concentrations but no correlation between the VPA doses and the plasma ammonia concentrations. Our findings demonstrated a correlation between plasma VPA concentrations and plasma ammonia concentrations (r=0.198, p=0.02), which is in agreement with the result from the Murphy and Marquardt study. We cannot extrapolate plasma ammonia concentrations from the VPA dose. Taking all of the above findings together, it appears that it might be necessary to conduct therapeutic drug monitoring for VPA-treated patients in order to avoid side effects from VPA.
Two studies [6,7] of Japanese epilepsy patients showed that risk factors for hyperammonemia associated with VPA were VPA dose, female gender, and the concomitant use of phenytoin, phenobarbital, or topiramate. VPA was used over 40% of the present study’s subjects for a mood disorder, schizophrenia, or organic (including symptomatic) mental disorders (Figure 2). VPA was generally used broadly as a mood stabilizer combined with an antidepressant or antipsychotic. Antidepressants were most prescribed (28.8%), especially SSRIs, as the combination drug with VPA (Figure 3). Some antidepressants such as amitriptyline and fluoxetine prolong the half-life of VPA or increase VPA concentrations [13-15]. We observed that the co-administrated antidepressant was associated with plasma ammonia levels, but not the plasma VPA levels (data not shown). We were unable to determine which antidepressants interact with VPA because of the relatively small number of patients examined, and further studies are needed regarding potential interactions of VPA with antidepressants.
The question of whether abnormal liver function is related to the ammonia concentrations in the blood of patients being treated with VPA is controversial [16,17]. In our study, there was no correlation between liver function parameters such as AST and ALT and ammonia concentrations. By conducting a regression statistical analysis, we observed that the antidepressant dose and plasma VPA concentrations were associated with plasma ammonia.
Our study has several limitations. First, the study was conducted to investigate the correlations among VPA doses, VPA concentrations and ammonia concentrations in the blood, but the patients’ diagnoses were not confirmed due to the retrospective design of the study. Second, most of the patients took antidepressants and/ or VPA, which might have influenced the results via pharmacokinetic and/or pharmacodynamic pathways. Third, we did not measure free VPA concentrations. Our findings regarding total VPA concentrations and the VPA doses should be interpreted with caution in light of the ceiling effect due to saturation of plasma protein binding.
In conclusion, the plasma VPA concentrations of patients being treated with VPA should be monitored, especially when high plasma VPA concentrations are observed, and concomitant with antidepressant, because ammonia neurotoxicity can be present even in patients who are asymptomatic.
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the publication of this paper.
References
- Tseng YL, Huang CR, Lin CH, et al. Risk factors of hyperammonemia in patients with epilepsy under valproic acid therapy. Medicine (Baltimore) 93(11), e66 (2014).
- Sharma S, Galati S, Kabra M, et al. Blood ammonia levels in epileptic children on 2 dose ranges of valproic acid monotherapy: a cross-sectional study. J. Child. Neurol 26(), 109-112 (2011).
- Haidukew ych D, John G, Zielinski JJ, et al. Chronic valproic acid therapy and incidence of increases in venous plasma ammonia. Ther. Drug. Monit 7(3), 290-294 (1985).
- Holroyd S, Overdyke JT. Hyperammonemia associated with valproic acid use in elderly psychiatric patients. J. Neuropsy. Clin. Neurosci 24(3), 372-374 (2012).
- Pegg EJ, Zaman F. Sodium valproate-related hyperammonaemic encephalopathy. BMJ. Case. Rep (2014)
- Yamamoto Y, Takahashi Y, Imai K, et al. Risk factors for hyperammonemia in pediatric patients with epilepsy. Epilepsia 54(6), 983-989 (2013).
- Yamamoto Y, Takahashi Y, Suzuki E, et al. Risk factors for hyperammonemia associated with valproic acid therapy in adult epilepsy patients. Epilepsy. Res 101(3), 202-209 (2012).
- Tseng YL, Huang CR, Lin CH, et al. Risk factors of hyperammonemia in patients with epilepsy under valproic acid therapy. Medicine (Baltimore) 93(11), e66 (2014).
- Zhang JF, Zhang ZQ, Dong WC, et al. A new derivatization method to enhance sensitivity for the determination of low levels of valproic acid in human plasma. J. Chromatogr. Sci 52(10), 1173-1180 (2014).
- Inada T, Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry. Clin. Neurosci 69(8), 440-447 (2015).
- Raja M, Azzoni A. Valproate-induced hyperammonemia. J. Clin. Psychopharmacol 22(1), 631-633 (2002).
- Murphy J, Marquardt K. Asymptomatic hyperammonemia in patients receiving valproic acid. Arch. Neurol 39(9), 591-592 (1982).
- Pisani F, Primerano G, Amendola D’ Agostino A, et al. Valproic acid-amitriptyline interaction in man. Ther. Drug. Monit 8(3), 382-383 (1986).
- Wong SL, Cavanaugh J, Shi H, et al. Effects of divalproex sodium on amitriptyline and nortriptyline pharmacokinetics. Clin. Pharmacol. Ther 60(1), 48-53 (1996).
- Lucena MI, Blanco E, Corrales MA, et al. Interaction of fluoxetine and valproic acid. Am. J. Psychiatry 155(4), 575 (1998).
- Hamer HM, Knake S, Schomburg U, et al. Valproate-induced hyperammonemic encephalopathy in the presence of topiramate. Neurology 54(1), 230-232 (2000).
- Carr RB, Shrewsbury K. Hyperammonemia due to valproic acid in the psychiatric setting. Am. J. Psychiatry 164(7), 1020-1027(2007).