Review Article - (2024) Volume 14, Issue 4
Overview of the Multifaceted Functions of lncRNA HOTAIR in Glioblastoma.
- Corresponding Author:
- Mohammed A Azab
Department of Neurosurgery, Cairo University Hospital, Cairo, Egypt
E-mail: [email protected]
Received date: 18-Jul-2024, Manuscript No. NPY-24-142181; Editor assigned: 22-Jul-2024, PreQC No. NPY-24-142181 (PQ); Reviewed date: 05-Aug-2024, QC No. NPY-24-142181; Revised date: 12-Aug-2024, Manuscript No. NPY-24-142181 (R); Published date: 19-Aug-2024, DOI: 10.37532/1758-2008.2024.14(4).717
Abstract
Glioblastoma is an aggressive primary brain tumor. Recurrence is a major clinical problem. Several biological features favor recurrence of these tumors following surgery. Therapies to prolong survival are not completely effective. Non-coding genetic elements play a key role in the process of gliomagenesis. Non-coding RNAs are novel regulatory RNAs that play key roles in various processes as gene regulation, cell differentiation, and proliferation. Interestingly, some lncRNAs may act as tumor suppressors while others are oncogenic. In this review, we are going to illustrate the role of a well-known lncRNA HOX Transcript Antisense Intergenic RNA (HOTAIR) in glioma and highlight the possible functions in glioma.
Keywords
Glioblastoma, long non-coding RNAs, microRNAs, brain tumor
Introduction
Glioblastoma Multiforme (GBM) is a primary brain tumor notorious for aggressive behavior [1]. The survival rate after one year is about 39.7% with a high rate of recurrence [2]. The recurrence of GBM is a complex multifactorial process. The best outcome reported was related to the European Organization for Research and Treatment of Cancer (EORTC) and National Cancer Institute of Canada (NCIC) clinical trials [3]. Epigenetics are extensively involved in the virulence of GBM. Several factors contribute to treatment failure such as the heterogeneity of the GBM microenvironment, repository of stem cells with great regenerative activity, and developing resistance to common therapies.
Non-coding RNAs (ncRNAs) are recent classes of RNA molecules that play essential roles in different processes as gene regulation, cell differentiation and growth [4]. The non- coding elements represent a large moiety of the human genome, however, its main functions are poorly understood [5,6]. The mechanism through which ncRNA regulates biological functions needs to be more elucidated. Non- coding RNA is classified into short and long types according to the nucleotide length. Small ncRNAs (20-200 nucleotides) include microRNAs (miRNAs), small nuclear RNAs (snRNAs), small interfering RNAs (siRNAs), small nucleolar RNAs (snoRNAs), Piwi- interacting RNAs (piRNAs) [7].
Long non-coding RNAs are composed of more than 200 nucleotides and could control genes that regulate the cell cycle, apoptosis, and cellular growth [8]. Mounting research suggests a possible role of lncRNAs in different cancers including glioma [9]. For example, a lncRNA Nuclear Enriched Abundant Transcript 1 (NEAT1) enhances invasion of GBM cells and inhibits apoptosis [9]. Another lncRNA H19 was found to correlate with glioma grade and invasiveness [10]. The Tumor Suppressor Candidate 7 (TUSC7), is a LncRNA that inhibits invasion and migration of glioma cells and correlates with prognosis [10,11]. In this review article, we are going to highlight the potential roles of a well-known lncRNA HOX Transcript Antisense Intergenic RNA (HOTAIR) in glioma.
Literature Review
Mechanism of action
The lncRNA HOX Transcript Antisense Intergenic RNA (HOTAIR) was the first lncRNA to be identified [12]. It relates to the homeobox super-families and comprises 2158 nucleotides. It is transcribed from the HOXC locus on chromosome 12q13.13 [13]. Polycomb-Repressive Complex 2 (PRC2) is a chromatin modifying complex and a binding target for HOTAIR [14]. PRC2 complex induces lysine methylation on histone H3. H3K27 methylation is considered a gene silencing way and is assisted by histone methyl transferase (Enhancer of Zeste Homolog 2 (EZH2) [15]. Through interaction with histone lysine-specific demethylase (KDM1), HOTAIR can silence different genes [16].
KDM1 can combine with RE1-Silencing Transcription factor (REST) and cofactor Corepressor for Element-1-Silencing Transcription Factor (CoREST) to promote gene silencing. In early embryo life, HOTAIR is expressed in certain locations such as hind limb bud, and posterior trunk. HOTAIR also can regulate the cell cycle proteins through controlling Cyclin-Dependent Kinase 2 (CDK2), CDK4, and Cyclin D1 [17]. Aberrant HOTAIR expression has been correlated with growth, and recurrence by affecting downstream targets [18-20].
Molecular interactions involving HOTAIR in GBM
HOTAIR exhibited oncogenic potential in breast and renal cancer by enhancing cell proliferation, suppressing apoptosis, and promoting invasion [21,22]. HOTAIR was expressed in glioma at a high rate compared to normal brain tissues [23]. A certain study showed that HOTAIR knockdown dismantled GBM mouse model [24]. HOTAIR is highly expressed in both classic and mesenchymal glioma subtypes compared to neural and proneural subtypes [25]. HOTAIR was identified as a marker that correlates for tumor grade and outcome given the fact that low- grade glioma has lower expression levels of HOTAIR compared with high-grade tumors [25]. Studies evaluating the role of HOTAIR in GBM are summarized.
HOTAIR activity could be controlled by other ncRNAs. Homeobox Protein A9, (HOXA9) stimulates the expression of HOTAIR in glioma.
The upregulation of HOXA9 was associated with abnormally aggressive behavior [26]. As mentioned before, HOTAIR can induce gene silencing depending on EZH2, meanwhile, HOXA9 is regulated by the Phosphatidylinositol 3-Kinase (PI3K) pathway and the inhibition of EZH2-mediated histone methylation [23,27].
Another study evaluated the role of Programmed Cell Death Protein 4 (PDCD4) in the progression of GBM. The overexpression of PDCD4 in glioma cells down regulated cellular proliferation suggesting that PDCD4 could function as a tumor suppressor.
Lower expression levels of PDCD4 are associated with upregulated Histone H3 methylation mediated by HOTAIR [28]. Exposure of glioma cells to a Bromodomain and Extra-Terminal (BET inhibitor) (I-BET151) downregulated the expression of HOTAIR and halted cell proliferation through cell cycle arrest. Moreover, the upregulation of HOTAIR abolished the anti-cancer effect of I-BET151. [29].
The role of HOTAIR as a tumor suppressor gene needs further scrutinization [30].
HOTAIR can influence cell-cycle related genes in GBM
Long non-coding RNAs (lncRNAs) can regulate the cell cycle through several ways [31,32]. Antisense Noncoding RNA in the INK4 Locus (ANRIL), for example, downregulates p15INK4B expression, and Metastasis Associated Lung Adenocarcinoma Transcript (MALAT1) controls B-MYB that controls cell cycle progression [33,34].
In LN229 and U87 cells, the downregulating HOTAIR resulted in G0 or G1 stage block [35]. The downregulation of Cyclin D1, Cyclin E, Cyclin-Dependent Kinase 2 (CDK2), CDK4, and the enhanced expression of other proteins such as p21 and p16 was associated with HOTAIR downregulation. HOTAIR regulates a group of 18 genes that constitute a cell-cycle related mRNA network. HOTAIR controls cell cycle in glioma cells by regulating Forkhead Box Protein M1 (FoxM1) and Aurora Kinase B (AURKB) that are involved in mitosis. Several genes such as ASPM, NCAPG, CDC6, CHEK1, CEP55 play a role in gliomagenesis, through their effect on cell cycle progression [36-39].
HOTAIR affected the expression of some cell- cycle related genes such as CDC6, NCAPG, CENPE, and PLK4. As mentioned earlier, HOTAIR can induce gene silencing depending on EZH2 through histone methylation [40]. EZH2 inhibition was reported to stop cell cycle progress at the G0 or G1 phase of GBM cells favoring it as a therapeutic target.
Prominent interactions of HOTAIR with micro-RNA in GBM
lncRNAs can control the activity of several mRNAs [41]. lncRNAs can compete with micro RNAs displacing them from binding sites [42]. In breast cancer, HOTAIR miR- 7 relation is a clear example and in gastric cancer, its pro oncogenic effect was through competing with miR-331-3p [42,43].
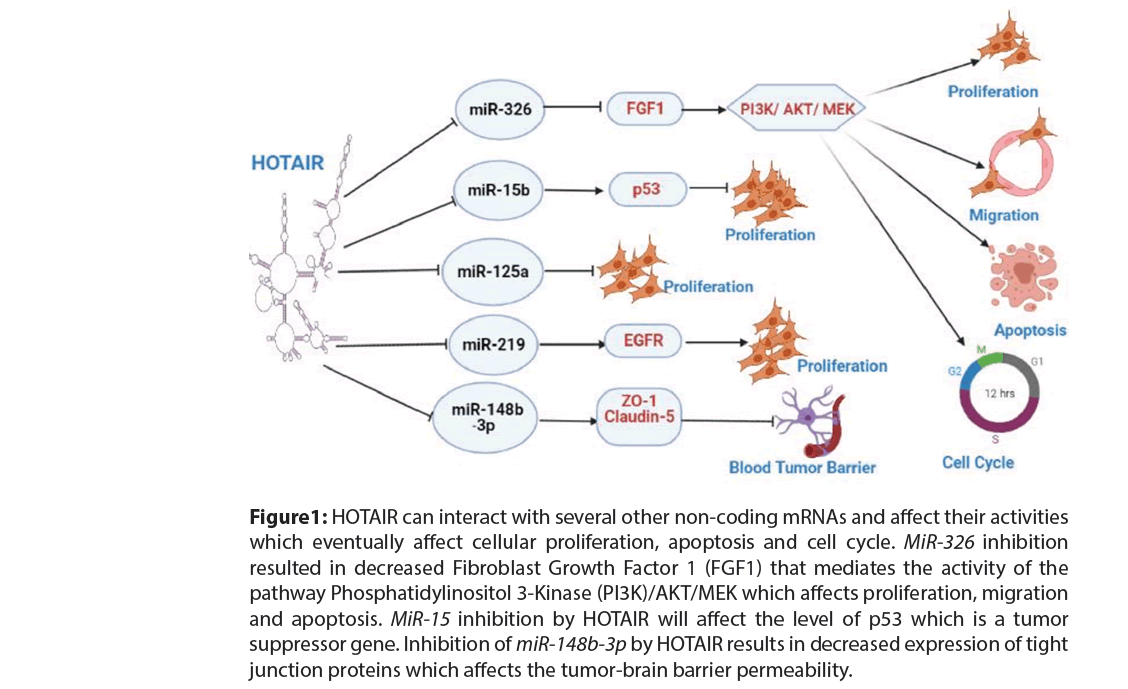
HOTAIR/miR-326: A study has shown that the expression of miR-326 is downregulated in glioma tissues. Knocking down HOTAIR resulted in the overexpression of miR-326 which resulted in downregulating Fibroblast Growth Factor 1 (FGF1) in U87 cells impacting cellular proliferation (Figure 1).
Figure 1: HOTAIR can interact with several other non-coding mRNAs and affect their activities which eventually affect cellular proliferation, apoptosis and cell cycle. MiR-326 inhibition resulted in decreased Fibroblast Growth Factor 1 (FGF1) that mediates the activity of the pathway Phosphatidylinositol 3-Kinase (PI3K)/AKT/MEK which affects proliferation, migration and apoptosis. MiR-15 inhibition by HOTAIR will affect the level of p53 which is a tumor suppressor gene. Inhibition of miR-148b-3p by HOTAIR results in decreased expression of tight junction proteins which affects the tumor-brain barrier permeability.
HOTAIR/miR-15b: A study found that HOTAIR reduced miR-15b expression in glioma cells which may have oncogenic potential [44]. miR-15b could upregulate p53 expression. HOTAIR, miR-15b, and p53 is a closed loop that controls glioma progression.
HOTAIR/miR-125a: miR-125a-5p was reported to inhibit glioblastoma cell proliferation, and HOTAIR has been demonstrated to reduce miR-125a expression [45,46]. Schisandrin B, an herbal extract, reduced HOTAIR expression in glioma cell lines by targeting the mammalian Target of Rapamycin (mTOR) expression [47].
HOTAIR/miR-219: miR-219-5p inhibits glial cell proliferation by targeting tyrosine kinase and Epidermal Growth Factor Receptor Mutation (EGFR) [48]. HOTAIR has been also shown to inhibit miR-219 in U87 cells, resulting in increased Cyclin D1 levels and cellular proliferation [49].
Discussion
HOTAIR and angiogenesis
Angiogenesis is controlled by hypoxia mediators, the most well-known ones are Hypoxia Inducible Factor (HIF) and Vascular Endothelial Growth Factor (VEGF) [50,51]. Both HIF and VEGF work together to promote a vascular niche for glioma cells. In nasopharyngeal carcinoma cells, HOTAIR enhanced angiogenesis by activating the transcription promoter of Vascular Endothelial Growth Factor A (VEGFA) [52]. It may act through the formation of extracellular vesicles as it was detected in the supernatant of GBM culture [53]. Comprehensive studies are needed to evaluate the role of HOTAIR in terms of glioma vascularization.
Potential use of HOTAIR as a diagnostic marker in GBM
An absolute need for a non-invasive accurate marker for clinical implications in patients diagnosed with high-grade gliomas is demanding. The possibility for certain body fluid markers to be used for clinical prediction of glioma is still under investigation. Markers that can monitor response to therapy are essential especially for an aggressive disease like GBM. Differentiating true GBM recurrence from pseudo-progression seems difficult and technically challenging. Conventional Magnetic Resonance Imaging (MRI) could not easily pick the exact differences between both conditions. A serum biomarker could be a tool to aid in the clinical differentiation in both situations.
Glial Fibrillary Acidic Protein (GFAP), lactate, miR-504, have been reported as potential candidates for diagnosing GBM [54-56]. The stability of lncRNAs secondary structures makes them perfect biomarkers [57]. HOTAIR has been identified as a possible serum marker in other cancers [58,59]. Its concentration was lower after the surgical treatment of a recurrent GBM and the reduction was more noticeable further weeks after surgery. Further experimental and clinical work should be implemented to evaluate the sensitivity and predictability of HOTAIR as a novel serum biomarker in patients diagnosed with GBM.
HOTAIR as a potential therapeutic target in GBM
As discussed earlier, HOTAIR can regulate glioma progression in an EZH2-dependent manner through epigenetic role. Therefore, targeting of HOTAIR-EZH2 interaction may be utilized as a possible therapeutic approach. AC1Q3QWB that targeted HOTAIR-EZH2, was found to inhibit glioma cell proliferation, with a resultant increase in CWF19L1 that works as a tumor suppressor gene [60,61]. The Bromodomain and Extra-Terminal (BET) proteins are epigenetic modulators that have been used as therapeutic tools for some cancers with profound epigenetic changes [62]. In a published study, I-BET151 treatment and BRD4 depletion reduced the overexpression of HOTAIR in glioma cells through an effect on transcription factors [63].
RNAi are tools that could inhibit specific genes, including short interfering RNAs (siRNAs) which are short double-stranded RNAs targeting complementary RNA molecules, resulting in gene suppression [64]. Carriers of nucleic acids could be used to deliver these siRNAs into tumor cells. Due to their high stability, iron oxide nanoparticles and specifically Super Paramagnetic Iron Oxide Nanoparticles (SPIONs) have been used widely in the delivery [65]. A study has demonstrated the successful delivery of siHOTAIR that subsequently inhibited glioma stem cell proliferation [66]. In a study by Zhang et al., deleting the HOTAIR regulatory element improved the sensitivity of glioma cells to Temozolomide. [67].
In Temozolomide-resistant GBM cells, HOTAIR was upregulated, while temozolomide resistance was enhanced upon the exosomemediated transfer of HOTAIR by a mechanism involving miR-519a-3p downregulation [68-70]. Poor penetration of the blood-brain barrier and failure to achieve a maximal intratumoral concentration is a common hurdle facing chemotherapy. HOTAIR knockdown resulted in improving brain-tumor barrier permeability by a mechanism involving the miR-148b-3p targeting. miR-148b-3p affects the microvascular endothelial cells which control the expression of proteins involved in Blood-Brain Barrier (BBB) integrity as Zonula Occludens (ZO-1), Claudin-5, and Occludin [71-75] (Table 1).
| Role of HOX Transcript Antisense Intergenic RNA (HOTAIR) |
Reference |
|---|---|
| HOTAIR inhibits the transcription of Neuroleukin (NLK) in U87, Glioblastoma Multiforme (GBM) cells, regulate Wnt/β-catenin pathway, inhibit cell cycle arrest and promote cell migration. | [70] |
| HOTAIR mRNA levels are increased in A172 glioma cells compared to normal astrocytes. | [71] |
| miR-141 directly binds to the 3 UTR of HOTAIR in U251 and U87 glioma cells, inhibiting its expression. | [72] |
| miR-148b-3p downregulates the expression of tight junction-related proteins including ZO-1, clauidin-5, and occludin. | [69] |
| HOTAIR rs920778 and rs12826786 frequencies do not differ between glioma patients and controls. | [73] |
| HOTAIR levels positively correlate with Matrix Metalloproteinase-7 (MMP-7), Matrix Metalloproteinase-9 (MMP-9), and Vascular Endothelial Growth Factor (VEGF) levels in human glioma. | [74] |
| HOTAIR upregulates the expression of hexokinase 2 by downregulating miR-125. | [75] |
| HOTAIR is upregulated in temozolomide-resistant GBM cells. Serum exosome HOTAIR levels are higher in GBM patients’ resistant to temozolomide compared with responders. | [68] |
Table 1: A sample of experimental studies investigating HOTAIR in glioblastoma
Conclusion
There is a compelling need for clinical studies that could uncover the HOTAIR role in GBM. Therapies to prolong survival in patients diagnosed with GBM are traditional and their effect on survival is not remarkable. More understanding of the biology of HOTAIR will enable researchers to develop new strategies and diagnostic markers that will eventually apply in clinical trials. Elevated expression of HOTAIR in glioma correlates with higher tumor grade and poor prognosis. Mechanistically, HOTAIR influences the expression of several cell cycle-related genes and interacts with various microRNAs, contributing to tumor growth, resistance to apoptosis, and increased invasion. Targeting HOTAIR-EZH2 interactions, utilizing RNA interference strategies, and employing BET inhibitors like I-BET151 have shown potential results in preclinical models.
Conflict of Interest
The authors certify that there is no conflict of interest with any financial organization about the material described in the manuscript.
Funding
The authors did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
References
- Bush NA, Chang SM, Berger MS. Current and future strategies for treatment of glioma. Neurosurg Rev 40, 1-4 (2017).
- Sherrod BA, Gamboa NT, Wilkerson C, et al. Effect of patient age on glioblastoma perioperative treatment costs: A value driven outcome database analysis. Neurooncol 143, 465-473 (2019).
- Stupp R, Mason WP, Van Den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10), 987-996 (2005).
- Kapranov P, Cheng J, Dike S, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 316(5830), 1484-1488 (2007).
- Wahlestedt C. Targeting long non-coding RNA to therapeutically upregulate gene expression. Nat Rev Drug Discov 12(6), 433-446 (2013).
- Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol 10(6), 924-933 (2013).
- Gomes AQ, Nolasco S, Soares H. Non-coding RNAs: Multi-tasking molecules in the cell. Int J Mol Sci 14(8), 16010-16039 (2013).
- Liu SJ, Nowakowski TJ, Pollen AA, et al. Single-cell analysis of long non-coding RNAs in the developing human neocortex. Genome Biol 1-7 (2016)
- Huang D, Bi C, Zhao Q, et al. Knockdown long non-coding RNA ANRIL inhibits proliferation, migration and invasion of HepG2 cells by down-regulation of miR-191. BMC cancer 18, 1-9 (2018).
- Shi Y, Wang Y, Luan W, et al. Long non-coding RNA H19 promotes glioma cell invasion by deriving miR-675. PloS one 9(1):e86295 (2014).
- Shang C, Guo Y, Hong Y, et al. Long non-coding RNA TUSC7, a target of miR-23b, plays tumor-suppressing roles in human gliomas. Front Cell Neurosci 10,235 (2016).
- Bhan A, Mandal SS. LncRNA HOTAIR: A master regulator of chromatin dynamics and cancer. Biochim Biophys Acta 1856(1), 151-164 (2015).
- Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464(7291), 1071-1076 (2010).
- Am K. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci USA 106,11667-11672 (2009).
- Bhan A, Mandal SS. Long noncoding RNAs: Emerging stars in gene regulation, epigenetics and human disease. ChemMedChem 9(9), 1932-1956 (2014).
- Tsai MC, Manor O, Wan Y, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 329(5992), 689-693 (2010).
- Mozdarani H, Ezzatizadeh V, Rahbar Parvaneh R. The emerging role of the long non-coding RNA HOTAIR in breast cancer development and treatment. J Transl Med 18(1), 152 (2020).
- Shi J, Dong B, Cao J et al. Long non-coding RNA in glioma: Signaling pathways. Oncotarget 8(16):27582 (2017).
- Cheng C, Qin Y, Zhi Q, et al. Knockdown of long non-coding RNA HOTAIR inhibits cisplatin resistance of gastric cancer cells through inhibiting the PI3K/Akt and Wnt/β-catenin signaling pathways by up-regulating miR-34a. Int J Biol Macromol 107, 2620-2629 (2018).
- Chen J, Lin C, Yong W, et al. Calycosin and genistein induce apoptosis by inactivation of HOTAIR/p-Akt signaling pathway in human breast cancer MCF-7 cells. Cell Physiol Biochem 35(2):722-728 (2015).
- Liang H, Huang W, Wang Y, et al. Overexpression of MiR-146a-5p upregulates lncRNA HOTAIR in triple-negative breast cancer cells and predicts poor prognosis. Technol Cancer Res Treat 18, 1533033819882949. (2019).
- Wu Y, Liu J, Zheng Y, et al. Suppressed expression of long non-coding RNA HOTAIR inhibits proliferation and tumourigenicity of renal carcinoma cells. Tumour Biol 35, 11887-11894 (2014).
- Xavier-Magalhães A, Gonçalves CS, Fogli A, et al. The long non-coding RNA HOTAIR is transcriptionally activated by HOXA9 and is an independent prognostic marker in patients with malignant glioma. Oncotarget9(21), 15740 (2018).
- Huang K, Sun J, Yang C, et al. HOTAIR upregulates an 18-gene cell cycle-related mRNA network in glioma. Int J Oncol 50(4):1271-1278 (2017).
- Zhang JX, Han L, Bao ZS, et al. Chinese Glioma Cooperative G. HOTAIR, a cell cycle-associated long noncoding RNA and a strong predictor of survival, is preferentially expressed in classical and mesenchymal glioma. Neuro Oncol 15,1595-1603 (2013).
- Pojo M, Gonçalves CS, Xavier-Magalhães A, et al. A transcriptomic signature mediated by HOXA9 promotes human glioblastoma initiation, aggressiveness and resistance to temozolomide. Oncotarget 6(10), 7657 (2015).
- Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature455(7216), 1061-1068 (2008).
- Chen YA, Bian Y, Zhao S, et al. Suppression of PDCD4 mediated by the long non-coding RNA HOTAIR inhibits the proliferation and invasion of glioma cells. Oncol Lett 12(6), 5170-5176 (2016).
- Xia S, Ji R,0020Zhan W. Long noncoding RNA Papillary Thyroid Carcinoma Susceptibility Candidate 3 (PTCSC3) inhibits proliferation and invasion of glioma cells by suppressing the Wnt/β-catenin signaling pathway. BMC Neurol 17,1-1(2017).
- Xiao D, Cui X, Wang X. LncRNA PTCSC3 inhibits cell proliferation in laryngeal squamous cell carcinoma by down-regulating lncRNA HOTAIR. Biosci Rep 39(6), BSR20182362 (2019).
- Visconti R, Della Monica R, Grieco D. Cell cycle checkpoint in cancer: A therapeutically targetable double-edged sword. J Exp Clin Cancer Res 35, 1-8 (2016).
- Bucher N, Britten CD. G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer. Br J Cancer 98(3), 523-528 (2008).
- Kotake Y, Nakagawa T, Kitagawa K, et al. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15INK4B tumor suppressor gene. Oncogene 30(16), 1956-1962 (2011).
- Tripathi V, Shen Z, Chakraborty A et al. Long noncoding RNA MALAT1 controls cell cycle progression by regulating the expression of oncogenic transcription factor B-MYB. PLoS Genet 9(3), e1003368 (2013).
- Ke J, Yao YL, Zheng J, et al. Knockdown of long non-coding RNA HOTAIR inhibits malignant biological behaviors of human glioma cells via modulation of miR-326. Oncotarget 6(26), 21934 (2015).
- Stangeland B, Mughal AA, Grieg Z, et al. Combined expressional analysis, bioinformatics and targeted proteomics identify new potential therapeutic targets in glioblastoma stem cells. Oncotarget 6(28), 26192 (2015).
- Liang ML, Hsieh TH, Ng KH, et al. Downregulation of miR-137 and miR-6500-3p promotes cell proliferation in pediatric high-grade gliomas. Oncotarget 7(15):19723-19738 (2016).
- Tang Y, Dai Y, Grant S, et al. Enhancing CHK1 inhibitor lethality in glioblastoma. Cancer Biol Ther 13(6):379-388 (2012).
- Varambally S, Dhanasekaran SM, Zhou M, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419(6907), 624-629 (2002).
- Zhang R, Wang R, Chang H, et al. Downregulation of Ezh2 expression by RNA interference induces cell cycle arrest in the G0/G1 phase and apoptosis in U87 human glioma cells. Oncol Rep 28(6), 2278-2284 (2012).
- Jalali S, Bhartiya D, Lalwani MK, et al. Systematic transcriptome wide analysis of lncRNA-miRNA interactions. PloS one 8(2), e53823 (2013).
- Liu XH, Sun M, Nie FQ, et al. lncRNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer 13, 1-4 (2014).
- Zhang H, Cai K, Wang J, et al. MiR-7, inhibited indirectly by lincRNA HOTAIR, directly inhibits SETDB1 and reverses the EMT of breast cancer stem cells by downregulating the STAT3 pathway. Stem cells 32(11):2858-2868 (2014).
- Sun G, Wang Y, Zhang J, et al. MiR‐15b/HOTAIR/p53 form a regulatory loop that affects the growth of glioma cells. J Cell Biochem 119(6):4540-4547 (2018).
- Yuan J, Xiao G, Peng G, et al. MiRNA-125a-5p inhibits glioblastoma cell proliferation and promotes cell differentiation by targeting TAZ. Biochem Biophys Res Commun 457(2), 171-176 (2015).
- Tang L, Shen H, Li X, et al. MiR-125a-5p decreases after long non-coding RNA HOTAIR knockdown to promote cancer cell apoptosis by releasing caspase 2. Cell Death Dis 7(3), e2137(2016).
- Jiang Y, Zhang Q, Bao J, et al. Schisandrin B inhibits the proliferation and invasion of glioma cells by regulating the HOTAIR–micoRNA-125a–mTOR pathway. Neuroreport 28(2), 93-100 (2017).
- Rao SA, Arimappamagan A, Pandey P, et al. miR-219-5p inhibits receptor tyrosine kinase pathway by targeting EGFR in glioblastoma. PloS one 8(5), e63164 (2013).
- Li H, Guan C. HOTAIR inhibits the proliferation of glioblastoma cells by targeting miR-219. Cancer Biomark 28(1), 41-47 (2020).
- Plate KH, Breier G, Weich HA, et al. Vascular endothelial growth factor and glioma angiogenesis: Coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer 59(4), 520-529.
- Azab MA, Alomari A, Azzam AY. Featuring how calcium channels and calmodulin affect glioblastoma behavior A review article. Cancer Treat Res Commun 25,100255 (2020).
- Fu WM, Lu YF, Hu BG, et al. Long noncoding RNA Hotair mediated angiogenesis in nasopharyngeal carcinoma by direct and indirect signaling pathways. Oncotarget 7(4), 4712 (2016).
- Subramaniam SR, Federoff HJ. Targeting microglial activation states as a therapeutic avenue in Parkinson’s disease. Front Aging Neurosci 9,176 (2017).
- Cata JP, Bhavsar S, Hagan KB, et al. Intraoperative serum lactate is not a predictor of survival after glioblastoma surgery. J Clin Neurosci 43, 224-228 (2017).
- Jin Z, Jin RH, Ma C, et al. Serum expression level of miR-504 can differentiate between glioblastoma multiforme and solitary brain metastasis of non-small cell lung carcinoma. J buon 22(2), 474-480 (2017).
- Vietheer JM, Rieger J, Wagner M, et al. Serum concentrations of Glial Fibrillary Acidic Protein (GFAP) do not indicate tumor recurrence in patients with glioblastoma. J Neurooncol 135(1), 193-199 (2017).
- Tan SK, Pastori C, Penas C, et al. Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol cancer 17, 1-7 (2018).
- Cantile M, Scognamiglio G, Marra L, et al. HOTAIR role in melanoma progression and its identification in the blood of patients with advanced disease. J Cell Physiol 232(12), 3422-3432 (2017).
- Wang W, He X, Zheng Z, et al. Serum HOTAIR as a novel diagnostic biomarker for esophageal squamous cell carcinoma. Mol Cancer 16, 1-5 (2017).
- Li Y, Ren Y, Wang Y, et al. A compound AC1Q3QWB selectively disrupts HOTAIR-mediated recruitment of PRC2 and enhances cancer therapy of DZNep. Theranostics 9(16), 4608 (2019).
- Shi J, Lv S, Wu M, et al. HOTAIR‐EZH2 inhibitor AC1Q3QWB upregulates CWF19L1 and enhances cell cycle inhibition of CDK4/6 inhibitor palbociclib in glioma. Clin Transl Med 10(1), 182-198 (2020).
- Filippakopoulos P, Knapp S. Targeting bromodomains: Epigenetic readers of lysine acetylation. Nat Rev Drug Discov 13(5), 337-356 (2014).
- Pastori C, Kapranov P, Penas C, et al. The Bromodomain protein BRD4 controls HOTAIR, a long noncoding RNA essential for glioblastoma proliferation. Proc Natl Acad Sci U S A. 112(27):8326-8331 (2015).
- Li CH, Chen Y. Targeting long non-coding RNAs in cancers: Progress and prospects. Int J Biochem Cell Biol 45(8), 1895-1910 (2013).
- Bruniaux J, Allard-Vannier E, Aubrey N, et al. Magnetic nanocarriers for the specific delivery of siRNA: Contribution of breast cancer cells active targeting for down-regulation efficiency. Int J Pharm 569, 118572 (2019).
- Fang K, Liu P, Dong S, et al. Magnetofection based on superparamagnetic iron oxide nanoparticle-mediated low lncRNA HOTAIR expression decreases the proliferation and invasion of glioma stem cells. Int J Oncol 49(2), 509-518 (2016).
- Zhang L, He A, Chen B, et al. A HOTAIR regulatory element modulates glioma cell sensitivity to temozolomide through long-range regulation of multiple target genes. Genome Res 30(2), 155-163 (2020).
- Yuan Z, Yang Z, Li W, et al. Exosome-Mediated Transfer of Long Noncoding RNA HOTAIR Regulates Temozolomide Resistance by miR-519a-3p/RRM1 Axis in Glioblastoma. Cancer Biother Radiopharm 24 (2020).
- Sa L, Li Y, Zhao L, et al. RETRACTED: The Role of HOTAIR/miR-148b-3p/USF1 on Regulating the Permeability of BTB. Front Mol Neurosci 10, 194 (2017).
- Zhou X, Ren Y, Zhang J, et al. HOTAIR is a therapeutic target in glioblastoma. Oncotarget 6(10), 8353 (2015).
- Wang G, Li Z, Tian N, et al. miR‑148b‑3p inhibits malignant biological behaviors of human glioma cells induced by high HOTAIR expression. Oncol Lett 12(2), 879-886 (2016).
- Bian EB, Ma CC, He XJ, et al. Epigenetic modification of miR-141 regulates SKA2 by an endogenous ‘sponge’HOTAIR in glioma. Oncotarget 7(21), 30610 (2016).
- Xavier-Magalhães A, Oliveira AI, de Castro JV, et al. Effects of the functional HOTAIR rs920778 and rs12826786 genetic variants in glioma susceptibility and patient prognosis. J Neurooncol 132, 27-34 (2017).
- Zhao WH, Yuan HY, Ren XY, et al. Association between expression of HOTAIR and invasiveness of gliomas, and its predictive value. Adv Clin Exp Med 28(9), 1179-1183 (2019).
- Zhang J, Chen G, Gao Y, et al. HOTAIR/miR‐125 axis‐mediated Hexokinase 2 expression promotes chemoresistance in human glioblastoma. J Cell Mol Med 24(10), 5707-5717 (2020).