Review Article - Neuropsychiatry (2018) Volume 8, Issue 1
Neurosteroids and Oxysterols as Potential Therapeutic Agents for Glaucoma and AlzheimerâÃâ¬Ãâ¢s Disease
- *Corresponding Author:
- Makoto Ishikawa
Department of Ophthalmology, Akita University Graduate School of Medicine, 1-1-1 Hondo, Akita 010-8543, Japan
Tel: +81-18-834-1111
Fax: +81-18-836-2621
Abstract
Abstract
Glaucoma is one of the most frequent causes of visual impairment worldwide and involves selective damage to retinal ganglion cells (RGCs) resulting in degeneration of neural pathways connecting retina to visual cortex. It is of interest that similarities in pathological changes have been described in Alzheimer’s disease (AD), the most common cause of progressive memory loss and dementia in older people. Accumulation of amyloid-beta (Abeta) and hyperphosphorylated tau is thought to contribute to apoptotic neuronal death in Alzheimer’s disease, and similar changes have been linked to apoptotic RGC death in glaucoma. Both glaucoma and Alzheimer’s disease also suffer from a lack of effective treatments prompting a search for novel therapeutic interventions. Neurosteroids (NSs) (including oxysterols) are endogenous molecules synthesized in the nervous system from cholesterol that can modulate glutamate and GABA receptors, the primary mediators of fast excitatory and inhibitory neurotransmission in the brain, respectively. Because changes in the glutamate and GABA neurotransmitter systems contribute to the pathogenesis of AD and glaucoma, NSs are possible therapeutic targets for these disorders. In this review, we present recent evidence supporting pathological links between Alzheimer’s disease and glaucoma, and focus onthe possible role of NSs in these diseases and how NSs might be developed for therapeutic purposes.
Keywords
Glaucoma, Alzheimer disease, Neuroprotection, Neurosteroid, Allopregnanolone, 24(S)- Hydroxycholesterol, NMDA receptors, GABAA receptors
Introduction
Despite its peripheral location, the retina is actually part of the central nervous system (CNS). Embryologically, the retina is derived from an out-pocketing of the neural tube, which is the precursor to the CNS including the brain and spinal cord [1]. Therefore, pathological processes and therapeutic strategies affecting the brain can be applicable to the retina [2]. Glaucoma is considered to be a neurodegenerative disease, and is one of the primary causes of visual impairment worldwide among older people [3,4]. Retinal ganglion cells (RGCs) transmit visual information from photoreceptor cells to the brain, and glaucoma is characterized by apoptotic death of RGCs [5]. Glaucoma and neurodegenerative disease such as Alzheimer’s disease, the most common cause of progressive memory loss and dementia in older people, have several factors in common such as selective loss of neurons and deposition of amyloid-β peptide or highly soluble microtubuleassociated protein (tau) [6,7]. Family history is also a significant risk factor for both diseases [8].
Neurosteroids including oxysterols are endogenously generated from cholesterol within the nervous system, and effectively modulate major neurotransmitter systems (particularly the glutamatergic and GABAergic systems) [9]. Although the precise pathological mechanisms have remained unclear, recent evidence indicates that neurosteroids may contribute to the pathogenesis of both glaucoma and Alzheimer’s disease [10]. In this review, we will discuss the association of glaucoma and Alzheimer’s disease, and focus on the possible role of neurosteroids in these illnesses, and how neurosteroids might be developed for therapeutic use.
Glaucoma and Alzheimer’s Disease
Based on the results of retrospective chart review, Alzheimer’s disease patients have a significantly increased incidence of glaucoma (24.5%) compared to controls (6.5%) [11]. A subsequent study from the same research group [12] found similar results with a prevalence of glaucoma in Alzheimer’s disease patients and controls of 25.9% and 5.2%, respectively. Consistent with this, a cross sectional epidemiologic study revealed that the prevalence of primary open angle glaucoma (POAG), the most common type of glaucoma, was 23.8% in Alzheimer’s disease patients compared to 9.9% in controls (p = 0.0002) [13].
Sartucci et al. [14] have shown an impairment of visual responses arising from the magnocellular pathway of visual processing using pattern electroretinograms and visual evoked potentials in patients with Alzheimer’s disease, suggesting that the largest RGCs (M cells) are primarily affected in the illness. M-cells are more sensitive to contrast stimuli than other types of RGCs, and known to mediate specific visual functions for detecting quick motion. M-cells have larger receptive field, and make up approximately 5-10% of all RGCs.
Retinal involvement in early stage Alzheimer’s disease is also suggested by results from imaging studies using optical coherence tomography (OCT). Parisi et al. [15] examined seventeen Alzheimer’s disease patients and fourteen age-matched controls by OCT, and reported that in Alzheimer’s disease patients, there was a significant reduction of nerve fiber layer (NFL) thickness compared to controls (59.5 ± 16.70 μm vs. 99.9 ± 8.95 μm, p<0.01). This morphological abnormality correlated with retinal dysfunction as revealed by abnormal pattern electroretinogram recordings [15]. A significant reduction of macular volume in Alzheimer’s disease patients was also reported compared to controls using OCT (p<0.05), and the reduction in macular volume was related to the severity of cognitive impairment [16].
Conversely, the incidence of Alzheimer’s disease in patients having glaucoma has also been investigated. Lin et al. [17] retrospectively analyzed a population-based cohort consisting of patients older than 60 years with POAG to investigate the risk developing Alzheimer’s disease. After 8 years following a diagnosis of POAG, the prevalence rates of Alzheimer’s disease were 2.85 (95% CI: 2.19-3.70) and 1.98 (95% CI: 1.68-2.31) per 1000 person-years in patients with and without POAG, respectively. Kaplan-Meier survival curves and the logrank test revealed that POAG patients had an increased risk of developing Alzheimer’s disease compared to controls without POAG (log-rank test, P=0 .0189).
A population-based longitudinal cohort study (Three‑City‑Bordeaux‑Alienor study) showed that participants with POAG have a four-fold increased risk of developing dementia (odds ratio=3.9, 95% confidence interval (CI) =1.5– 10.4, p=0.0054) during a 3-year follow-up period [18]. An increased risk of dementia was also associated with 2 parameters of glaucoma progression (increase in vertical cup to disk ratio and decrease in rim to disk ratio). Recently, Lai et al. [19] calculated the odds ratio for developing Alzheimer’s disease as 1.50 in subjects with glaucoma (95% CI=1.19-1.89) using a multivariable unconditional logistic regression model.
Other studies, however, have reported opposite results concerning subsequent risk of developing Alzheimer’s disease in patients with glaucoma. Ou et al. [20] examined a nationally representative sample of persons in the U.S. with POAG newly diagnosed prior to 1994 and determined whether these persons have subsequent risk of developing Alzheimer’s disease or other dementia (Alzheimer’s disease/dementia) compared to a well-matched control population without glaucoma over a 14 year follow up period. Their analysis revealed that individuals older than 68 years diagnosed with POAG have a decreased risk of Alzheimer’s disease compared to control patients who were not diagnosed with POAG. In a nationwide case register study of patients with hospital admission or outpatient contact during the period from 1977 to 2001 in Denmark, the rate of subsequent Alzheimer’s disease for patients with a diagnosis of POAG was compared with the rate for patients with primary angle-closure glaucoma (PACG), cataract, and osteoarthritis (OA) along with the rate for the general population [21]. This study reported that patients having POAG showed no increased risk of developing Alzheimer’s disease compared with the other groups. Therefore, the association of glaucoma with Alzheimer’s disease remains equivocal based on epidemiological data.
Histologically, Hinton et al. [22] showed remarkable decreases in optic nerve fiber density (from 50% to 33%) in 8 of 10 postmortem Alzheimer’s disease patients compared with controls. The retinas in Alzheimer’s disease patients showed disappearance of RGCs and appearance of reactive gliosis in the ganglion cell layer. These histological changes in the retina of Alzheimer’s disease patients were markedly different from the findings in 10 age-matched controls. However, amyloid was not detected in the retinal and optic nerve. Blanks et al. [23] analyzed RGC density in 9 Alzheimer’s disease patients, and found that a 43% decrease in RGC density occurred in the fovea. In the more peripheral region of the macula, RGC decreased by 24-26%. Sadun and Bassi [24] reported similar degeneration in the RGCs. Optic nerves from ten patients with Alzheimer’s disease were histologically examined and compared with those from 5 age-matched controls. Morphometric analysis suggested that optic nerves in Alzheimer’s disease showed predominant loss of the largest RGCs (M-cells). These results were consistent with results obtained using pattern electroretinograms and visual evoked potentials [14]. These results suggest that glaucoma and Alzheimer’s disease likely share some common pathogenetic features.
In the next sections, we focus on the pathological roles of neurotoxic factors such as amyloid-β peptide and tau, both of which contribute to neuronal degeneration in Alzheimer’s disease and glaucoma [25-27].
▪ Amyloid-dependent mechanisms in glaucoma
Generation of neurotoxic amyloid-β peptide from sequential amyloid precursor protein (APP) proteolysis is a crucial step in the pathogenesis of Alzheimer’s disease. APP is a transmembrane protein preferentially expressed in the brain, and metabolized by proteases including γ- and β-secretase complexes. Amyloid-β peptide is a polypeptide containing 37 to 49 amino acid residues, and its amyloid fibrillar form is the main component of the senile plaques that are characteristic of Alzheimer’s disease lesions [28].
Both Alzheimer’s disease and glaucoma are associated with apoptotic neuronal degeneration [29-31], and the activation of caspases is a central mechanism driving this cell death [29]. Caspase-3 induces abnormal APP processing and increases expression of amyloid-β peptide in RGCs [32] along with decreases in vitreous amyloid-β peptide levels (consistent with retinal amyloid-β peptide deposition) [33]. These findings suggest that APP exerts neuroprotection for RGCs, while APP fragments such as amyloid-β peptide are toxic in glaucoma. Presenilin 1 (PS1) and PS2 proteins play important roles in caspase-3- mediated abnormal APP processing. Most cases of early-onset familial Alzheimer’s disease are caused by mutations in the genes encoding the PS1 and PS2 proteins, both of which undergo regulated endoproteolytic processing [34]. Consistent with this, Ning et al. [35] demonstrated significant age-dependent deposition of amyloid-β peptide in the retinal nerve fiber layer (NFL) and an agedependent increase in TUNEL-positive RGC using two strains of bi-transgenic APP/presenilin 1 (PS1) mice. Amyloid-β peptide therefore may offer a novel therapeutic target in the treatment of glaucomatous neurodegeneration. An α2 adrenergic receptor agonist (brimonidine), used to lower intraocular pressure, has been recently reported to prevent RGC death through its effects on the amyloid-β peptide pathway (reduction of amyloid-β peptide) and soluble APPα (increase in APPα) in an in vivo rat glaucoma model [36].
Neuroinflammation is another possible contributor to the pathogenesis of Alzheimer’s disease [37]. An increase in amyloid-β peptide deposition induces the activation of astrocytes as well as microglia [38]. Activated glia can secrete inflammatory chemical mediators and induce chronic inflammation. This inflammation is induced by amyloid-β peptide deposition, and accelerates to generate more amyloid-β peptide while weakening mechanisms responsible for its elimination [39].
These results were interpreted to indicate that agents affecting the amyloid-β peptide pathway may become possible neuroprotectants in glaucoma.
Guo et al. [40] examined three ways to target amyloid-β peptide in experimental glaucoma, including: (i) administration of a beta-secretase inhibitor to reduce amyloid-β peptide, (ii) administration of anti- amyloid-β peptide antibody to inhibit amyloid-β peptide deposition, and (iii) administration of Congo red to inhibit amyloid-β peptide aggregation and neurotoxic effects. These authors found that a combination of triple agents was most effective compared to other possible combination therapies.
However, the clinical outcomes of these amyloid-β peptide-based therapies have been disappointing so far [41]. It is possible that these agents may not target the misfolded Tau and neurofibrillary tangles (NFTs) that contribute significantly to the pathology of Alzheimer’s disease [42].
▪ Tau-dependent mechanisms in glaucoma
Tau protein is typically localized to axons where it binds to and stabilizes microtubules [43] in physiological conditions [43,44]. Aggregation of hyperphosphorylated tau induces impairments of axonal transport and neuronal degeneration [45]. NFTs are pathological hallmarks of Alzheimer’s disease [46] and are comprised of intracellular filamentous hyperphosphorylated tau [47].
In the retina, tau is physiologically present and involved in axonal development and survival of RGCs [48]. Hyperphosphorylation of tau is increased in the retina of elderly persons [49]. In the vitreous from 8 glaucoma patients, tau levels were found to be quite high (113.6 ± 43.1 pg/ml) compared to levels in 13 control subjects (3.3 ± 3.2 pg/ml) despite low levels of betaamyloid [33]. In surgically excised specimens from glaucoma patients, hyperphosphorylated tau, which is not seen in control specimens, is detected in the inner nuclear layer, most likely in horizontal cells [50]. In a rat glaucoma model with unilateral intraocular pressure (IOP) elevation, a marked tau increase is observed in the inner plexiform layer rather than GCL, suggesting that the primary site of tau accumulation is in RGC dendrites [51].
The use of transgenic mouse models harboring gene mutations (APP, PS1, or P301S) that cause familial early-onset Alzheimer’s disease confirmed either the development of amyloid-β peptide plaques and/or neurofibrillary tangles with subsequent local neuroinflammation, characterized by microglial infiltration, astrogliosis, and disruption of inner parts of the retina [52,53].
In double transgenic APP/PS1 mice, hyperphosphorylation of retinal tau is accompanied by a preceding increase in calpain [54], and hypoxia induces abnormal calpain activation, which in turn increases ER stress-induced apoptosis in Alzheimer’s disease pathogenesis [55]. Prominent activation of microglia was also observed in this transgenic model [56]. Microgliosis is induced even in the early phase of Alzheimer’s disease, and is involved in the degradation of accumulated amyloid-β peptide. Additionally, microgliosis can induce neuronal inflammation, which may contribute to functional alterations in electroretinograms [57].
In the P301S mutant human tau transgenic mice, hyperphosphorylation and aggregation of tau were associated with reduced axonal transport in the optic nerve [58,59].
In Tg2576 transgenic mice, which carry a transgene derived from a Swedish family with early onset Alzheimer’s disease, hyperphosphorylated tau is observed in the vicinity of amyloid-β peptide deposition [60]. In Tg4510 transgenic mice with the P301L mutation [61], activation of caspase induces tangle formation in neurons, and while tangle-bearing neurons are long-lived, soluble extracellular tau is remarkably toxic. Although soluble extracellular tau in the CSF can be detected in healthy individuals, Alzheimer’s disease patients shows significant amounts of this protein [62,63]. Soluble extracellular tau is also toxic to cell-to-cell communication, disrupting synaptic plasticity and resulting in subsequent cognitive impairment [64-66]. Additionally, oligomeric extracellular tau can bind to APP [67], and APP knock-out mice were resistant to oligomeric extracellular tauinduced impairments in long term potentiation (LTP). Thus, these authors suggested that APP may be a therapeutic target against Alzheimer’s disease. There is increasing interest in developing tau-based therapies for treating tauopathies including Alzheimer’s disease [68].
Transcription factor EB (TFEB) is a molecule that plays a central role in cellular degradative processes. TFEB effectively reduces neurofibrillary tangle pathology and rescues behavioral and synaptic deficits and neuronal degeneration in the Tg4510 transgenic mouse [69]. Phosphatase and tensin homolog is a direct target of TFEB and is required for TFEB-dependent aberrant Tau clearance. The specificity and efficacy of TFEB in mediating the clearance of toxic Tau species makes it an attractive therapeutic target for treating tauopathies. Consistent with this, selenomethionine (Se-Met), a major bioactive form of selenium (Se) with significant antioxidant capacity, has recently been reported to reduce the levels of total tau and hyperphosphorylated tau and to ameliorate cognitive deficits in younger triple transgenic Alzheimer’s disease mice (three mutations associated with familial Alzheimer’s disease genes; APP Swedish, MAPT P301L, and PSEN1 M146V) (3xTg-AD mouse) [70].
▪ ApoE-dependent mechanisms in glaucoma
Apolipoprotein E (ApoE) is a very low-density lipoprotein, responsible in part for removing cholesterol from the bloodstream. In humans, the APOE gene exists as three different polymorphic alleles (ε2, ε3 and ε4), which engender six different genotypes (ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4 and ε4/ε4). ε3 is the most common allele (77%) and ε2 the least (8%) common allele [71]. APOE polymorphisms cause changes in cholesterol transport. The ε4 allele frequency is about 15% in general populations, but is ca. 40% in Alzheimer’s disease patients. Having one ε4 allele increases the risk of developing Alzheimer’s disease 3- to 4-fold compared to controls without ε4 alleles [72,73]. In addition, receptors recognizing ApoE are also widely expressed in the Alzheimer’s disease brain [74,75]. Although ApoE mediates amyloid-β peptide clearance by binding amyloid-β peptide and forming a stable complex, ApoE may also stimulate amyloid-β peptide aggregation and amyloid deposition, as well as tau hyperphosphorylation [76-79]. Moreover, among the mechanisms that might explain the effects of ApoE on the brain of Alzheimer’s disease subjects, ApoE ε4 and its receptors are also reported to be involved in APP trafficking and its processing to amyloid-β peptide [80]. Additionally, ApoE may mediate amyloid-β peptide cell internalization, by binding to the LDL receptor-related protein (LRP) [81].
A number of epidemiological studies also report that individuals with hypercholesterolemia have increased risk of developing Alzheimer’s disease. The risks to develop Alzheimer’s disease are significantly increased by having the APOE ε4 genotype, which may influence cholesterol metabolism and the formation of cholesterol oxidation products, known as oxysterols [82,83]. Inoue et al. [84] reported that glaucoma patients have elevated levels of ApoE in the aqueous humor using a multiplex bead immunoassay. Although several reports suggest that APOE genotype may not be a risk factor for glaucoma [85-87], the association of APOE allelic isoforms (ε2, ε3, and ε4) has been investigated as a possible risk for different forms of glaucoma [88]. POAG patients with lower IOP (38.0%) and higher IOP (34.2%) had higher expression of ε4 alleles compared to controls (18.9%). The odds of ε4 carriers having normal tension glaucoma were also significantly greater than for ε3 homozygotes (odds ratio 2.45, 95% confidence interval [1.02- 5.91]). Thus, inheritance of the ε4 allele appears to increase risk for glaucoma.
Furthermore, two types of single-nucleotide polymorphisms (SNPs) of APOE, APOE(- 219G) and APOE(-491T), are associated with Alzheimer’s disease, and modify the phenotype of POAG [89]. The presence of APOE(- 219G) increased cupping of the optic disc, and exacerbated visual field impairments. APOE(- 491T) interacts strongly with a SNP in the MYOC promoter, and is associated with IOP elevation or resistance to IOP-lowering therapy. Despite these positive reports, it is important to note that there are other results indicating that APOE genotypes do not constitute risk factors for developing glaucoma [85-87].
Furthermore, two types of single-nucleotide polymorphisms (SNPs) of APOE, APOE(- 219G) and APOE(-491T), are associated with Alzheimer’s disease, and modify the phenotype of POAG [89]. The presence of APOE(- 219G) increased cupping of the optic disc, and exacerbated visual field impairments. APOE(- 491T) interacts strongly with a SNP in the MYOC promoter, and is associated with IOP elevation or resistance to IOP-lowering therapy. Despite these positive reports, it is important to note that there are other results indicating that APOE genotypes do not constitute risk factors for developing glaucoma [85-87].
Table 1 summarizes the common characteristics between Alzheimer’s disease and glaucoma.
| Methods | Outcome measure | Results and references |
|---|---|---|
| Epidemiology | Occurrence of glaucoma in AD patients Occurrence of AD in glaucoma patients | Increase [11-13] Increase [17-19] No increase [20,21] |
| Elecroretinogram | RGCs (especially M cells) in AD | Affected [14] |
| Optical coherence tomography | Nerve fiber layer thickness in AD | Significantly decreased [15,16] |
| Histology | Nerve fiber density Disappearance of RGC Reactive gliosis RGC density M-cells |
Decrease in both AD and glaucoma [22] Disappearance of RGCs in both AD and glaucoma [22] Induction of reactive gliosis in the GCL in both AD and glaucoma [22] Decrease in both AD and glaucoma [23] Decrease in both AD and glaucoma [24] |
Table 1: Common characteristics between glaucoma and Alzheimer’s disease.
Table 2 summarizes the common pathological features between glaucoma and Alzheimer’s disease.
| Mechanism | Outcome measure | Results and references |
|---|---|---|
| 1. Apoptosis | Apoptotic neuronal degeneration Caspase | Activated in glaucoma [29-31] Activated in glaucoma [29] |
| 2. Amyloid-dependent mechanism | Amyloid β peptide (Abeta) | Increased in glaucoma [32] Caspase-3-mediated abnormal APP processing induced apoptosis in glaucoma [34,35] |
| Presenilin 1 (PS1) and PS2 | Gene mutataion induced abnormal amyloid precursor protein, and activated caspase-3-mediated abnormal APP processing in glaucoma model [34] | |
| Amyloid precursor protein (APP) | Neuroprotective in glaucoma model [33] | |
| 3. Tau-dependent mechanism | Tau | High level in vitreous of glaucoma patient [33] Accumulated in the RGC axon in the rat glaucoma model [51] |
| Hyperphosphorylation of tau | Highly expressed in the aging human retina [49] Mainly localized in the horizontal cell in glaucoma patient retina [50] |
|
| 4. Induction of Transgenic mouse | Characteristics of APP/presenilin 1 mouse | Age-dependent deposition of Abeta in the NFL [35] Age dependent increase in TUNEL-positive RGC [35] Microglial infiltration, astrogliosis of inner retina [52,53] |
| Characteristics of P301S mutant human tau transgenic mice | Reduced axonal transport in the optic nerve [58,59] |
Table 2: Common patholohical features between glaucoma and Alzheimer’s disease.
Neurosteroids and Alzheimer’s disease
Neurosteroids are endogenous modulators generated in the nervous system. Neurosteroids are synthesized from cholesterol and are potent modulators of excitatory glutamatergic and inhibitory GABAergic neurotransmitter systems [91,92]. As examples, 24(S)-HC and allopregnanolone (AlloP) are positive modulators of glutamate N-methyl-D-aspartate receptors (NMDARs) and GABAA receptors, respectively. Recently, neurosteroids have been considered as putative therapeutic agents for multiple neurodegenerative disorders [93-95].
▪ Oxysterols
Oxysterols are divided into two classes: sterol-ring oxidized oxysterols such as 7-ketochoelsterol or 7α/β-hydroxycholesterol, and side-chain oxidized oxysterols such as 24(S)-HC, 25-hydroxycholesterol (25-HC), or 27-hydroxycholesterol (27-HC). In general, the former class is produced by reactive oxygen species (ROS), and the latter is enzymatically generated from cholesterol [96].
25-HC appears to be a weak partial agonist at NMDARs and has antagonizing effects against 24(S)-HC on NMDARs [97]. It has been reported that 25-HC induces apoptosis of neuronal cells at high concentrations [98]. Cholesterol 25-hydroxylase is the enzyme that catalyzes formation of this oxysterol. In Alzheimer’s disease, 25-HC enhances amyloid-β peptide insertion into cell membranes (the peptide penetration into membrane by 25- HC was confirmed by experiments using artificial model membranes [99]), thus affecting mitochondria or endosomes, and subsequently leading to oxidative stress-mediated apoptosis [100]. Recently, 25-HC was reported to induce neuronal death in amyotrophic lateral sclerosis (ALS), another major neurodegenerative illness, via effects on neuronal apoptosis. Studies examining the potential role of 25-HC in ALS may be instructive for understanding the role of oxysterols in other neurodegenerative conditions such as Alzheimer’s disease and glaucoma. Kim et al. [101] demonstrated that serum 25-HC concentrations were significantly higher in ALS patients (5.39 ± 1.94 ng/ml) than in controls (4.27 ± 1.18 ng/ml). Serum 25-OH levels were negatively correlated with ALSFRSr score (the revised ALS functional rating scale score) (-0.591; 95% CI -5.385, -0.673; p = 0.014) by multivariate regression analysis. Additionally, 25-HC also triggered neuronal apoptosis by activation of the glycogen synthase kinase-3 beta (GSK-3β)/ liver X receptor (LXR) pathways in in vitro ALS models.
27-HC is a metabolite of cholesterol formed mainly in the periphery that is capable of passing into the brain. In contrast with 24S-HC, an effective inhibitor of the amyloid-β peptide formation [102], 27-HC increases amyloid-β peptide and oxidative stress in in vitro [103]. To date most information about 27-HC in neurodegenerative disorders comes from studies of ALS. A large and comprehensive genome-wide association study was performed in an attempt to find novel genetic variants and candidate genes for sporadic ALS, and reported that the gene coding for the enzyme CYP27A1 (cholesterol 27-hydroxylase) that converts cholesterol into 27-HC, may be a risk factor for sporadic ALS [104]. Abdel-Khalik et al. [105] quantified non-esterified cholesterol and its metabolites in CSF and serum from ALS patients compared with a group of healthy controls using LC/MS. Comparing results of cholesterol metabolites between CSF and serum, it was suggested that impaired activity of CYP27A1 may lead to a failure of the CNS to remove excess cholesterol, which may in turn be toxic to neuronal cells, compounded by a reduction in neuroprotective 3β,7α- dihydroxycholest-5-enoic acid and other LXR ligands. Mateos et al. [106] demonstrated that 27-HC decreases activityregulated cytoskeleton-associated protein (Arc) levels as well as NMDAR expression in rat primary hippocampal neurons. Arc is thought to contribute to the molecular mechanisms underlying learning and memory. Findings with 27-HC noted above promoted interest in measuring plasma levels of 27-HC in ALS patients. Wuolikainen et al. [107] measured the level of 27-HC using isotope dilution mass spectrometry and found that 27-HC was significantly lower in male ALS patients (p = 0.008) compared to male controls (fold ALS/controls: 0.81). The lower levels could not be explained by a correlation with cholesterol. When testing 27-HC in males normalized against BMI, the difference was still significant (p = .03).
Cholesterol is mainly removed from the brain by enzymatic conversion into 24(S)-HC, which diffuses across the blood–brain barrier. Therefore, 24(S)-HC is proposed to be a marker of brain cholesterol metabolism. 24(S)-HC is synthesized from cholesterol by CYP46A1 (cholesterol 24-hydroxylase), a brain and neuron specific enzyme, coded by CYP46A1 gene. Although 24(S)-HC involvement in the etiology of Alzheimer’s disease has also been investigated [108], the association of 24(S)-HC with Alzheimer’s disease remains equivocal. There are a number of reports showing that 24(S)-HC levels are elevated or lowered in CSF and plasma of Alzheimer’s disease patients as compared to control [109-111], although some of these discrepancies may relate to stage of illness. Papassotiropoulos et al. [109] reported that 24(S)-HC in CSF is significantly elevated at early stages of Alzheimer’s disease compared to control (Alzheimer’s disease vs. control = 2.6 ± 1.1 ng/ml vs. 1.6 ± 0.6 ng/ml, p<0.001) using combined gas-chromatography and mass spectrometer. It is possible that elevated cholesterol levels induced by myelin destruction causes an increase in 24(S)-HC level. By contrast, patients with advanced Alzheimer’s disease had significantly reduced 24(S)-HC levels in the plasma [112]. As Alzheimer’s disease progresses, 24S-HC levels in plasma and CSF decline, possibly reflecting extensive neuronal loss.
CYP46A1 may be preferentially expressed in degenerating neurites surrounding senile plaque in brains of Alzheimer’s disease patients [102]. Furthermore, association of CYP46A1 polymorphisms with risk of Alzheimer’s disease and elevated amyloid-β peptide load has been reported [113,114]. These results suggest that 24(S)-HC abnormally synthesized via CYP46A1 may be more directly involved in Alzheimer’s disease pathogenesis.
Conversely, upregulation of CYP46A1 is also found to be neuroprotective in animal models of Huntington’s disease, another major neurodegenerative illness [115]. Under physiological conditions, 24(S)-HC derived from neurons may signal astrocytes to increase production of lipidated ApoE particles in order to supply neurons with cholesterol during synaptogenesis or neuritic remodeling [116]. Moreover, alterations in the transcriptional regulation role of 24(S)-HC on ApoE-mediated cholesterol efflux may affect the progression of neurodegenerative diseases including Alzheimer’s disease [116]. Although there is still controversy, CYP46A1 and 24(S)-HC have possibilities to exert neuroprotection in neurodegenerative diseases.
▪ Allopregnanolone
AlloP is an endogenous neurosteroid synthesized in the CNS from cholesterol, and a potent and effective positive modulator of the major inhibitory neurotransmitter, GABA. Previous studies reported that astrocytes or other glial cells synthesized AlloP under pathological conditions [117-119]. Recently, increasing evidence indicates that principal excitatory neurons synthesize GABAergic neurosteroids [120]. In the process of endogenous AlloP synthesis, cholesterol translocation to mitochondrial inner membrane by translocator protein 18 kD (TSPO) [121] and the catalytic reaction by 5α-reductase (5aRD) [122,123] are considered rate-limiting steps. AlloP potentiates the activity of GABAA receptors [124], and exerts neuroprotective properties both in in vitro cell culture [125-128] and in vivo animal models [129-131]. AlloP also increases myelination [132-134], enhances neurogenesis [135], decreases inflammation [134,136,137], and reduces apoptosis [138-140]. Deficits in AlloP could induce excitotoxicity [141-144], neurodegeneration [145-147], dysregulation in myelination [148,149], neurogenesis [150,151], apoptosis [152,153], and inflammation [154], possibly contributing to Alzheimer’s disease pathophysiology. A reduction of AlloP in temporal cortex of Alzheimer’s disease patients is reported to be negatively associated with disease progression [155].
The presence of the APOE ε4 allele, a risk factor for developing Alzheimer’s disease, is associated with reduction in AlloP levels. AlloP median levels in temporal cortex are significantly decreased in patients homozygous or heterozygous for the APOE ε4 allele (2.86 ng/g, n=36) compared to patients not carrying an APOE ε4 allele (5.23 ng/g, n=44) (Mann Whitney p=0.04) [156].
Wang et al. [135] showed that AlloP significantly increased proliferation of hippocampusderived neural progenitor cells and cerebral cortex-derived neural stem cells. Additionally, AlloP upregulated genes promoting mitosis and downregulated genes that repress cell proliferation. Proliferation by AlloP was inhibited by nifedipine, an antagonist of L-type voltagegated calcium channels, suggesting that AlloP induces proliferation of neural progenitor cells and neural stem cells via a mechanism dependent upon L-type calcium channels. The 3xTgAD mouse carries mutations in two human familial Alzheimer’s disease genes (APPSwe, PS1M146V) and one frontal temporal dementia-linked tau mutation (tauP301L), and manifests agedependent neuropathology that includes both β-amyloid plaques and neurofibrillary tangles. Wang et al. [95] examined the neuroproliferative effects of AlloP in the hippocampal subgranular zone (SGZ) and the reversibility of learning and memory deficits in 3xTgAD transgenic mice. At 3 months of age, AlloP induced a significant increase in progenitor cell proliferation with subcutaneous injection of 10 mg/kg AlloP in 3xTgAD male mice (P < 0.05). AlloP also ameliorated cognitive impairment with learning and memory defects improving to normal levels. These findings suggest that AlloP may be able to prevent or delay cognitive deficits associated with early onset Alzheimer’s disease [155].
The same research group [157] also examined the efficacy of potential treatment regimens with AlloP in the 3xTgAD male mouse model. Three different AlloP treatment regimens were compared; (1) 1/month single injection, (2) 3/week x 3 months and (3) 1/week x 6 months paradigms. In each regimen, AlloP was subcutaneously injected at 10 mg/kg body weight. Results indicate that AlloP administered 1/week for 6 months was most effective in increasing survival of newly generated neurons and simultaneously reducing amyloid-β peptide pathology in 3xTgAD male mice.
Taken together, these findings provide preclinical evidence that AlloP administration has the potential to promote regeneration and decrease amyloid-β peptide production [157].
Glaucoma and Neurosteroids
▪ Oxysterols
We did not find any papers examining an association between 25-HC and glaucoma. The gene expression profile analysis of human trabecular meshwork cells revealed that the 27-HC synthesizing enzyme (CYP27A1) was differentially expressed in response to steroid [158]. However, functional studies of CYP27A1 in glaucoma remain to be done.
Fourgeux et al. [159] found that the frequency of rs754203 SNP in the CYP46A1 intron 2 was significantly higher in POAG patients compared to controls (POAG vs. control = 61.3% vs. 48.3%, p<0.05). However, a subsequent epidemiologic study failed to replicate the association between rs754203 SNP and POAG [160], making it unclear whether there is a genetic association between CYP46A1 and glaucoma.
CYP46A1 specifically located in RGCs, the cells most affected by elevated IOP in glaucoma. This localization of CYP46A1 supports the idea that 24(S)-HC may play a role in glaucoma pathogenesis. Fourgeux et al. [161] reported that CYP46A1 expression was induced early in response to IOP elevation, but CYP46A1 up-regulation is only transient and returned to baseline within a few days in a rodent experimental glaucoma model.
In our previous study, we examined the role of 24(S)-HC in glaucoma using a rat ex vivo model in which pressure was adjusted 10 mmHg and 75 mmHg for 24 hours to simulate physiological IOP and conditions during an acute angle closure glaucoma attack, respectively. This glaucoma model has the advantage that it avoids baseline ischemic degeneration and the influence of circulating steroids [162,163]. In this ex vivo model, we found that 24(S)-HC production is increased in RGCs, while cholesterol concentration is reduced following pressure elevation [164].
Based on studies in hippocampus, Sodero and colleagues [165,166] proposed a model, in which stress- and aging-induced activation of glutamate receptors promotes translocation of CYP46A1 from the endoplasmic reticulum to the cell surface, resulting in acceleration of 24(S)-HC synthesis, and subsequent induction of neuronal survival pathways. Consistent with this, we found that administration of 1 μM 24(S)-HC diminished apoptotic RGC death and axonal injury in the hyperbaric condition in our ex vivo model [164]. We also observed that the CYP46A1 inhibitor, voriconazole, was toxic at 10 mmHg and 75 mmHg [164]. These findings indicate that 24(S)-HC is likely an endogeneous neuroprotectant under glaucomatous conditions.
However, the neuroprotective effects of 24(S)- HC are contradictory, because it has been reported that this oxysterol is a potent allosteric NMDAR modulator in CNS, and would thus be expected to promote excitotoxic damage [167,168]. Previous studies found that 24(S)- HC plays a complex role under pathological conditions [169] with another endogenous oxysterol, 25-HC, dampening the effects of 24(S)-HC on NMDAR [97,170]. In addition, cholestane-3β, 5α, 6β-triol, another oxysterolk is a negative allosteric modulator of NMDARs [170,171]. Thus, further studies are needed to understand the possible interactions among oxysterols under pathological conditions such as pressure elevation.
▪ Allopregnanolone
In our acute model [172,173], pressure elevation induced expression of two key participants in AlloP synthesis, TSPO and 5aRD (mostly type II), in RGC. Furthermore, several other acute neuronal stressors such as acute forced swim [174] ammonia [175], ethanol [176] and acetaldehyde [177], induce AlloP synthesis in the brain. Since IOP elevation is a form of acute stress, the findings with elevated pressure in the retina are consistent with what has been observed elsewhere in brain.
Atriol, an agent that preferentially inhibits TSPO [178], and dutasteride, a broader spectrum 5aRD inhibitor, suppressed pressureinduced AlloP synthesis and induced excitotoxic retinal degeneration in hyperbaric condition. These findings indicate that upregulation of endogenous AlloP and GABAA receptor activation are critical to maintain the integrity of the retina during the stress of pressure elevation. Exogenous administration of AlloP also inhibited axonal swelling, indicating that AlloP can be a neuroprotectant against glaucomatous changes. Picrotoxin, a GABAA receptor antagonist, attenuated the neuroprotective effects of AlloP, supporting the hypothesis that neuroprotection by AlloP involves GABAA receptors.
The TSPO agonist, Ro5-4864, has been found to be neuroprotectant in a mouse model of Alzheimer’s disease (3xTgAD mice) [179]. Furthermore, the TSPO ligand, PK11195, has at least partial agonist activity at TSPO, depending upon cell type and drug concentration [180,181], and, in the rat ex vivo glaucoma model, PK11195 clearly enhanced actions mediated by TSPO, preventing pressureinduced RGC injury and apoptotic RGC death. Thus, PK11195 or other TSPO agonists may be useful agents in protecting RGCs from pressureinduced excitotoxic degeneration via effects on AlloP synthesis [182].
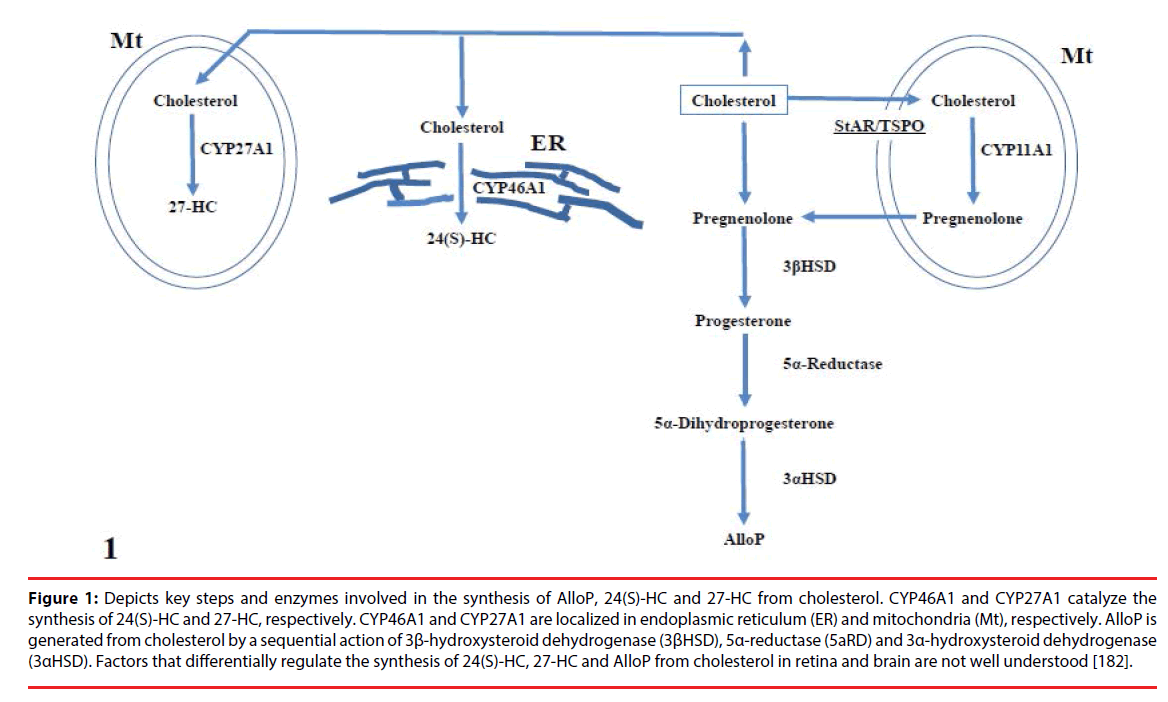
Table 3 summarizes the neurosteroids in Alzheimer’s disease and glaucoma. Figure 1 depicts key steps and enzymes involved in the synthesis of AlloP and 24(S)-HC from cholesterol.
| Neurosteroids and their generating enzymes | Alzheimer disease (results and references) | Glaucoma (results and references) |
|---|---|---|
| 25-Hydroxycholesterol | Upregulate Abeta insertion into membrane [99] | - |
| (25-HC) | ||
| Promoted peptide penetration into membranes [99] | - | |
| Induce oxidative stress and apoptosis [98,100] | - | |
| 27-Hydroxycholesterol | Dampen beneficial effects of 24(S)-HC inhibiting Abeta | - |
| (27-HC) | formation [102] | |
| Increases Abeta and oxidative stress [103] | - | |
| Decrease activity-regulated cytoskeleton-associated protein | - | |
| (Arc) levels [106] | ||
| 27-HC generating enzyme | Risk factor for sporadic ALS [104] | - |
| (CYP27A1) | ||
| 24(S)-Hydroxycholesterol | Efficient inhibitor of the formation of Abeta peptides [102] | Increase in pressure-dependent manner [164] |
| (24(S)-HC) | ||
| Increase in CSF of early stages [109] and decrease in | Neuroprotective agaist IOP elevation [164] | |
| plasma and CSF of advanced stage [112] | ||
| 24(S)-HC generating enzyme | Preferentially expressed in degenerating neurites | Increase in pressure-dependent manner [164] |
| (CYP46A1) | surrounding senile plaque of AD [102] | |
| CYP46A1 polymorphisms increase risk of AD and elevated | ||
| Abeta load [113,114] | ||
| CYP46A1 activation results in cholesterol loss and | ||
| subsequent stimulation of neuroprotection in excitotoxicity | ||
| in hippocampus [165,166]. | ||
| Allopregnanolone (AlloP) | Decrease in temporal cortex in AD patient [155] | Increase in pressure-dependent manner [172,173] |
| Decrease with the presence of the APOE4 allele, a risk | Neuroprotective agaist IOP elevation [172,173] | |
| factor for late onset AD)[156] | ||
| Increase proliferation of neural progenitor cells in | ||
| transgenic mouse (APP/PS1/P301L) [95,135,155] | ||
| AlloP generating sequence | TSPO agonist Ro5-4864 is neuroprotective in a mouse | Increase in pressure-dependent manner [173] |
| (TSPO,α-Reductase)5 | model of Alzheimer’s disease (3xTgAD) [179] | |
| PK11195, TSPO agonist, is neuroprotective against | ||
| pressure-dependent injury [173] | ||
Table 3: Neurosteroids and oxysterols in Alzheimer’s disease and glaucoma.
Figure 1: Depicts key steps and enzymes involved in the synthesis of AlloP, 24(S)-HC and 27-HC from cholesterol. CYP46A1 and CYP27A1 catalyze the synthesis of 24(S)-HC and 27-HC, respectively. CYP46A1 and CYP27A1 are localized in endoplasmic reticulum (ER) and mitochondria (Mt), respectively. AlloP is generated from cholesterol by a sequential action of 3β-hydroxysteroid dehydrogenase (3βHSD), 5α-reductase (5aRD) and 3α-hydroxysteroid dehydrogenase (3αHSD). Factors that differentially regulate the synthesis of 24(S)-HC, 27-HC and AlloP from cholesterol in retina and brain are not well understood [182].
Conclusion
Elevated IOP is the predominant risk factor for glaucoma, and, to date, IOP lowering therapies are the only proven method to treat glaucoma. However, recent evidence indicates that the progression of RGC death cannot be prevented despite effective lowering of IOP. Therefore, alternative treatments will likely be required to delay or prevent progressive RGC damage. The term neuroprotection implies mechanisms that protect neurons from apoptotic or necrotic degeneration, and neuroprotection in glaucoma is aimed at protecting RGC that are damaged by glaucomatous processes. Based on common pathogenetic features between Alzheimer’s disease and glaucoma, several possibilities exist to develop novel therapeutic strategies. Neurosteroids and oxysterols are synthesized from cholesterol in the CNS and offer a potential new avenue for treatment development. Among the neurosteroids,24(S)-HC and AlloP are positive modulators of NMDA and GABAA receptors, respectively. In Alzheimer’s disease and aging models, upregulation of CYP46A1, the 24(S)- HC generating enzyme, appears to be neuroprotective. Additionally, AlloP enhances neurogenesis, decreases inflammation, and reduces apoptosis, and thus could prevent neurodegenerative and cognitive deficits associated with Alzheimer’s disease. In our ex vivo glaucoma model, pressure loading increases activation of CYP46A1 and 24(S)- HC synthesis, resulting in protection of RGC against pressure-induced damage. AlloP production is also increased in hyperbaric conditions, and also exerts neuroprotection against IOP elevation-induced axonal injury through mechanisms that are likely distinct from 24(S)-HC. As the next step of future research, the effects of these neurosteroids on cognitive function in transgenic mice modeling Alzheimer’s disease and tau aggregation should be examined with paralleled studies in their retinas in order to determine the possible link between Alzheimer’s disease and glaucoma. Further work is also needed to determine whether AlloP and 24S-HC are neuroprotective in different in vivo Alzheimer’s disease models. Following from commonalities in the pathophysiology of glaucoma and Alzheimer’s disease, it will also be interesting to determine how the retinas of mice expressing genes associated with Alzheimer’s disease respond to changes in IOP and the effects of neurosteroids and oxysterols. Taken together, available data suggest that neurosteroids and oxysterols warrant further consideration as potential therapeutic approaches in both glaucoma and Alzheimer’s disease.
Acknowledgement
The authors thank Sanae Takaseki for technical support and Kathiresan Krishnan for synthesizing the atriol in the laboratory of D.F.C.
Grant information: This work was supported in part by JSPS KAKENHI Grant No. 15K10888 to M.I., and National Institute of Health grants MH077791 and MH101874, and the Bantly Foundation to C.F.Z.
A conflict-of-interest statement: CFZ serves on the Scientific Advisory Board of Sage Therapeutics. CFZ and DFC own stock in Sage Therapeutics. MI and TY receive lecture fees from Santen, Pfizer, Kowa, and Alcon. The other authors have no financial conflicts of interest.
References
- London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat. Rev. Neurol 9(1), 44-53 (2013).
- Wostyn P, Audenaert K, De Deyn PP. Alzheimer's disease and glaucoma: is there a causal relationship? Br. J. Ophthalmol 93(12), 1557-1559 (2009).
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol 90(3), 262–267 (2006).
- Tham YC, Wong TY, Quigley HA, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121(11), 2081-2090 (2014).
- Quigley HA. Neuronal death in glaucoma. Prog. Retin. Eye Res 18(1), 39–57 (1999).
- Gupta N, Yücel YH. Glaucoma as a neurodegenerative disease. Curr. Opin. Ophthalmol 18(2),110-114 (2007).
- McKinnon SJ, Lehman DM, Kerrigan-Baumrind LA, et al. Caspase activation and amyloid precursor protein cleavage in rat ocular hypertension. Invest. Ophthalmol. Vis. Sci 43(4), 1077-1087 (2002).
- Tsolaki F, Gogaki E, Tiganita S, et al. Alzheimer’s disease and primary open-angle glaucoma: is there a connection? Clin. Ophthalmol 5, 887–890 (2011).
- Baulieu EE. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog. Horm. Res 52, 1–32 (1997).
- Zorumski CF, Paul SM, Izumi Y, et al. Neurosteroids, stress and depression: Potential therapeutic opportunities. Neurosc. Biobehav. Rev 37(1), 109–122 (2013).
- Bayer AU, Keller ON, Ferrari F, et al. Association of glaucoma with neurodegenerative diseases with apoptotic cell death. Alzheimer's disease and Parkinson's disease. Am. J. Ophthalmol 133(1), 135–137 (2002).
- Bayer AU, Ferrari F, Erb C. High occurrence rate of glaucoma among patients with Alzheimer’s disease. Eur. Neurol 47(3), 165–168 (2002).
- Tamura H, Kawakami H, Kanamoto T, et al. High frequency of open-angle glaucoma in Japanese patients with Alzheimer’s disease. J. Neurol. Sci 246(1-2), 79–83 (2006).
- Sartucci F, Borghetti D, Bocci T, et al. Dysfunction of the magnocellular stream in Alzheimer’s disease evaluated by pattern electroretinograms and visual evoked potentials. Brain Res. Bull 82(3-4), 169–176, (2010).
- Parisi V, Restuccia R, Fattapposta F, et al. Morphological and functional retinal impairment in Alzheimer's disease patients. Clin. Neurophysiol 112(10), 1860–1867 (2001).
- Iseri PK, Altinas O, Tokay T, et al. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J. Neuroophthalmol 26(1), 18–24 (2006).
- Lin IC, Wang YH, Wang TJ, et al. Glaucoma, Alzheimer's disease, and Parkinson's disease: an 8-year population-based follow-up study. PLoS One 9(9), e108938 (2014).
- Helmer C, Malet F, Rougier MB, et al. Is there a link between open‑angle glaucoma and dementia? The Three‑City‑Alienor cohort. Ann. Neurol 74(2), 171‑179 (2013).
- Lai SW, Lin CL, Liao KF. Glaucoma may be a non-memory manifestation of Alzheimer's disease in older people. Int. Psychogeriatr 29, 1-7 (2017).
- Ou Y, Grossman DS, Lee PP, et al. Glaucoma, Alzheimer disease and other dementia: A longitudinal analysis. Ophthalmic Epidemiol 19(5), 285‑292 (2012).
- Kessing LV, Lopez AG, Andersen PK, et al. No increased risk of developing Alzheimer disease in patients with glaucoma. J. Glaucoma 16:47‑51 (2007).
- Hinton DR, Sadun AA, Blanks JC, et al. Optic‑nerve degeneration in Alzheimer’s disease. N Engl J Med 315(8), 485‑487 (1986).
- Blanks JC, Torigoe Y, Hinton DR, et al. Retinal pathology in Alzheimer's disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol. Aging, 1pp. 377-384 (1996).
- Sadun AA, Bassi CJ. Optic nerve damage in Alzheimer’s disease. Ophthalmology 97(1), 9‑17 (1990).
- Criscuolo C, Fabiani C, Cerri E, et al. Synaptic Dysfunction in Alzheimer's Disease and Glaucoma: From Common Degenerative Mechanisms Toward Neuroprotection. Front. Cell Neurosci 11, 53 (2017).
- Sivak, JM. The aging eye: common degenerative mechanisms between the Alzheimer’s brain and retinal disease. Invest. Ophthalmol. Vis. Sci 54(1), 871–888, (2013).
- Jain S, Aref AA. Senile Dementia and Glaucoma: Evidence for a Common Link. J. Ophthalmic Vis. Res 10(2), 178-183 (2015).
- Pepys MB. Amyloidosis. Annu. Rev. Med 57, 223–241 (2006).
- Vigneswara V, Akpan N, Berry M, et al. Combined suppression of CASP2 and CASP6 protects retinal ganglion cells from apoptosis and promotes axon regeneration through CNTF-mediated JAK/STAT signaling. Brain 137(Pt 6), 1656-1675 (2014).
- Ramalho RM, Viana RJ, Castro RE, et al. Apoptosis in transgenic mice expressing the P301L mutated form of human tau. Mol. Med 14(5-6), 309-317 (2008).
- Nickells RW. Apoptosis of retinal ganglion cells in glaucoma: an update of the molecular pathways involved in cell death. Surv. Ophthalmol 43(Suppl 1), S151-S161 (1999).
- McKinnon SJ. Glaucoma: ocular Alzheimer's disease? Front. Biosci 8, s1140-156 (2003).
- Yoneda S, Hara H, Hirata A, et al. Vitreous fluid levels of beta-amyloid((1-42)) and tau in patients with retinal diseases. Jpn. J. Ophthalmol 49, 106–108 (2005).
- Kim TW, Pettingell WH, Jung YK, et al. Alternative cleavage of Alzheimer-associated presenilins during apoptosis by a caspase-3 family protease. Science 277(5324), 373–376 (1997).
- Ning A, Cui J, To E, et al. Amyloid-beta deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Invest. Ophthalmol. Vis. Sci 49(11), 5136-5143 (2008).
- Nizari S, Guo L, Davis BM, et al. Non-amyloidogenic effects of α2 adrenergic agonists: implications for brimonidine-mediated neuroprotection. Cell Death Dis 7(12), e2514 (2016).
- Verkhratsky A, Parpura V, Pekna M, et al. Glia in the pathogenesis of neurodegenerative diseases. Biochem. Soc. Trans 42(5), 1291-301 (2014).
- Ramirez AI, de Hoz R, Salobrar-Garcia E, et al. The Role of Microglia in Retinal Neurodegeneration: Alzheimer's Disease, Parkinson, and Glaucoma. Front. Aging Neurosci 9, 214 (2017)
- Nixon RA. Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer's disease: inseparable partners in a multifactorial disease. FASEB J 31(7), 2729-2743 (2017).
- Guo L, Salt TE, Luong V, et al. Targeting amyloid-β in glaucoma treatment. Proc. Natl. Acad. Sci. U S A 104(33), 13444-13449 (2007).
- Callaway E. Alzheimer's drugs take a new tack. Nature 489(7414), 13-14 (2012).
- Gendron TF, Petrucelli L. The role of tau in neurodegeneration. Mol. Neurodegener 4, 13 (2009).
- Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb. Perspect. Med 2, a006247 (2012).
- Hoeprich GJ, Mickolajczyk KJ, Nelson SR, et al. The axonal transport motor kinesin-2 navigates microtubule obstacles via protofilament switching. Traffic 18(5), 304-314 (2017).
- Kosik KS, Finch EA. MAP2 and tau segregate into dendritic and axonal domains after the elaboration of morphologically distinct neurites: an immunocytochemical study of cultured rat cerebrum. J. Neurosci 7(10), 3142–3153 (1987).
- Grundke-Iqbal I, Iqbal K, Tung YC, et al. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. U S A. 83(13), 4913-4917 (1986).
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu. Rev. Neurosci 24, 1121–1159 (2001).
- Scholz T, Mandelkow E. Transport and diffusion of tau protein in neurons. Cell Mol. Life Sci 71(16), 3139 –3150 (2014).
- Leger F, Fernagut PO, Canron MH, et al. Protein aggregation in the aging retina. J. Neuropathol. Exp. Neurol 70(1), 63–68 (2011).
- Gupta N, Fong J, Ang LC, et al. Retinal tau pathology in human glaucomas. Can. J. Ophthalmol 43(1), 53–60 (2008).
- Chiasseu M, Vargas JLC, Destroismaisons L, et al. Tau accumulation, altered phosphorylation, and missorting promote neurodegeneration in glaucoma. J Neurosci 36(21), 5785–5798 (2016).
- Chiu K, Chan TF, Wu A, et al. Neurodegeneration of the retina in mouse models of Alzheimer's disease: what can we learn from the retina? Age (Dordr) 34(3), 633-649 (2012).
- Gasparini L, Crowther RA, Martin KR, et al. Tau inclusions in retinal ganglion cells of human P301S tau transgenic mice: effects on axonal viability. Neurobiol. Aging 32(3), 419-433 (2011).
- Zhao H, Chang R, Che H. et al. Hyperphosphorylation of tau protein by calpain regulation in retina of Alzheimer’s disease transgenic mouse. Neurosc. Lett 551, 12– 16 (2013).
- Wang CY, Xie JW, Wang T, et al. Hypoxia-triggered m-calpain activation evokes endoplasmic reticulum stress and neuropathogenesis in a transgenic mouse model of Alzheimer's disease. CNS Neurosci. Ther 19(10), 820-833 (2013).
- Perez SE, Lumayag S, Kovacs B, et al. Beta-amyloid deposition and functional impairment in the retina of the APPswe/PS1DeltaE9 transgenic mouse model of Alzheimer’s disease. Invest. Ophthalmol. Vis. Sci 50(2), 793–800 (2009).
- Krasodomska K, Lubinski W, Potemkowski A, et al. Pattern electroretinogram (PERG) and pattern visual evoked potential (PVEP) in the early stages of Alzheimer’s disease. Doc. Ophthalmol 121, 111–121 (2010).
- Bull ND, Guidi A, Goedert M, et al. Reduced axonal transport and increased excitotoxic retinal ganglion cell degeneration in mice transgenic for human mutant P301S tau. PLoS One 7(4), e34724 (2012).
- Onishi T, Matsumoto Y, Hattori M, et al. Early-onset cognitive deficits and axonal transport dysfunction in P301S mutant tau transgenic mice. Neurosci. Res 80(1), 76-85 (2014).
- Liu B, Rasool S, Yang Z, et al. Amyloid-peptide vaccinations reduce {beta}-amyloid plaques but exacerbate vascular deposition and inflammation in the retina of Alzheimer's transgenic mice. Am. J. Pathol 175(5), 2099-2110 (2009).
- de Calignon A, Fox LM, Pitstick R, et al. Caspase activation precedes and leads to tangles. Nature 464(7292), 1201-1204 (2010).
- Sengupta U, Portelius E, Hansson O, et al. Tau oligomers in cerebrospinal fluid in Alzheimer's disease. Ann. Clin. Transl. Neurol 4(4), 226-235 (2017).
- Takeda S, Commins C, DeVos SL, et al. Seed-competent high^molecular –weight tau species accumulates in the cerebrospinal fluid of Alzheimer’s disease mouse model and human patients. Ann. Neurol 80(3), 355-367.
- Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav. Brain Res 192(1), 106-113 (2008).
- Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, et al. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci. Rep 2:700 (2012).
- Fá M, Puzzo D, Piacentini R, et al. Extracellular Tau oligomers produce an immediate impairment of LTP and memory. Sci. Rep 6, 19393 (2016).
- Puzzo D, Piacentini R, Fa' M, et al. LTP and memory impairment caused by extracellular Aβ and Tau oligomers is APP-dependent. Elife 6. pii: e26991 (2017).
- Brunden KR, Trojanowski JQ, Lee VM. Advances in tau-focused drug discovery for Alzheimer’s disease and related tauopathies. Nat. Rev. Drug Discov 8(10), 783–793 (2009).
- Polito VA, Li H, Martini-Stoica H, et al. Selective clearance of aberrant tau proteins and rescue of neurotoxicity by transcription factor EB. EMBO Mol. Med 6(9), 1142-1160 (2014).
- Zhang ZH, Wu QY, Zheng R, et al. Selenomethionine mitigates cognitive decline by targeting both tau hyperphosphorylation and autophagic clearance in an Alzheimer's disease mouse model. J. Neurosci 37(9), 2449-2462 (2017).
- Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240(5852), 622–630 (1988).
- Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261(5123), 921–923 (1993).
- Bertram L, Tanzi RE. Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat. Rev. Neurosci 9(10), 768–778 (2008).
- Bu G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat. Rev. Neurosci 10(5), 333-344 (2009).
- Kim J, Basak JM, Holtzman DM, et al. The role of apolipoprotein E in Alzheimer's disease. Neuron 63(5), 287-303 (2009).
- Liraz O, Boehm-Cagan A, Michaelson DM, et al. ApoE4 induces Aβ42, tau, and neuronal pathology in the hippocampus of young targeted replacement apoE4 mice. Mol. Neurodegener 8, 16 (2013).
- Jiang Q, Lee CY, Mandrekar S, et al. ApoE promotes the proteolytic degradation of Abeta. Neuron 58(5), 681-693 (2008).
- Sagare A, Deane R, Bell RD, et al. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat. Med 13(9), 1029-1031 (2007).
- Cam JA, Bu G. Modulation of beta-amyloid precursor protein trafficking and processing by the low density lipoprotein receptor family. Mol. Neurodegener 1, 8 (2006).
- Herz J, Beffert U. Apolipoprotein E receptors: linking brain development and Alzheimer's disease. Nat. Rev. Neurosci 1(1), 51-58 (2000).
- Liu Q, Zerbinatti CV, Zhang J, et al. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron 56(1), 66-78 (2007).
- Jenner AM, Lim WL, Ng MP, et al. The effect of APOE genotype on brain levels of oxysterols in young and old human APOE epsilon2, epsilon3 and epsilon4 knock-in mice. Neuroscience 169(1), 109-115 (2010).
- Gamba P, Testa G, Sottero B, et al. The link between altered cholesterol metabolism and Alzheimer's disease. Ann. N. Y. Acad. Sci 1259, 54-64 (2012).
- Inoue T, Kawaji T, Tanihara H. Elevated levels of multiple biomarkers of Alzheimer's disease in the aqueous humor of eyes with open-angle glaucoma. Invest. Ophthalmol. Vis. Sci 54(8):5353-5358, (2013).
- Ressiniotis T, Griffiths PG, Birch M, et al. The role of apolipoprotein E gene polymorphisms in primary open-angle glaucoma. Arch. Ophthalmol 122(2), 258-261 (2004).
- Lake S, Liverani E, Desai M, et al. Normal tension glaucoma is not associated with the common apolipoprotein E gene polymorphisms. Br. J. Ophthalmol 88(4), 491–493 (2004).
- Saglar E, Yucel D, Bozkurt B, et al. Association of polymorphisms in APOE, p53, and p21 with primary open-angle glaucoma in Turkish patients. Mol. Vis 15, 1270-6 (2009)
- Vickers JC, Craig JE, Stankovich J, et al. The apolipoprotein epsilon4 gene is associated with elevated risk of normal tension glaucoma. Mol Vis 8, 389-93 (2002).
- Copin B, Brezin AP, Valtot F, et al. Apolipoprotein E-promoter single-nucleotide polymorphisms affect the phenotype of primary open-angle glaucoma and demonstrate interaction with the myocilin gene. Am. J. Hum. Genet 70(6), 1575–1581 (2002).
- Gambert S, Gabrielle PH, Masson E, et al. Cholesterol metabolism and glaucoma: Modulation of Muller cell membrane organization by 24S-hydroxycholesterol. Chem Phys Lipids (2017) Jun 4. pii: S0009-3084(17)30020-8. [Epub ahead of print]
- Locci A, Pinna G. Neurosteroid biosynthesis downregulation and changes in GABAA receptor subunit composition: A biomarker axis in stress-induced cognitive and emotional impairment. Br. J. Pharmacol (2017) Apr 29. doi: 10.1111/bph.13843. [Epub ahead of print]
- Yoshizawa K, Okumura A, Nakashima K, et al. Role of allopregnanolone biosynthesis in acute stress-induced anxiety-like behaviors in mice. Synapse 71(8), (2017).
- Zorumski CF, Mennerick S, Isenberg KE, et al. Potential clinical uses of neuroactive steroids. Curr. Opin. Investig. Drugs 1(3), 360-369 (2000).
- Irwin RW, Wang JM, Chen S, et al. Neuroregenerative mechanisms of allopregnanolone in Alzheimer’s disease. Front. Endocrinol (Lausanne) 2, 1–14 (2012).
- Wang JM, Singh C, Liu L, et al. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U S A 107(14), 6498–6503 (2010).
- Björkhem I, Cedazo-Minguez A, Leoni V, et al. Oxysterols and neurodegenerative diseases. Mol. Aspects Med 30(3), 171–179 (2009).
- Linsenbardt AJ, Taylor A, Emnett CM, et al. Different oxysterols have opposing actions at N-methyl-D-aspartate receptors. Neuropharmacology 85, 232-242 (2014).
- Panini SR, Yang L, Rusinol AE. Arachidonate metabolism and the signaling pathway of induction of apoptosis by oxidized LDL/oxysterol. J. of Lipid Res 42(10), 1678-1686 (2001).
- Phan HT, Hata T, Morita M, et al. The effect of oxysterols on the interaction of Alzheimer's amyloid beta with model membranes. Biochim. Biophys. Acta 1828(11), 2487-2495 (2013).
- Liu Q, Zhou QH, Ji SR, et al. Membrane localization of β-amyloid 1–42 in lysosomes: a possible mechanism for lysosome labilization J. Biol. Chem 285(26), 19986–19996 (2010).
- Kim SM, Noh MY, Kim H, et al. 25-Hydroxycholesterol is involved in the pathogenesis of amyotrophic lateral sclerosis. Oncotarget 8(7), 11855-11867 (2017).
- Brown J 3rd, Theisler C, Silberman S, et al. Differential expression of cholesterol hydroxylases in Alzheimer’s disease. J. Biol. Chem 279(33), 34674–34681 (2004).
- Dasari B, Prasanthi JR, Marwarha G, Singh BB, Ghribi O. The oxysterol 27-hydroxycholesterol increases β-amyloid and oxidative stress in retinal pigment epithelial cells. BMC Ophthalmol 10, 22 (2010)
- Diekstra FP, Saris CG, van Rheenen W, et al. Mapping of gene expression reveals CYP27A1 as a susceptibility gene for sporadic ALS. PLoS One 7, e35333 (2012).
- Abdel-Khalik J, Yutuc E, Crick PJ, et al. Defective cholesterol metabolism in amyotrophic lateral sclerosis. J. Lipid Res 58(1), 267-278 (2017).
- Mateos L, Akterin S, Gil-Bea FJ, et al. Activity-regulated cytoskeleton-associated protein in rodent brain is down-regulated by high fat diet in vivo and by 27-hydroxycholesterol in vitro. Brain Pathol 19(1), 69-80 (2009).
- Wuolikainen A, Acimovic J, Lövgren-Sandblom A, et al. Cholesterol, oxysterol, triglyceride, and coenzyme Q homeostasis in ALS. Evidence against the hypothesis that elevated 27-hydroxycholesterol is a pathogenic factor. PLoS One 9(11), e113619 (2014).
- Rebeck GW. Cholesterol efflux as a critical component of Alzheimer's disease pathogenesis. J. Mol. Neurosci 23(3), 219-224 (2004).
- Papassotiropoulos A, Lutjohann D, Bagli M, et al. 24S-hydroxycholesterol in cerebrospinal fluid is elevated in early stages of dementia. J. Psychiatr. Res 36(1), 27–32 (2002).
- Popp J, Lewczuk P, Kolsch H, et al. Cholesterol metabolism is associated with soluble amyloid precursor protein production in Alzheimer's disease. J. Neurochem 123(2), 310–316 (2012).
- Bjorkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol 24(5), 806–815 (2004).
- Leoni V, Masterman T, Mousavi F, et al. Diagnostic use of cerebral and extracerebral oxysterols. Clin. Chem. Lab. Med 42(2), 186–191 (2004).
- Lenhard B, Brookes AJ, Pedersen NL, et al. Variants of CYP46A1 may interact with age and APOE to influence CSF Abeta42 levels in Alzheimer's disease. Hum. Genet 114(6), 581-587 (2004).
- Papassotiropoulos A, Streffer JR, Tsolaki M, et al. Increased brain beta-amyloid load, phosphorylated tau, and risk of Alzheimer disease associated with an intronic CYP46 polymorphism. Arch Neurol 60(1), 29–35 (2003).
- Boussicault L, Alves S, Lamazière A, et al. CYP46A1, the rate-limiting enzyme for cholesterol degradation, is neuroprotective in Huntington’s disease. Brain 139(Pt 3), 953–970 (2016).
- Abildayeva K, Jansen PJ, Hirsch-Reinshagen V, et al. 24(S)-hydroxycholesterol participates in a liver X receptor-controlled pathway in astrocytes that regulates apolipoprotein E-mediated cholesterol efflux. J. Biol. Chem 281(18), 12799-808 (2006).
- Casellas P, Galiegue S, Basile AS. Peripheral benzodiazepine receptors and mitochondrial function. Neuroche. Int 40(6), 475–486 (2002).
- Chen M-K, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol. Ther 118(1), 1–17 (2008).
- Rupprecht R, Papadopoulos V, Rammes G, et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov 9(12), 971–988 (2010).
- Chisari M, Eisenman LN, Krishnan K, et al. The influence of neuroactive steroid lipophilicity on GABAA receptor modulation: evidence for a low affinity interaction. J. Neurophysiol 102(2), 1254–1264 (2009).
- Papadopoulos V, Fan J, Zirkin B Translocator protein (18 kDa): an update on its function in steroidogenesis. J. Neuroendocrinol (2017) Jul 1. doi: 10.1111/jne.12500. [Epub ahead of print]
- Dong E, Matsumoto K, Uzunova V, et al. Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc. Natl. Acad. Sci. U S A 98(5), 2849–2854 (2001).
- Agis-Balboa RC, Pinna G, Pibiri F, et al. Downregulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc. Natl. Acad. Sci. U S A 104(47), 18736–18741 (2007).
- Weir CJ, Ling AT, Belelli D, et al. The interaction of anaesthetic steroids with recombinant glycine and GABAA receptors. Br. J. Anaesth 92(5), 704–711 (2004).
- Kajta M, Budziszewska B, Lason W. Allopregnanolone attenuates kainate-induced toxicity in primary cortical neurons and PC12 neuronal cells. Pol. J. Pharmaco 51(6), 531–534 (1999).
- Frank C, Sagratella S. Neuroprotective effects of allopregnenolone on hippocampal irreversible neurotoxicity in vitro. Prog. Neuropsychopharmacol Biol. Psychiatr 24(7), 1117–1126 (2000).
- Ardeshiri A, Kelley MH, Korner IP, et al. Mechanism of progesterone neuroprotection of rat cerebellar Purkinje cells following oxygen-glucose deprivation. Eur. J. Neurosci 24(9), 2567–2574 (2006).
- Ishihara Y, Kawami T, Ishida A, et al. Allopregnanolone-mediated protective effects of progesterone on tributyltin-induced neuronal injury in rat hippocampal slices. J. Steroid Biochem. Mol. Biol 135, 1-6 (2013).
- Ciriza I, Azcoitia I, Garcia-Segura LM. Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. J. Neuroendocrinol 16(1), 58–63 (2004).
- He J, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor. Neurol Neurosci. 22(1), 19–31 (2004).
- Chesnoy-Marchais D. Progesterone and allopregnanolone enhance the miniature synaptic release of glycine in the rat hypoglossal nucleus. Eur. J. Neurosci 30(11), 2100–2111 (2009).
- Azcoitia I, Leonelli E, Magnaghi V, et al. Progesterone and its derivatives dihydroprogesterone and tetrahydroprogesterone reduce myelin fiber morphological abnormalities and myelin fiber loss in the sciatic nerve of aged rats. Neurobiol. Aging 24(6), 853–860 (2003).
- Ghoumari AM, Ibanez C, El-Etr M, et al. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J. Neurochem 86(4), 848–859 (2003).
- Liao G, Cheung S, Galeano J, et al. Allopregnanolone treatment delays cholesterol accumulation and reduces autophagic/lysosomal dysfunction and inflammation in Npc1−/− mouse brain. Brain Res 1270, 140–151 (2009).
- Wang JM, Johnston PB, Ball BG, et al. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J. Neurosci 25(19), 4706–4718 (2005).
- He J, Evans CO, Hoffman SW, et al. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp. Neurol 189(2), 404–412 (2004).
- VanLandingham JW, Cekic M, Cutler S, et al. Neurosteroids reduce inflammation after TBI through CD55 induction. Neurosci. Lett 425, 94–98 (2007).
- Charalampopoulos I, Alexaki VI, Tsatsanis C, et al. Neurosteroids as endogenous inhibitors of neuronal cell apoptosis in aging. Ann. N. Y. Acad. Sci 1088, 139–152 (2006).
- Charalampopoulos I, Tsatsanis C, Dermitzaki E, et al. Dehydroepiandrosterone and allopregnanolone protect sympathoadrenal medulla cells against apoptosis via antiapoptotic Bcl-2 proteins. Proc. Natl. Acad. Sci. U S A 101(21), 8209–8214 (2004).
- Xilouri M, Papazafiri P. Anti-apoptotic effects of allopregnanolone on P19 neurons. Eur. J. Neurosci 23(1), 43–54 (2006).
- Francis PT. The interplay of neurotransmitters in Alzheimer's disease. CNS Spectr 10 (Suppl 18), 6–9 (2005).
- Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer's disease. Neurochem. Int 45(5), 583–595 (2004).
- Lipton SA. The molecular basis of memantine action in Alzheimer's disease and other neurologic disorders: low-affinity, uncompetitive antagonism. Curr Alzheimer Res 2(2), 155–165 (2005).
- Wenk GL, Parsons CG, Danysz W. Potential role of N-methyl-D-aspartate receptors as executors of neurodegeneration resulting from diverse insults: focus on memantine. Behav. Pharmacol 17(5-6), 411–424 (2006).
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet 368(9533), 387–403 (2006).
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297(5580), 353–356 (2002).
- Marx CE, Trost WT, Shampine LJ, et al. The neurosteroid allopregnanolone is reduced in prefrontal cortex in Alzheimer's disease. Biol. Psychiatry 60(12), 1287-1294 (2006).
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol. Aging 25(1), 5–18; author reply 49–62 (2004).
- Benes FM. A disturbance of late myelination as a trigger for Alzheimer's disease. Neurobiol. Aging 25(1), 41–43 (2004).
- Lejri I, Grimm A, Miesch M, et al. Allopregnanolone and its analog BR 297 rescue neuronal cells from oxidative stress-induced death through bioenergetic improvement. Biochim Biophys Acta 1863(3), 631-642 (2017).
- Irwin RW, Solinsky CM, Loya CM, et al. Allopregnanolone preclinical acute pharmacokinetic and pharmacodynamic studies to predict tolerability and efficacy for Alzheimer's disease. PLoS One 10(6), e0128313 (2015).
- Calissano P, Matrone C, Amadoro G. Apoptosis and in vitro Alzheimer disease neuronal models. Commun. Integr. Biol 2(2), 163–169 (2009).
- Rohn T. The role of caspases in Alzheimer's disease; potential novel therapeutic opportunities. Apoptosis. 15(11), 1403-1409 (2010).
- Zotova E, Nicoll JA, Kalaria R, et al. Inflammation in Alzheimer's disease: relevance to pathogenesis and therapy. Alzheimers Res. Ther 2(1), 1 (2010).
- Irwin RW, Brinton RD. 2013. Allopregnanolone as regenerative therapeutic for Alzheimer’s disease: translational development and clinical promise. Prog. Neurobiol 113, 40-55 (2014).
- Naylor JC, Kilts JD, Hulette CM, et al. Allopregnanolone levels are reduced in temporal cortex in patients with Alzheimer's disease compared to cognitively intact control subjects. Biochim. Biophys. Acta 1801(8), 951-959 (2010).
- Chen S, Wang JM, Irwin RW, et al. Allopregnanolone promotes regeneration and reduces beta-amyloid burden in a preclinical model of Alzheimer’s disease. PLoS One 6(8), e24293 (2011).
- Fan BJ, Wang DY, Tham CC, et al. Gene expression profiles of human trabecular meshwork cells induced by triamcinolone and dexamethasone. Invest Ophthalmol Vis Sci 49(5), 1886-1897 (2008).
- Fourgeux C, Martine L, Björkhemet I, et al. Primary open-angle glaucoma: association with cholesterol 24S-hydroxylase (CYP46A1) gene polymorphism and plasma 24-hydroxycholesterol levels. Invest. Ophthalmol. Vis. Sci 50(12), 5712–5717 (2009).
- Mossböck G, Weger M, Faschinger C, et al. Role of cholesterol 24S-hydroxylase gene polymorphism (rs754203) in primary open angle glaucoma. Mol. Vis 17, 616-620 (2011).
- Fourgeux C, Martine L, Pasquis B et al. Steady-state levels of retinal 24S-hydroxycholesterol are maintained by glial cells intervention after elevation of intraocular pressure in the rat. Acta Ophthalmol. 90(7), e560-e567 (2012).
- Ishikawa M, Yoshitomi T, Zorumski CF, et al. Effects of acutely elevated hydrostatic pressure in the rat ex vivo retinal preparation. Invest. Ophthalmol. Vis. Sci. 51(12), 6414-6423 (2010).
- Ishikawa M, Yoshitomi T, Zorumski CF, et al. Down regulation of glutamine synthetase via GLAST suppression induces retinal axonal swelling in a rat ex vivo hydrostatic pressure model. Invest. Ophthalmol. Vis. Sci 52(9), 6604-6616 (2011).
- Ishikawa M, Yoshitomi T, Zorumski CF, et al. 24(S)-Hydroxycholesterol protects the ex vivo rat retina from injury by elevated hydrostatic pressure. Sci. Rep 6, 33886, (2016).
- Sodero AO, Trovò L, Iannilli F, et al. Regulation of tyrosine kinase B activity by the Cyp46/cholesterol loss pathway in mature neurons: relevance for neuronal survival under stress and aging. J Neurochem 116(5), 747–755 (2011).
- Sodero AO, Vriens J, Ghosh D, et al. Cholesterol loss during glutamate-mediated excitotoxicity. EMBO J 31 (7), 1764–1773 (2012).
- Paul SM, Doherty JJ, Robichaud AJ, et al. The major brain cholesterol metabolite 24(S)-hydroxycholesterol is a potent allosteric modulator of N-methyl-Daspartate receptors. J. Neurosci 33(44), 17290–17300 (2013).
- Sun MY, Izumi Y, Benz A, et al. Endogenous 24S-hydroxycholesterol modulates NMDAR mediated function in hippocampal slices. J. Neurophysiol 115(3), 1263–1272 (2016).
- Sun MY, Linsenbardt AJ, Emnett CM, et al. 24(S)-Hydroxycholesterol as a modulator of neuronal signaling and survival. Neuroscientist 22(2), 132–144 (2016).
- 122. Sun MY, Taylor A, Zorumski CF, et al. 24S-hydroxycholesterol and 25-hydroxycholesterol differentially impact hippocampal neuronal survival following oxygen-glucose deprivation. PLoS One 12(3), e0174416 (2017).
- Hu H, Zhou Y, Leng T, et al. The major cholesterol metabolite cholestane-3β ,5α ,6β-triol functions as an endogenous neuroprotectant. J. Neurosci 34(34), 11426–11438 (2014).
- Ishikawa M, Yoshitomi T, Zorumski CF, et al. Neurosteroids are endogenous neuroprotectants in an ex vivo glaucoma model. Invest Ophthalmol Vis Sci 55(12), 8531-8541 (2014).
- Ishikawa M, Yoshitomi T, Covey DF, et al. TSPO activation modulates the effects of high pressure in a rat ex vivo glaucoma model. Neuropharmacology 111, 142-159 (2016).
- Purdy RH, Morrow AL, Moore PH Jr, et al. Stress-induced elevations of c-aminobutyric acid type A receptor-active steroids in the rat brain. Proc. Natl. Acad. Sci U S A 88, 4553–4557 (1991).
- Vallee M, Rivera JD, Koob GF, et al. Quantification of neurosteroids in rat plasma and brain following swim stress and allopregnanolone administration using negative chemical ionization gas chromatography/mass spectrometry. Anal. Biochem 287(1), 153–166 (2000).
- Tokuda K, Izumi Y, Zorumski CF. Ethanol enhances neurosteroidogenesis in hippocampal pyramidal neurons by paradoxical NMDA receptor activation. J. Neurosci 31(27), 9905-9909 (2011).
- Izumi Y, Svrakic N, O’Dell K, et al. Ammonia inhibits long-term potentiation via neurosteroid synthesis in hippocampal pyramidal neurons. Neuroscience 233, 166–173 (2013).
- Midzak A, Akula N, Lecanu L, et al. Novel androstenetriol interacts with the mitochondrial translocator protein and controls steroidogenesis. J. Biol. Chem 286(11), 9875-9887 (2011).
- Rupprecht R, Rammes G, Eser D, et al. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science 325(5939), 490–493 (2009).
- Shany E, Hochhauser E, Halper P. et al. Ro5-4864 has a negative inotropic effect on human atrial muscle strips that is not antagonized by PK11195. Eur. J. Pharmacol 253(3), 231–236 (1994).
- Choi J, Ifuku M, Noda M, et al. Translocator protein (18 kDa) peripheral benzodiazepine receptor specific ligands induce microglia functions consistent with an activated state. Glia 59(2), 219–230 (2010).
- Liao W-L, Heo G-Y, Dodder NG, et al. Quantification of cholesterol-metabolizing P450s CYP27A1 and CYP46A1 in neural tissues reveals a lack of enzyme-product correlations in human retina but not human brain. J. Proteome Res 10(1), 241-248 (2011).