Review Article - Neuropsychiatry (2018) Volume 8, Issue 1
Neurochemicals, Behaviours and Psychiatric Perspectives of Neurological Diseases
- *Corresponding Author:
- Amarendranath Choudhury, PhD
Jaipal Homes, Hightension Road, Kondapur Hyderabad-500084
India & Alumnus: Department of Life Science and Bioinformatics
Assam University, Silchar-788011, India
Tel: +91-7003017920
Abstract
Structural changes in different regions of the brain have become clinically relevant and regarded as the signature phenomenon for neurological diseases. Morphological changes in brain are also associated with neuronal and neurochemical alterations. Studies have showed that minute changes in neurochemical levels may have marked impact on the psychobehaviourof the subject. Several neurological disease profiles have been reported with such specific psychobehavioural expression. However, application of behavioural abnormalities as possible disease progress markers has not been considered and emphasised much. Reports suggestedthat- the subjects, who have already entered into the terminal stage of the disease, used to show cardinal behavioural signs for a specific disease profile. However, psychologicalexpressions are comparatively early expressive. As most of the neurodegenerative disordersare unidirectional and progressive by nature, therapeutic intervention at the right time isessential for attaining the desired outcome. Moreover, early diagnosis can aid in managingthe disease progression also. Psychobehavioural analysis could meet the expected outcomeof disease diagnosis if implemented properly and timely. In the present review, we have amalgamated the reported behavioural anomalies with the supportive background fromneurochemical basis. Further, we have concluded that behaviour centric studies could b ea p ote ntial diagnostic tool for the early diagnosis of major neurological diseases such asAlzheimer disease, Parkinson’s disease, Amyotrophic lateral sclerosis (ALS), Bipolar disorder,Schizophrenia, Impulse control disorder (ICD) and Obsessive-compulsive disorder (OCD).
Keywords
Neurotransmitters, Psychology, Neurodegenerative disorders, Behaviour
Introduction
The brain is the supreme authority that controls all behaviours based on endogenous and exogenous influences. Such behavioural manifestation is the outcome of neuronal communications between neurons and other cells regulated through a chemo-electrical messenger system [1]. Neurotransmitters are chemical messengers released from neurons which regulate neuronal function by mediating adequate receptor-ligand interactions [2]. Subtle changes in the quality and quantity of neurotransmitters may exert severe alterations in behaviour [3]. Such changes may occur as a result of genetic mutation or toxicity [4]. Moreover, research is being conducted to investigate such abnormalities for effective design and formulation of new therapeutics. A plethora of experimental evidences showed that the distribution and function of neurotransmitters are positively correlated with psychological events [5,6]. Molecular mechanisms associated with electrical gradients have been reported to alter the neurophysiology that impacts the psychometrics of the patient [7]. It is noteworthy that, functionally not all neurotransmitters are uniform in nature; neurotransmitter abundance also depends on the regional differences in the brain [8,9]. Among neurotransmitters, dopamine, serotonin, nor-adrenalin, and acetylcholine contribute profoundly to the psycho-behavioural events in humans. However, the significant role of several other proteins and peptides such as neurotrophic factors, growth factors, and endogenous chemical compounds are also recognized in this regard. Each of these entities plays selective roles in the maintenance of proper brain function, such as, the feedback mechanisms of the synthesis of particular neurotransmitters [10].
Among the major neurotransmitters, dopamine is known to control most of the psychological events in human [11]. Dopamine- ‘the molecule of happiness’, is responsible for movement, memory, cognition, attention, pleasure, reward, motivation, sleep regulation, creativity, and personality determination. Chronic decrease in dopamine levels is an indicator of the neurodegenerative pathology of Parkinson’s disease (PD) [12]. Besides neurodegeneration, scarcity of dopamine is also associated with depression and mood swings [13]. Serotonin or 5-hydroxytryptamine (5-HT) is another important neurotransmitter which has a diverse set of functions. Serotonin functions sometimes overlap with that of dopamine. Serotonin regulates social behaviour, mood, sleep, digestion, appetite, memory, and sexual desire. Serotonin deficiency has been linked with depression and anxiety [12]. Large quantity (80-90%) of serotonin is available in the gastrointestinal tract, where it regulates the appetite and bowel movement [11]. As brain and systemic serotonin are not inter-exchangeable due to the bloodbrain- barrier, the brain’s own serotonin regulates the functions in the central nervous system [13]. Serotonin is also associated with breast milk production, liver regeneration and bone metabolism. Serotonin assists in blood clotting following release from platelets. It also plays a role in vasoconstriction [12]. Reports suggest that low blood serotonin levels are associated with higher libido. This fact has been leveraged in the sexual dysfunction therapeutics [11]. Dopa decarboxylase (Figure 1) is responsible for converting L-DOPA to Dopamine and Serotonin. On the contrary, norepinephrine (noradrenaline) has a dual role of a hormone and a neurotransmitter. This particular molecule is effective for ‘fight-or-flight situation’ and controls stressful events through regulating the central nervous system [9,11]. Norepinephrine is associated with the occurrence of Attentiondeficit hyperactivity disorder (ADHD), depression, and low blood pressure. The role of norepinephrine and serotonin was found to be crucial in depression [12]. Medication related to serotonin-norepinephrine reuptake inhibitors is a well-known treatment for chronic depression [10- 14]. Unlike norepinephrine, epinephrine mostly functions as a hormone [13]. Acetylcholine is an organic compound formed by the esterification of acetic acid and choline. Acetylcholine plays a crucial role in memory and cognitive aptitude related tasks. Acetylcholine deficiency has been reported in the disease profiles of AD, PD, and Myasthenia Gravis [14]. Another neurotransmitter glutamate, has an essential role in learning and memory related functions [15,16].
Aging plays a crucial role in the synthesis of neurotransmitters. It has been reported that dopamine synthesis in the striatal region of the brain is known to be affected by aging [17,18]. Decreased expression of specific receptors (like NMDA, AMPA) also occurs during aging [19]. Apart from these receptors, neurochemical alterations in glutamate, GABA, aspartate, glutamine, taurine, glycine, and arginine levels remain the hallmark of psychological and behavioural changes during neurodegeneration [19,20]. Table 1 delineates the familiar neurotransmitters and endogenous neuroactive components along with their respective functions.
| Sl. No. | Neurotransmitters/neuropeptides/Neuroactive molecules | Role in psychology |
|---|---|---|
| 1 | 2-Arachidonoylglycerol | Memory; Anxiety-like responses |
| 2 | Acetylcholine | Memory; Behavior; Cognition |
| 3 | Anandamide | Memory; Behavior |
| 4 | Bombesin | Memory; Behavior; Cognition |
| 5 | Carbon monoxide | Memory |
| 6 | Cholecystokinin | Behavior; Cognition |
| 7 | Cocaine- and amphetamine-regulated transcript | Memory; Behavior; Cognition |
| 8 | Dynorphin | Memory |
| 9 | Endomorphin | Memory |
| 10 | Endorphin | Memory; Behavior; Cognition |
| 11 | Enkephalin | Memory; Behavior; Cognition |
| 12 | Epinephrine(adrenaline) | Memory; Behavior; Cognition |
| 13 | Galanin | Memory |
| 14 | Gamma-aminobutyric acid | Memory; Behavior; Cognition |
| 15 | Gastrin releasing peptide | Memory; Behavior; Cognition |
| 16 | Glucagon-like peptide 1 | Memory; Cognition |
| 17 | Growth hormone–releasing hormone | Memory; Behavior; Cognition |
| 18 | Histamine | Memory; Behavior; Cognition |
| 19 | Hydrogen sulfide | Behavior; Cognition |
| 20 | Kisspeptin | Memory; Behavior |
| 21 | Neurokinin | Memory; Behavior; Cognition |
| 22 | Neuropeptide B | Memory |
| 23 | Neurophysin | Memory; Behavior; Cognition |
| 24 | Nitric oxide | Memory; Behavior; Cognition |
| 25 | Norepinephrine(noradrenaline) | Memory; Behavior; Cognition |
| 26 | Octopamine | Behavior |
| 27 | Orexins | Memory; Behavior; Cognition |
| 28 | Oxytocin | Memory; Cognition |
| 29 | Pancreatic polypeptide | Behavior; Cognition |
| 30 | Secretin | Behavior; Cognition |
| 31 | Somatostatin | Memory; Cognition |
| 32 | Synephrine | Memory; Cognition |
| 33 | Tryptamine | Memory; Cognition |
| 34 | Tyramine | Memory |
| 35 | Vasoactive intestinal peptide | Cognition |
Table 1: List of some important neurochemicals related to memory, cognition and behaviours.
Therapeutics for neurodegeneration has been always challenged by the unidirectional and progressive nature of the diseases [21]. Moreover, very few supportive evidences exist related to aging associated plausible neurogenesis in the human brain [22]. Hence, behavioural and psychological anomalies, which occur due to neurochemical alterations, could be used as a possible tool for the diagnosis of different neurodegenerative disease profiles at an early stage. This might identify the course of pathological progression and provide a better solution. In the present review, we have attempted to accumulate available reports on behavioural anomalies with the supportive background from neurochemical understanding. Moreover, relevance of psycho-behavioural study as a diagnostic tool for neurological diseases has been discussed.
▪ Neurochemicals in Psychological Events
Psychological events follow a complex cascade of molecular mechanism with the crucial involvement of neurotransmitters. Psychological events and their associated neurotransmitters have been described in the following sections.
▪ Major Neurotransmitters and their Functions
Neurotransmitters function as crucial connecting molecules in the transmission of neural signals with significant precision and intensity. Such activation of signals occurs either at the presynaptic or postsynaptic level [6]. Blocking the normal functioning of the neurotransmitters might hamper the propagation of the active potential in a designated neural path [5,23]. Neurotransmitters are categorized in different manners based on the super-families, families or the structural features and sites of action [5]. The most commonly accepted types are plasma membrane bound neurotransmitters and vesicular membrane neurotransmitters. Further, neurotransmitters are also classified as amino acid based neurotransmitters such as glycine, glutamate [16], γ-aminobutyric acid (GABA), D-serine, and aspartate. The other important classes of neurotransmitters are, the monoamines such as epinephrine (adrenaline), norepinephrine (noradrenaline), dopamine (DA), serotonin, histamine etc. [19,20]. Apart from these major classes there are gasotransmitters, such as, carbon monoxide, nitric oxide etc.; trace amines including tyramine, phenethylamine, octopamine etc.; certain peptides like substance P, somatostatin, etc.; and certain purines like adenosine [24].
It was observed that neurotransmitters have a direct impact on the regular functioning of the central and peripheral nervous systems [25]. Moreover, several psychological and mental disorders are associated with the proper operation of neurotransmitters [26].
▪ Role of Dopamine in psychobehavioural manifestation
Dopamine deficiency has been linked with several psycho-behavioural anomalies and related disease pathologies. Scarcity of dopamine is the hallmark pathological sign for PD [27]. PD patients also suffer from depression, anxiety, and memory related complications, reports have shown a significant correlation between dopamine deficiency in such psycho-behavioural anomalies [28]. Interestingly, pathological gambling, compulsive shopping, and hypersexuality are also evident in PD patients at different stages of the disease. Whether such behaviours are triggered by dopamine deficiency or due to the adverse effect of PD medications, is still debatable [29]. During substance abuse dopamine level rises transiently, providing reward-like feelings and urge for such events. This situation makes the patient compulsive and habitual for addiction. Such addictions in PD patients are reported and presence of addiction along with other psychological anomalies provides a notion about the actual scenario of the disease [30]. Though, PD patients with less interest in cigarettes and alcohol have also been reported [30]. Further studies are needed to get a clear insight about such contention. The dopamine D3 receptor also has a crucial role in sensitization and development of addictive behavioural syndrome [31]. Additionally, high levels of dopamine lead to vigorous thinking, which causes delusion and hallucination. Such events are evident in Schizophrenia, and bipolar disorder, where dopamine lowering treatment is the only prescribed approach, reported so far [32]. Errors in the dopamine signal indicate the differences between predicted and actually received rewards along with assessing learning and decision making abilities [33]. Anhedonia, the hallmark of major depressive disorder, occurs due to dysregulation of the dopamine system. Abnormalities in the regulatory afferent circuits of the dopaminergic system remain the centre point of such chronic depression [34]. Lack of motivation, fatigue, inability to experience pleasure, insomnia, mood swings, forgetfulness, inability to focus and concentrate, inability to connect with others, low libido, sugar cravings, caffeine cravings, inability to handle stress, and inability to lose weight are all associated with dopamine deficiency [35]. Obesity, thyroid disorders, chronic inflammation, hormone imbalance, bipolar disorder, and ADHD are related to dopamine deficiency [11,36]. Dopamine replenishment therapy results in pathological improvement and psycho-behavioural recovery [37] proving the involvement of dopamine in the psychological manifestations.
▪ Role of Serotonin in psycho-behavioural manifestation
Serotonin or 5-hydroxytryptamine (5-HT) is one of the most important monoamine neurotransmitters. The precursor of serotonin is L-Tryptophan [35,37]. L-Tryptophan gets converted into 5-Hydroxy-L-tryptophan (5- HTP) which in turn generates Serotonin through the activity of specific decarboxylase (Figure 2) [38].
Figure 2: (A) [PDB ID: 4IAQ, Crystal structure of the chimeric protein of 5-HT1B-BRIL in complex with dihydroergotamine (PSI Community Target), Wang et al., 2013]. (B) [PDB ID: 4IB4, Crystal structure of the chimeric protein of 5-HT2B-BRIL in complex with ergotamine, Wacker et al., 2013]. (C) [PDB ID: 4PIR, X-ray structure of the mouse serotonin 5-HT3 receptor, Hassaine et al., 2014]. (D) [PDB ID: 5TUD, Structural Insights into the Extracellular Recognition of the Human Serotonin 2B Receptor by an Antibody, Ishchenko et al., 2017].
Serotonin is associated with mood and decision making process. Balanced levels of serotonin represent calmness, maturity, and decisive behaviour [39] and are associated with several neurological and psychological behaviours including learning, memory, anxiety, cognition, depression, aggression etc. The same neurotransmitter is involved in regular physiological processes such as maintaining appetite, regular movement of bowels and GI tract, and digestion [39-41]. Serotonin was found to play an important role in bone metabolism and development of organs like brain [42].
Serotonin acts via cell membrane bound 5-HT receptors and the same mechanism is also evident in nerve cells in humans [43]. The 5-HT receptors are of 7 types, namely, 5-HT1 through 5-HT7. 5-HT1 and 5-HT5 show inhibitory action potential, whereas all others follow excitatory potential [42]. Most of these receptors act by modulating the cAMP level where 5-HT1 and 5-HT5 reduce the cAMP levels, but 5-HT2, 5-HT4, 5-HT6, and 5-HT7 receptors elevate the cAMP levels [40-43]. Except 5-HT3, which is ion channel dependent, all other receptors are G-protein coupled receptors. Serotonin reuptake that occurs through the SERT or serotonin transporter can be terminated by various serotonin reuptake inhibitors (SSRIs) [44].
Serotonin is known to maintain the chemical balance in the brain and regulate the proper functioning of the central nervous system [42]. The involvement of serotonin in several other peripheral functions is also crucial [45]. Serotonin is indispensable for the normal functioning of the system. Imbalance in serotonin levels is directly associated with different neurological and psychological conditions [44,45]. In many countries, selective SSRIs are considered as effective antidepressants [46]. There are two plausible types of functional polymorphisms which occur in the Serotonin transporter gene as per the research observations. Repetitive length variation (20-30 nucleotides) in the 5′ upstream of the SLC6A4 gene develops the serotonin associated transporter polymorphic region (5-HTTLPR) [47,48]. The second type of polymorphism occurs as a result of a variable number of tandem repeats (VNTR) being present within the second intron. This polymorphism is represented as 5-HTTVNTR [49]. Functionally, these two polymorphisms modulate the 5-HTT protein expression by altering the transcript ratio. Several studies were able to link these polymorphisms of serotonin to neurological disease conditions [50]. Investigations have been made to understand the relation between 5-HTTLPR and mental conditions such as anxiety [51], autism [52], depression [53], alcoholism [54], and schizophrenia [55].
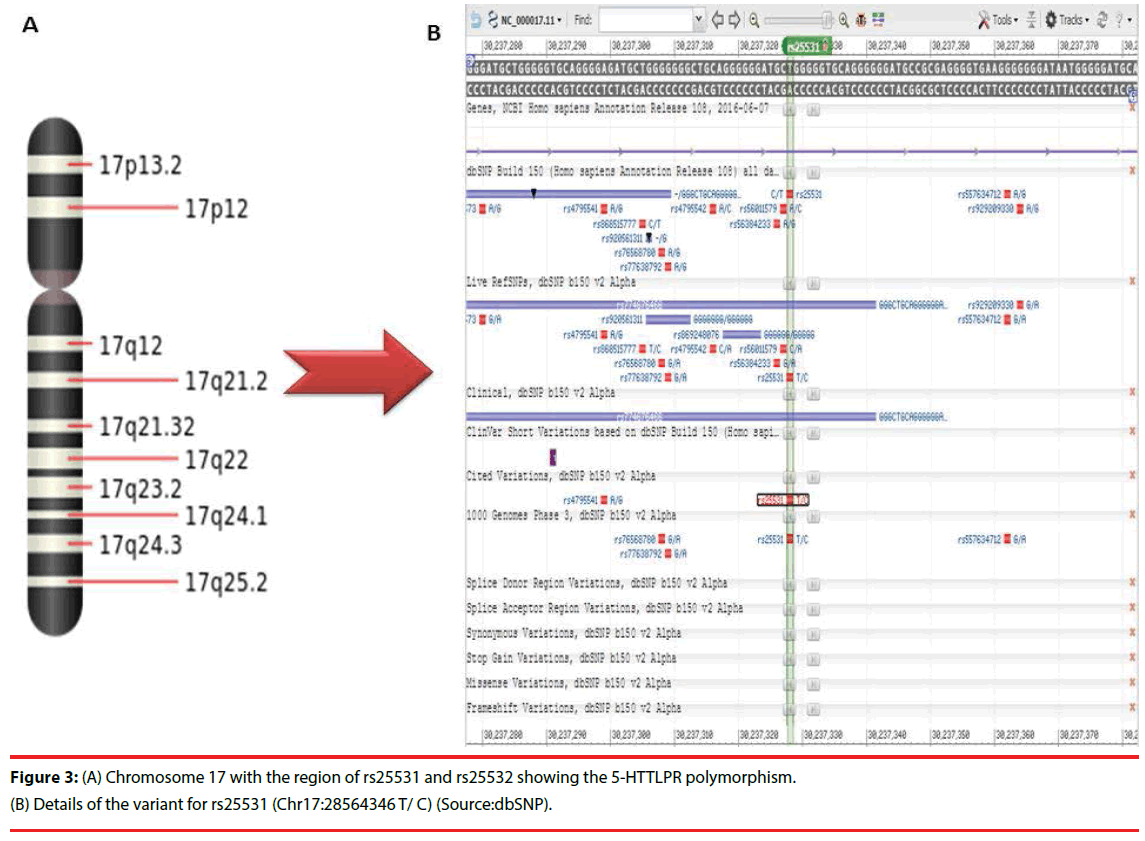
Recent research progress has depicted the association of 5-HTTLPR with different types of phenotypical, genotypical and behavioural manifestation, which has highlighted several crucial aspects of serotonin function [56]. Important functional polymorphism (5-HTTLPR) in the SLC6A4 gene is linked with one or the other symptoms of major psychoses [57]. These SNPs in this gene are present on chromosome 17 at rs25531 and rs25532 regions (Table 2). The representation of the same on the 17th chromosome is provided in Figure 3.
| Population | Allele Count | Allele Number | Number of Homozygotes | Allele Frequency |
|---|---|---|---|---|
| African | 370 | 1320 | 50 | 0.2803 |
| East Asian | 45 | 166 | 10 | 0.2711 |
| Other | 22 | 154 | 2 | 0.1429 |
| European (Finnish) | 163 | 1164 | 9 | 0.1400 |
| European (Non-Finnish) | 244 | 2120 | 13 | 0.1151 |
| Ashkenazi Jewish* | 3 | 38 | 0 | 0.07895 |
| Latino | 14 | 182 | 1 | 0.07692 |
| South Asian | 0 | 0 | 0 | NA |
| Total | 861 | 5144 | 85 | 0.1674 |
Table 2: Population Frequencies of the alleles related to the rs25531 (Chr17:28564346 T/ C) SNP. [Source: (The Genome Aggregation Database (gnomAD)]
The involvement of suicidal tendency and serotonergic system has been studies vividly and experimental evidences of such direct association assisted in the related therapeutics [58]. Further, efforts have been poured towards establishing a link between the serotonin levels and extensive behavioural problems. Support from neuroimaging techniques has drawn attention to the interplay between serotonin concentration and mental disorders [59]. Advanced imaging techniques such as positron-emission tomography (PET) and single photon emission computed tomography (SPECT) have been utilized with relevant radio ligands to unravel the relation between Major Depressive Disorder (MDD), Schizophrenia, addiction, mood disorders, anxiety disorders, and Attention Deficit Hyperactivity Disorder (ADHD) with serotonergic systems [59]. All these efforts remain inconclusive due to lack of direct experimental evidences.
Therefore, advanced methodology and innovative investigation approaches are required to reach conclusive outcome. Attempts have been made to understand the relation between the administration of selective serotonin reuptake inhibitors (SSRIs) and neurological development of the foetus during gestation [60]. SSRI exposure to the foetus during gestational period might be positively associated with depression but not with ADHD or autism spectrum disorders. Similar experiments provided hints regarding the role of serotonin in maintaining the mental balance and avoiding depression [61], but none of these studies successfully dissected the exact relation. Recently, association of compounds such as vitamin D, 2 marine omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), with serotonin release and control has been reported, confirming their indirect impact on mental disorders [62].
▪ Role of Noradrenaline in psychobehavioural manifestation
Norepinephrine or noradrenaline is an important hormone and neurotransmitter majorly secreted from the locus coeruleus present in the pons of brain stem. The locus coeruleus-norepinephrine (LC-NE) system is believed to perform multiple complex behavioural regulations. Dual mode of phasic and tonic activity was proposed by Aston- Jones and Cohen in 2005 [63].
In spinal cord and abdomen this neurotransmitter is used through the sympathetic ganglia. The alertness of an individual is profoundly influenced by this neurotransmitter. Norepinephrine secretion remains lowest during sleep whereas it reaches its peak during any alarming situation [64]. Increment of arousal and vigilance remains major activity of this hormone. Physiologically, increment of blood pressure, heart rate, and blood flow to the muscles are regulated by Norepinephrine. Norepinephrine influences the gastrointestinal motility when required [64,65].
Medically, norepinephrine levels are controlled for treating several disease conditions. Direct injection of norepinephrine is used for the treatment of low blood pressure. Several classes of neurotransmitter blockers are regularly used for the treatment of different type of disease conditions. In certain cardiovascular problems and glaucoma, beta blockers are used to block norepinephrine action [66]. Alpha blockers are used for psychiatric treatments. The mechanism of action of these two types of blockers, i.e., beta and alpha is markedly different [67]. The beta blockers function through β1, β2, and β3 receptors where elevation of cAMP is observed through the Adenylate Cyclase via Gs coupled protein system. On the contrary, the alpha blockers act through Gq and Gi/Go-coupled protein system with the help of α1 and α2 receptors. The mechanism differs from α1 to α2 receptor mediation where α1 activates the phospholipase C, thus, elevating the IP3 and calcium level. α2 plays an important role in preventing the function of Adenylate Cyclase, which in turn decreases the cAMP levels [68].
In several psychiatric and nervous system associated conditions, norepinephrine or noradrenaline supplementation is used extensively as a regular therapeutic. In such cases, the required concentration of vital neurotransmitter is regarded as the determining factor [69]. Conditions associated with the sympathetic nervous system such as sympathetic hyper activation occur along with symptoms such as palpitation, anxiety, sweating, alteration in blood pressure, and headache [70,71]. Chronic stress is also directly associated with sustained release of norepinephrine [70]. This situation hampers growth in children, alters the homeostasis of the body, negatively impacts the immunity and damages many other normal functions as well. ADHD is also treated with norepinephrine related drugs where stimulant class of medication is provided to the patient. Molecular imaging based evidences have established an obvious relationship between dopamine, norepinephrine and the pathophysiology of ADHD [71]. At the genetic level, persistent ADHD displays specific biomarkers with relation to the expression and processing of the norepinephrine [72]. Medication directly effecting norepinephrine has been shown to be beneficial for ADHD as compared to other classes of medication [73]. Reports are available in support of the role of Norepinephrine in dementia [74]. Deviation from the normal functioning of this neurotransmitter was also found to be associated with AD [75].
Numerous drugs, ranging from psychedelics to antidepressants such as mescaline, psilocybin, and various compounds of Dimethyltryptamine; alpha and beta blockers such as phentolamine, atenolol, and metoprolol are extensively used. Studies suggest that there are several side effects associated with such treatments [76,77]. Questions have been raised pertaining to the application of single versus multiple antipsychotic agents as therapeutics [78]. Therefore, search for safer, effective alternative therapy is the need of the time.
▪ Role of Acetylcholine in psychobehavioural manifestation
Acetylcholine (Ach) is the most abundant neurotransmitter. It was discovered by Hallett Dale in the year 1914. It is a chemical messenger that primarily acts during muscle contraction and therefore exerts profound effects on behavioural manifestation. Ach controls majority of the autonomic nervous system [14,79]. Ach function is directly associated with memory and cognition. Lack of Ach has been observed in several disease pathologies associated with memory related complications like AD, ADHD, etc. [80]. In the central nervous system, Ach plays excitatory roles, assisting in learning, memory, arousal, and neuroplasticity. The psycho-behavioural anomalies like sleep disorders and rapid eye movement during sleep have been linked with Ach function. Ach dysregulation is responsible for Myasthenia Gravis, chronic fatigue and depression [81,82]. Auto-antibodies against acetylcholine receptors cause muscle weakness and fatigue in Myasthenia Gravis. This situation results in inhibition of proper acetylcholine signal transmission [83]. Deficiency of Ach is generally expressed through several psycho-behavioural signs like low energy levels, fatigue, memory loss, cognitive decline, learning disabilities, muscle aches, nerve damage, and frequent mood swings [81,82].Generally, Ach level decreases with age; as a result, sporadic loss of short-term memory occurs. However, drastic loss of Ach (~90%) is evident in AD, where deteriorating cognition and behavioural function are evident. In general, lack of Ach is responsible for confusion in daily tasks and activities. Thus, Ach replenishment improves the memory function and cognition. Inhibition of Acetyl cholinesterase has become popular for the therapeutics of AD and other Ach deficit disorders [83,84].
▪ Neurotropic factors and related psychological abnormalities
Neurotrophic factors (NTFs) have a profound influence on the manifestation of psychobehavioural expressions. NTFs support the growth, differentiation, and survival of neurons [85]. They are also having utmost influence in the regulation of synaptic plasticity and in maintenance of long-term memories [86]. NTFs can be subdivided as: (1) neurotrophin family, (2) Ciliary neurotrophic factor (CNTF) family, and (3) Glial cell line-derived neurotrophic factor (GDNF) [87]. The neurotrophin family plays a vital role in the development of the nervous system and the regulation of neuronal plasticity [88]. In mammals, molecules of the neurotrophin family are comprised of: (1) brain-derived neurotrophic factor (BDNF), (2) nerve growth factor (NGF), (3) neurotrophin 3 (NT3), and (4) neurotrophin 4 (NT4). BDNF is the most abundant molecule out of these [89]. A number of psychiatric and neurodegenerative conditions are associated with varied levels of neurotrophic factors and their receptors [90]. For example, phenotypes similar to the neurodegeneration observed in AD are observed in mouse models in which the NGF levels have been halved [91]. In fact, AD associated and aged rat brains exhibit reduced levels of NGF in the basal forebrain cholinergic neurons (BFCNs) [92,93]. Down’s syndrome (DS) is also characterized by similar deficits in NGF signalling in the BFCNs [94].
NGF, the first identified neurotrophin, was remarkably able to reduce the neurodegeneration of cholinergic neurons in fimbria transection [95,96]. Additionally, it partially reduced the cholinergic atrophy in aged rodents [97]. Altered levels of BDNF and its receptor, Tropomyosin receptor kinase B (TrkB) in the entorhinal cortex (EC) and the frontal cortex (FC) are related to impairments in memory and cognition associated with AD [95], Down Syndrome [96], and aging [97]. PD is associated with reduced BDNF levels at both the transcript and protein levels in the dopaminergic neurons of the substantia nigra [98]. Similarly, in Huntington’s disease, hampering the transport of BDNF from the cortical to the striatal neurons results in the loss of striatal neurons and impairments in the voluntary muscle movements [99]. Furthermore, BDNF levels aid in determining the susceptibility towards non-neurodegenerative and psychiatric disorders, such as: bipolar disorder, anxiety, depression, and schizophrenia [100]. Drug abuse also alters BDNF and GDNF activities [101]. The exact role of BDNF and GDNF with respect to drug abuse is still unclear but development of drug-seeking behaviour and addiction has been reported. The relationship between drug abuse and NTFs seems to be bidirectional where drug intake and NTF expression can impact each other [102,103]. Ciliary neurotrophic factor (CNTF) on the other hand is thought to help in the maintenance of the mature motor system. It is abundantly found in myelinated Schwann cells in the sciatic nerve and striatal neurons [104]. The effect of CNTF on motor neurons has provided a basis for clinical and preclinical studies in neurodegenerative diseases such as Amyotrophic Lateral Sclerosis (ALS) and Huntington’s disease. Administration of CNTF in age-related macular degeneration (AMD) patients has resulted in promising results [105,106]. The association of neurotropic factor levels with neuronal diseases makes them potential biomarkers for neuronal disease diagnosis [107]. Epigenetic modifications of these factors imposes an additional complexity, influencing disease progression and pathogenesis [108,109].
▪ Nuclear respiratory factor and related psychological abnormalities
The Nuclear respiratory factor 1 (NRF1) is a transcription factor that regulates the expression of several genes involved in mitochondrial biogenesis and function. Increasing evidences suggest that mitochondrial function is severely hampered in neurodegenerative diseases where NRF1 might play a vital role. [110]. Integrated pathway analysis of an NRF1 ChIP-Seq dataset identified MAPT (tau), PAELR (GPR37), PARK2 (Parkin), PARK6 (Pink1), PARK7 (DJ-1), and PSENEN (Pen2) genes which are related to AD and PD. These could be novel targets of NRF1 [110]. Disruption of NRF1 orthologs in Drosophila and Zebrafish causes severe neurological defects confirming its role in functional maintenance of the nervous system. Age dependent behavioural abnormalities such as: impaired rotarod performance, abnormal leg-clasping reflex, and hyperactivity were observed in NRF1 deficient mice. Moreover, corresponding brain atrophy due to apoptosis in NRF1 deficient mice was observed [110-112]. Mutation in APOE4 that blocks NRF1 binding to the exon 4 of the gene has been recently linked to AD [113]. The Nuclear factor E2 (NRF2) is a master regulator which aids the expression of cytoprotective genes related to inflammation, mitochondrial biogenesis, antioxidant enzymes, and the proteasome pathway. NRF2 binds to the antioxidant response elements (AREs) in its target genes following nuclear translocation. Several evidences suggest an association between NRF2 deregulation and PD pathogenesis [114].
▪ Endocannabinoids and Anandamide
Endocannabinoid and related psychobehavioural manifestation has long been studied for different therapeutic approaches [114]. Alterations in the level of endocannabinoids induce specific psychotic symptoms in an individual [115]. Moreover, exogenous cannabinoids can exacerbate the pathological psychosis in patients suffering from psychobehavioural anomalies [115,116]. In case of endocannabinoids, the duration of psychosis is comparatively longer and sometimes found to be beneficial for improvement in the symptomatic anomalies in neurodegenerative disease profiles [117]. Administration of exogenous cannabinoids to influence endocannabinoids is equivocally documented where information related to causalities, dose-responses, direction, and biological plausibility is absent. Among the endocannabinoids, ‘Anandamide’ is the most notable one. It is a neurotransmitter which exhibits structural similarity to tetrahydrocannabinol [118]. Tetrahydrocannabinol serves as the active principle ingredient of several exogenous cannabinoids. It aids in the regulation of appetite, memory, pain, depression, and fertility [119]. The name ‘Anandamide’ comes from ‘Ananda’-a Sanskrit word symbolising ‘bliss’. Functionally, these molecules increase the “bliss” feeling [120]. Intriguingly, several exogenous API molecules have been reported to bind specific receptor counterpart in humans resulting in proper homeostatic outcomes. Further research has identified several such endogenous molecules in humans such as Enkephalins (equivalent to morphine) [121]. Such molecules have high solubility and can cross the bloodbrain- barrier easily resulting in rapid and robust action. Anandamide is abundant in the brain regions associated with memory and cognition [204] along with learning and movement regulation. Anandamide is one of the initial chemical messengers that connect the mother with the foetus [122,123]. However, overdose of Anandamide causes adverse effects like lack of concentration and memory related complications, equivalent to the effect of exogenous cannabinoid overdose. Experimental outcomes in rodent models have suggested that such an overdose alters the psycho-behavioural phenology of the offspring [123]. However, lack of Anandamide has been linked to the development of chronic depression and anxiety. Synthetic Anandamide like component AM1172 has showed promising results in overcoming such chronic psychobehavioural anomalies [124].
▪ Gut-Brain-Microbiome Axis and Psychobehavioural manifestation
The Gut-Brain-Axis (GBA) is a two-way interaction system between the emotional or cognitive centers of the brain with proper intestinal functioning [125]. The gut microbiome plays a crucial role in this interconnected system [124-127]. The interaction between microbiota and GBA involves exchange of molecular signaling mediators which functionally regulate the neural, endocrine, immune, and humoral systems [126]. GBA maintains gastro-intestinal homeostasis along with improvements in cognitive and motor functions [127]. In brief, neuro-immuno-endocrine mediators serve as the backbone for GBA networks that cover the CNS, automatic nervous system (ANS), enteric nervous system, and the hypothalamic pituitary adrenal axis (HPA) [128]. ANS controls toand- fro signals passing from CNS to the lumen or intestinal wall. HPA axis is the principal outward moving axis that controls adaptive responses like stress. Thereby, HPA is engaged in motor and emotional responses [127]. HPA is triggered via corticotropin releasing factor simulation. ACTH with relation to the increased systematic pro-inflammatory cytokines along with environmental stress subsequently triggers cortisol release by the adrenal glands. A combination of neural, hormonal, and gut microbiota network helps the brain regulate the effector cells [129,130]. Oral administration of antibiotics in subjects showing symptoms of hepatic encephalopathy exhibited significant improvement in microbiota-brain interaction. Experiments confirmed that probiotic microbiota have a protective role in chronic anxiety and depression. In disease pathology such as, irritable bowel syndrome, abnormal intestinal function destroys the GBA network [124]. GBA operates two major functions, namely, regulation of gut health, and controlling emotional and cognitive centers.
▪ Psycho-behavioural anomalies, neurochemical alterations and diseases
Neurochemical alterations resulting due to endocrine changes or exposure to some drug often tilt the fine balance of nervous system functioning and have been observed even in mood changes, depression and neurological diseases of significance.
▪ Necessity of behavioural typing for neurological disease diagnosis
Neurological disorders are usually multifaceted in manifestation. Many of them are characterized by the presence of neurodegeneration and memory related problems, cognitive dysfunctions and/or developmental delays or functional impairment [131]. Interestingly, all these factors are used as prognostic features, often in the advanced stages of the disease. Conversely, if we use them to diagnose or at least indicate the possibility of tentative occurrence of such neurological discrepancies, preventive measures can be implemented for better prognosis [132,133]. Unfortunately, the importance of assessment, analysis and correlation of such behavioural changes is understated. In addition, the general perceptions on common behavioural changes, such as memory loss, anxiety, sleep problems, etc. as natural outcomes of aging is extremely prevalent [134]. Therefore, it is wise to consider the repetitive pattern of such behavioural problems, which may indicate the underlying neurological discrepancies. Thus, early behavioural typing is recommended for the timely diagnosis or monitoring of the disease condition [135].
Similar behavioural alterations with reference to PD and AD are prominent. AD is a chronic neurological disease that causes a gradual deterioration of the patients’ memory skills and the ability to think and reason [136]. These symptoms are often accompanied by many other problems viz. behavioural changes, cognitive impairment, and psychiatric problems. Analyses of these factors and correlating them with neuro-anatomy using MRI or any other method usually forms the basis of AD diagnosis [137]. Apathy, agitation, irritability, anxiety, disinhibition, delusions, hallucinations, dysphoria, and aberrant motor behaviour are associated with AD [138].
Furthermore, mild behavioural impairment (MBI), an indicator of neuropsychiatric symptoms (NPS) syndrome progression, is also an indicator of future occurrence of dementia. NPS increases simultaneously with late onset of AD progression. Therefore, tracking NPS can help in effective diagnosis and prognosis of AD [139].
Moreover, AD often co-occurs with other behavioural problems such as Dysexecutive disorders. Godefroy et al., described the relative prevalence of Dysexecutive disorders in early stage AD patients [139] along with identifying specific features of this condition important for diagnosis. Ossenkoppele et al. in 2015 indicated that such behavioural patterns are actually the representatives of “frontal variant of AD” [140,141].
Like AD, PD is also a complex neurological condition that often manifests as a myriad behavioural discrepancy. Movement disorder due to motor system dysfunction in PD patients is already established. Such symptoms may be accompanied by tremor, postural instability, rigidity and sometimes bradykinesia. In addition, PD patients are prone to experience depression, anxiety and psychosis. Occasionally, hallucinations occur in advanced stages of the disease [142]. Recent researches related to behavioural manifestations of PD indicate that stereotyped behaviours also occur as the disease sets in. Similar behavioural changes are also observed in amphetamine and cocaine abusers. Therefore, it is imperative that these factors are collectively and correctly assessed before a final diagnosis is made [143,144]. A study conducted by Voon et al. indicated that PD patients have relatively high tendency for compulsive shopping and gambling [143]. Such patients also have a prevalence of pathologic hypersexuality as well as repetitive reward seeking behaviour. Furthermore, research suggests that significant percentage of people with Rapid Eye Movement (REM) sleep behaviour disorder (RBD) suffer from Parkinson’s disease [145].
Therefore, AD and PD associated behaviours clearly indicate that long before the identification of typical neurological manifestations of these diseases, patients start manifesting behavioural changes [145]. So, monitoring such typical patterns of behavioural changes can help in early diagnosis of these diseases. The conventional behavioural typing method is a multistep and elaborate procedure requiring multiple visits to the clinic for behavioural pattern and alteration assessment of the patient [140]. Such a process is time consuming and is prone to manual error during documenting and interpreting the behavioural patterns. Owing to the increasing necessity and indispensability of behavioural typing of patients, researchers from the Oregon Centre for Aging and Technology (ORCATECH) have initiated a novel process. The feasibility of a sensor based continuous in-home computerized monitoring system of daily activities of the patient is being attempted. The sensors are designed to detect and record typical features viz. mood swings, loneliness and changes in cognitive functions [146].
▪ Psycho-behavioural anomalies as diagnostic marker for neurological diseases
Several behavioral changes mark the onset and progression of neurological diseases. For instance, apathy and yawning are often associated with many neurological diseases.
▪ Alzheimer’s disease pathology and related psycho-behavioural anomalies
AD is a progressive neurodegenerative disorder characterized by cognitive and functional declination in an age-dependent manner. In the elderly, AD is recognized as the foremost cause of dementia [147]. The most common symptom of early stage AD is the difficulty in remembering recent events and newly learned information. The major pathological features of AD include progressive loss of cholinergic neurons in the basal forebrain, accumulation of extracellular senile plaques containing amyloid-β peptide (Aβ), and accumulation of intracellular neurofibrillary tangles (NFT), containing hyper phosphorylated tau (P-tau) [148]. Both Aβ plaques and tau NFTs are known to alter synaptic plasticity, leading to dysfunction of the neural network, synapse loss, and eventually neuron loss. However, the mechanism underlying the Aβ and tau induced neurodegeneration is still not clear [149]. Neuropathological changes associated with AD are initiated in the hippocampal formations and entorhinal cortex [150].
However, latest data suggests that Aβ pre-plaque monomers and oligomers, and not the aggregated plaques cause neuronal death. Therefore, large amyloid plaques might inhibit neuronal death by accumulating the deleterious amyloid-β monomers and oligomers [151]. Aβ oligomers induce morphologic and metabolic changes in the pyramidal neurons of the neo-cortex and the hippocampus. Changes in the prefrontal, cingulate cortices, and the hippocampus are observed since the early stages of the disease [17]. In addition to the degeneration of anatomical pathways17, genetic factors, environmental factors, mitochondrial dysfunction, immune dysfunction, vascular factors, infectious agents might also have a role in pathogenesis of AD [148]. However, inspite of our extensive understanding of AD, there is still no cure for this multifactorial disorder. Current diagnostic tools for the early detection of AD are insufficient.
Memory loss is amongst the first symptoms of AD. The first characteristic lesions of AD appear in the poorly myelinated neurons of the limbic system associated with memory and learning. Highly myelinated neurons are affected only in the terminal phases of the disease [150]. The pattern of impaired memory functions correlates with disruptions in structural or functional brain integrity [150]. In healthy individuals, the brain volume shrinks by nearly 0.2% to 0.41% each year, whereas the shrinkage rates in AD are almost ten times of that value. In regions like the hippocampal formation, the rates of atrophy might be even higher [139]. The hippocampus is responsible for memory formation, damage to this area is believed to underlie the memory loss in AD [152]. The hippocampal formation receives cortical as well as sub-cortical inputs. The latter arrives predominantly from the locus coeruleus (LC), the medial septal nucleus, nucleus basalis of Meynert, the raphe complex, and the ventral tegmental area (VTA). Neuropathological observations have demonstrated extensive disruption in the hippocampal formation and the extrinsic (cortical and sub-cortical) connections in the post-mortem AD brains. Thus, suggesting that the progressive brain damage might contribute towards worsening memory and cognition in AD [152]. Latest hypothesis suggests that the earliest pathology associated with AD is due to the accumulation of hyperphosphorylated tau in the neurons of the locus coeruleus (LC) [153]. Extensive degeneration of LC is amongst the earliest pathological impacts in AD and is nearly universal. LC neuropathology is detectable as early as 10 years prior to the appearance of neurocognitive symptoms. LC houses more than half of the noradrenergic neurons, from where they prominently project into the hippocampus, the thalamus, and the entorhinal and frontal cortices [154].
Thus, the pathology of AD interferes with memory formation from the molecular to the neural network levels. Memory complaints in AD may affect episodic memory, speech production, and/or visual orientation [150]. Reports suggest that both working memories and long-term declarative memories are affected early during the course of the disease. The synaptic loss in the AD cerebral cortex is found to involve 5-HT, ACh, and glutamate-containing synapses. Though the cholinergic abnormalities are a key feature of AD, the precise relationship between prolonged cholinergic dysfunction and risk of dementia is poorly understood. The levels of both nicotinic and the muscarinic acetylcholine receptors are known to be decreased in AD. Decline in the number of acetylcholine receptors happens prior to other pathological changes in AD [155]. Whole-genome RNA sequencing revealed that cholinergic failure leads to the deregulation of key transcripts related to AD such as betasecretase 1 (BACE1). Over expression of BACE1 enhances APP processing and accumulation of Aβ1-42. This process is accompanied by hyperphosphorylation of tau, increased neuronal death, decreased synaptic markers, and impaired cognition. Thus, suggested cholinergic loss facilitating AD-like pathology in mice [156]. The degeneration of cholinergic neurons across the basal forebrain, especially in the nucleus basalis of Meynert (NBM), is responsible for the cholinergic deficiency observed in the brains of AD patients [18]. The early and progressive degeneration of basal forebrain cholinergic neurons (BFCNs), characterized by decreased production of acetylcholine (ACh) substantially contributes to the gradual cognitive decline in AD [157].
Emerging studies suggest that the detrimental effects of Aβ in AD may be mediated in part by the excessive activation of the perisynaptic or extrasynaptic NMDARs. Soluble Aβ1–42 oligomers are thought to mimic the stimulation of eNMDARs by extracellular glutamate, and disrupt long-term potentiation and synaptic plasticity, eventually leading to synaptic loss [158]. Reports suggest, Aβ induced hyperexcitability is also associated with excessive secretion of glutamate from the neurons and astrocytes, leading to enhanced glutamate levels in the extrasynaptic space [149]. Additionally, individuals with AD were found to have decreased glutamate (Glu) in their hippocampal regions [159]. Aβ plaques and soluble Aβ oligomers cause excitotoxicity through different mechanisms including: (1) stimulation of glutamate release, (2) inhibition of glutamate uptake, and (3) alteration of pathways associated with activation of glutamatergic receptors [149]. Glutamate-induced excitotoxicity in the hippocampus has been correlated with dendritic branching, neuronal injury, and reduced neuronal regeneration. All these molecular events lead to impaired spatial learning [160]. Inhibition of glutamate uptake has been linked to reduced reward sensitivity, a symptom of depression [161]. Additionally, excess extracellular glutamate leads to cellular influx of Ca2+. Recent studies reveal that Ca2+ stimulates the oligomeriztion of Aβ [162]. Therefore, it is plausible that the protracted influx of Ca2+ via the activation of NMDA receptors can promote the generation of toxic Aβ oligomers, working as a sort of positive feedback loop [163].
Dopamine (DA) is known to modulate calcium signalling, especially in response to physiological glutamate concentrations. Dopamine acts as a neuroprotectant in glutamate-induced cell death at pathologic concentrations of glutamate. Dopamine helps in preventing calcium deregulation in hippocampal, cortical, and midbrain neurons [164]. Several disruptions in the dopaminergic (DAergic) system have been reported in AD. Reduced dopamine levels were found in the cingulate gyrus, nucleus amygdalae, nucleus caudatus, putamen, raphae, and substantia nigra in patients [165]. The reduced levels of dopamine receptors, especially the D2 subtype are associated with the pathology of AD [17]. Dopaminergic neurons are predominantly found in different areas of the midbrain. Dopamine from each area projects into various regions of the brain, exerting different functions. One of the primary sources of DA in the hippocampus which was initially thought to be the dopaminergic neurons located in the VTA17. However, reports suggest that dopaminergic neurons originating from the locus coeruleus (LC) are a key source of dopamine in the dorsal hippocampus [166]. Binding of this DA to the dopaminergic receptors, D1/D5 located in the dorsal hippocampus, is the key determinant of hippocampal synaptic plasticity and memory encoding [17]. Dopaminergic neurotransmission in the hippocampus also aids in the successful consolidation of long-term memories [167]. The dopaminergic neurons originated from the VTA are also known to target the cerebral cortex and nucleus accumbens (NAc). Thereby, mediating the regulation of reward processing and incentive motivation [168]. In mice models of AD, expressing the amyloid precursor protein (APP), progressive degeneration of the VTA dopaminergic neurons was observed. Interestingly, neurodegeneration of the VTA dopaminergic neurons occurs at the pre-plaque stage in this model [152]. Additionally, accumulation of Aβ in transgenic AD mice, or administration of Aβ1-42 oligomers in WT mice leads to reduced dopamine release in the cortex. This deregulation of DA resulted in conversion of long-term potentiation (LTP) into long-term depression (LTD) following high frequency stimulation (HFS), leading to impaired recognition of memory [169].
Additionally, increased density of dopamine D3 receptors in the nucleus acumbens and increased availability of striatal dopamine (D2/D3) has been positively correlated with AD related psychosis [170]. Surprisingly, the striatum, which is known to control motor activity, has high levels of DA and very little level of norepinephrine (NE). Comparatively, hippocampus shows little or no expression of dopamine transporter (DAT) as compared to the striatum. Therefore, the clearance of dopamine from the hippocampal regions is dependent on the uptake of the extracellular dopamine by the norepinephrine transporter (NET) present on the dopaminergic neurons of the VTA and the LC [171]. Unlike the VTA, which solely contains dopaminergic neurons, the neurons of the LC synthesize dopamine only as an intermediary in the synthesis of norepinephrine. Hypothesis suggests that the dorsal hippocampus, neuronal cells from the LC co-release DA along with NE. Thus, the dopaminergic system is tightly coupled to the noradrenergic system in the hippocampus [166].
By virtue of its extensive innervation of multiple regions of the forebrain, the noradrenergic system controls many behavioural and physiological processes [172]. Research in animal models indicates that the loss of NE results in: (1) the generation of a neurotoxic proinflammatory condition, (2) reduction of Aβ clearance, and (3) negative impacts on cognition. All these features recapitulate the key aspects of AD. Selective ablation of noradrenergic neurons in the AD mouse models increases the accumulation of Aβ [173], impairs spatial memory [174] and alters the α1, α2, and β1 adrenergic receptor (Figure 4) binding sites [174]. Such models also exhibited increased accumulation of hyperphosphorylated tau in the cerebral cortex [175]. Mice which are unable to synthesize norepinephrine have compromised maze performance and LTP [176]. These evidences suggest the loss of noradrenergic input to the cortex exacerbates, which results into AD-like behavioural and pathological implications [177]. Inspite of the AD associated loss of the noradrenergic neurons, there are conflicting reports regarding the levels of norepinephrine in the brain. High levels of norepinephrine were found to be associated with individuals suffering from advanced stages of AD. Similarly, elevated levels of NE and 3-methoxy- 4-hydroxyphenylglycol (MHPG), a metabolite of NE in the blood, plasma, and CSF were found in individuals with advanced AD [178]. Other post-mortem studies have found reduced levels of NE and serotonin in the frontal and temporal cortices of AD subjects. On the contrary, the concentrations of MHPG were found to be elevated in these subjects [179]. Surprisingly, in an ante-mortem study, no change was observed in the NE levels in the frontal cortex, but significant decrease was observed in the temporal cortex [180]. Loss of norepinergic neurons of the LC and subsequent loss of norepinephrine have a pro-inflammatory effect in animal models [181]. Moreover, observation suggests, stimulation of the microglia with norepinephrine leads to enhanced phagocytosis of amyloid-β (Aβ) [181]. Therefore, alterations of the norepinergic system contribute partially to the neuroinflammation associated with AD and the subsequent consequences [181]. The NE receptors and transporters may be associated with AD related behaviour. Role of α1-AR binding sites in the cortex and hippocampus are correlated with aggressive behaviour [182].
Figure 4: (A) Representation of human beta2 adrenergic receptor (Courtesy: PDB ID: 3D4S, Cholesterol bound form of human beta2 adrenergic receptor. Hanson et al., 2008. (B) Turkey Beta1 adrenergic receptor (Courtesy: PDB ID: 2VT4, Turkey Beta1 adrenergic receptor with stabilizing mutations and bound to Cyanopindolol, Warne et al., 2008).
In AD, several brain regions have a decreased concentration of 5-HT along with significant decrease in 5-HT1 and 5-HT2 receptor levels in the cerebral cortex [183]. Interestingly, reduced serotonin levels and increased 5-HT1A receptor density in the neo cortex are associated with cognitive impairment in AD. Whereas, the expression of 5-HT(6) and 5-HT(1B/1D) receptors are found to be reduced in the frontal and temporal cortex of the AD patients with low Mini-Mental State Examination (MMSE) scores [184]. In the hippocampus, a negative correlation exists between the activity of 5-HT1A receptors and verbal memory. Cognitive impairments observed in AD are known to be associated with increased 5-HT1A receptor density. Cirrito et al. [184] demonstrated that activation of serotonergic neurotransmission is beneficial in AD. Moreover, production of toxic Aβ proteins and plaques decreased upon administration of selective serotonin reuptake inhibitors (SSRIs) in AD mouse models. Similar results were obtained upon infusion of serotonin into the hippocampus of AD mice [184]. In humans, lower cortical amyloid levels were observed in participants who had been administered SSRIs in the past five years [186]. These transporters remain at much lower levels in patients having AD. These drugs cannot serve their purpose without their precise target [187].
It was shown that increased 5-HT1A receptor density is associated with the cognitive impairment observed in AD. Therefore, 5-HT1A receptor provided the basis for using antagonists in AD treatment [188]. Similarly, 5-HT1B/1D was also found to be associated with cognitive dysfunction in AD. A recent study confirmed that 5-HT1B/1D receptor density was significantly reduced in the frontal and temporal cortex of AD patients with impaired Mini-Mental State Examination (MMSE) scores [184].
Next to memory impairment, spatial disorientation is one of the earliest manifestations of AD. The severe cognitive impairment associated with AD interferes with abilities essential for smooth navigation. This includes reference frame translation, optic flow perception, scene matching, visual perceptual analyses, spatial planning, and possibly landmark recognition. These difficulties seem to stem from the wide spread damage in areas of the parietal lobes, the temporal cortex, retrosplenial cortex (RSC) and frontal lobes [189]. Various natural and synthetic medications have been used to cope up with such behavioural anomalies [190,191]. However, conclusive therapeutic intervention is still unachieved.
▪ Parkinson’s disease and related psychobehavioural anomalies
Parkinson disease (PD) is the second-most common neurodegenerative disorder, and affects 2–3% of the population ≥ 65 years of age. PD is characterized by loss of neurons in the substantia nigra leading to dopamine deficiency in the striatum and intracellular aggregation of α-synuclein called Lewy bodies (LBs). These physiological alterations are the hallmarks of Parkinson disease. LBs are considered to be the markers for neuronal degeneration, as neuronal loss is associated with sites rich in LBs [192]. Impairments in different cognitive domains such as language, memory, executive functions, and visio-spatial skills have been reported in the early stages of PD [193]. Though the clinical diagnosis of PD relies on bradykinesia and motor functions, many non-motor symptoms also append the overall disease load [194]. The nonmotor symptoms of PD range from abnormalities in sleep, such as Rapid Eye Movement behaviour disorder (RBD) to apathy, chronic fatigue, and cognitive dysfunction, which may arise from the non-dopaminergic neurodegeneration in PD [195]. The underlying molecular pathogenesis of PD involves multiple pathways including α-synuclein proteostasis, oxidative stress, mitochondrial function, axonal transport calcium homeostasis, and neuroinflammation [194,196-201].
PD is characterized by an imbalance in the neurotransmitters of the extrapyramidal system with a surplus of acetylcholine and glutamate and a deficiency of dopamine and GABA. Other neurotransmitters such as serotonin, neuroactive substances such as adenosine, and neuropeptides such as substance P and dynorphin are also involved in the disease pathophysiology [202]. Both motor and non-motor manifestations of PD exhibit robust diurnal oscillations [203]. Circadian deregulation has been recognized as a crucial cause of sleep disruption in most of the neurodegenerative disorders including PD and AD [204]. Importantly, circadian and sleep misalignment can influence the neurodegeneration itself [205]. A prime example of the bidirectional relationship between sleep/ circadian rhythm and neurodegeneration is AD. In AD, accumulation of Aβ disrupts sleep and enhances the risk of further Aβ accumulation and development of dementia [205]. The role of the circadian rhythm is not well studied in PD. However, diurnal variations in the levels of DA, its receptors, and some of its metabolites have been well-documented for PD [206].
Oxidative stress has a role in PD associated neurodegeneration, but the exact connection between these pathways and neurons is still unclear. Burbulla et al. [206] have identified that mitochondrial oxidative stress leads to accumulation of oxidized dopamine in the human substantia nigra pars compacta (SNc) dopaminergic neurons in a time-dependent manner. This ultimately leads to lysosomal dysfunction, reduced glucocerebrosidase activity, and α-synuclein accumulation, all of which are key features of PD [206]. The majority of dopaminergic neurons in the brain are located in the midbrain region, which project into the basal ganglia (nigrostriatal system), the cortical regions (mesocortical system), and the limbic regions (mesolimbic system) [207]. The largest group of dopaminergic neurons (A9 group) are located in the substantia nigra (SN), a region responsible for motor control and reward processing [208]. The ventro-lateral A9 neurons that project into the putamen, degenerate prior to the formation of LBs. Progression of PD is associated with increased degeneration of A9 nigrostriatal neurons that results in increased motor deficits. These deficits are ameliorated upon dopamine replacement [209,210]. Studies in animal models suggest that the loss of norepinephrinergic neurons might exacerbate dopaminergic neuronal damage in PD. Thus, norepinephrine might have neuroprotective effects [211].
Data suggests that a crosstalk exists between the dopaminergic system and other neurotransmitter systems in various parts of the brain. This affects the pathology of PD. In the striatum, depletion of dopamine leads to excessive release of acetylcholine which trims the neuronal spines of the indirect-pathway projections. Thereby, interrupting the transfer of information from motor command centres located in the cerebral cortex [212]. Serotonergic neurons also impair the release of striatal dopamine in PD. Dopamineserotonin interactions have been implicated in neuropsychiatric disorders and reward-related behaviour as well [213]. Moreover, Dopamine activity within the SNc is in-turn modulated by GABAergic and glutamatergic innervations [214].
Besides dopamine, the cholinergic system is thought to be significant in developing PDrelated symptoms. Cholinergic denervation was found to be related to the REM behaviour disorder, gait disorders, fall history, and cognitive dysfunction [215]. The muscarinic acetylcholine (ACh) receptor (AChRs) antagonists were used for treating akinetorigid disorders long before the recognition of PD as a disorder [216]. The cholinergic system was found to be related to the development of visual hallucinations in individuals with or without PD. Loss of grey matter in the thalamus and the cholinergic pedunculopontine nucleus region is associated with hallucinations in PD. An extensive decrease in the biochemical acetylcholine transferase in neocortex was found to be associated with hallucinations in PD [217]. The serotonin system might also be responsible for the development of psychosis in some cases of PD [218].
▪ Amyotrophic Lateral Sclerosis and related psycho-behavioural anomalies
Amyotrophic lateral sclerosis (ALS) otherwise referred to as motor neurone disease (MND)/ Lou Gehrig’s disease involves cognitive and behavioural impairment of the patient. A large proportion of such patients face chronic depression [219]. Experiencing executive function deficits, i.e., inability to maintain verbal fluency, working memory and problem solving skills, behavioural dysfunctions, are the chief indicators of improper functioning of the prefrontal brain circuits and occurrence of ALS. Frontal Systems Behaviour Scale (FrSBe) is one of the commonly adopted rating scales for the assessment of apathy, disinhibition, and executive dysfunction in ALS patients [220]. Most of these symptoms are overlapping and should be analysed carefully. For example, most behavioural changes seen in ALS patients are similar to those of patients diagnosed with behavioural variant fronto-temporal dementia (bvFTD) [221]. Woolley et al. [221] found that unlike non-demented ALS patients, significant lack of insight was observed in patients diagnosed with both ALS and FTD [221].
Rabkin et al. [222] clearly indicated that the degree of depression and the wish to die are directly dependent upon the presence or absence, and severity of behavioural and cognitive impairment of the patients [222]. Therefore, patients exhibiting early symptoms of ALS must be screened for dementia and depression for designing proper treatment regime.
▪ Bipolar disorder and related psychobehavioural anomalies
Once called ‘manic-depressive illness’, Bipolar disorder (BD), can be defined in terms of a single lifetime manic or mixed event [223]. BD is the sixth leading cause of disability worldwide. Before treatment, it is ensured that the disorder must have appeared for more than a week. BD symptoms (type I) are generally noted as irritability or euphoria apart from depressive feelings, sleeping problems, grandiose ideas, unusually talkative nature, impulsive behaviour, increased randomness and other related agitated behaviour [223]. However, not all BD cases experience periods of depression. BD is a tandem and severe disorder that causes lack of interest towards life, accompanied by loss of proper body functioning [224,225]. The genes responsible for BD could be related to schizophrenia. Emotional dysregulation together with unregulated dopamine and serotonin levels characterizes the pathological feature of BD [225,226].
Stress due to work, sudden shock, and emotion influences the course of illness significantly. Prescribed social interactions along with conventional medication enhance the longterm outcomes [227]. Interactive contributions of genetic, neurobiological, and psychosocial factors are involved in therapeutic regime of BD [224]. Such approach helps to understand the interactions between genes, neural pathways, and socio-environmental influences for depression or mania [226]. Some BD patients exhibited hypomanic nature along with chronic depression. Symptoms of BD (type II) are associated with functional impairment [225].
Major depression comprises two or more weeks of intense sadness or loss of interest with symptoms like insomnia, tiredness, psychomotor agitation or retardation, body weight fluctuation, cognitive dysfunction, low self-morale, and suicidal tendency [225]. The occupational and social dysfunction is evident through signs of depressive symptoms, excessive alcohol use, anxiety, abnormal symptoms and degraded socioeconomic status [227]. Functional impairment can be seen between episodes and most frequently in case of subsyndromal depressive symptoms [228,223]. Clinical studies on BD subjects revealed that only 48% chances of complete recovery with 24% chances of functional restoration are possible [228,230]. Studies also indicated that the after effects of manic episodes can be witnessed even at workplace, home and surroundings even after five years [222]. Elevated creativity and productivity was also observed in several BD patients. There are many similarities between BD patients and exceedingly creative people [226]. Norepinephrine, dopamine, and serotonin explain mood related illness in BD [227,228]. Low levels of dopamine and norepinephrine specify depression while higher levels indicate mania. On the other hand, low levels of serotonin lead to unregulated dopamine and norepinephrine levels [229]. Nonetheless, recent studies suggested that effects of amphetamine are instigated in BD patients even in the absence of increased dopamine binding. This rejects the former notion about mood disorders that refers binding levels of neurotransmitters at the synaptic cleft [228,230]. Apparently, sleep deprivation affects sensitivity of dopamine receptors. Almost 10% of mania affected people are found to develop symptoms immediately after a sleepless night. BD cases are also characterized by decreased serotonin sensitivity. Being the precursor molecule, manipulation in tryptophan levels affects serotonin systems critically [231].
▪ Schizophrenia and related psychobehavioural anomalies
Schizophrenia is a poorly understood, debilitating psychiatric disorder. This condition is known to have variable phenotypic symptoms and extremely complex etiology involving interplay of genetic and environmental factors [232]. Schizophrenia usually involves the presentation of typical symptoms of “positive (e.g., hallucinations and delusion), negative (e.g., social withdrawal and flat affect), and cognitive impairment” [233]. Schizophrenic patients have extremely high suicidal tendencies and violent behaviour [234]. Common yet atypical behavioural patterns of schizophrenia include laughing without reason, depression and anxiety and irrational anger and abnormal sleep patterns.
Frequent clinic visits for diagnostic issues usually makes it difficult for the patient to remain employed or having normal relationships with family and spouse. However, this usually occurs in advanced stages of the disease by which time the clinical and economic burden of the disease has already been amplified [234]. Therefore, identification and implication of psychobehavioural anomalies associated with the disease can help in diagnosing the disease, preferably in its early stages.
Often, schizophrenic patients start showing behavioural changes like difficulty in self-care and proper dressing in their adolescence [235]. Another distinctive behavioural symptom observed in early stages of schizophrenia is the “flat effect”, where the patient lacks normal human emotions [236]. Clinically, schizophrenic patients have altered facial activity, thus projecting unresponsiveness or flatness towards human emotions [237,238]. The “cognitive symptoms” of the disease include experiencing difficulty in remembering information [239,240]. Physically, people with schizophrenia experience catatonia (motor immobility), expressing state of stupor [241]. Changes in the verbal patterns of schizophrenic individuals usually involve halting mid-sentence and then resuming speaking on an entirely different topic. Sometimes, they may also say incredibly long but meaningless sentences, often referred to as “word salad.” Such “word salads” often contain rhyming words, in a pattern referred to as “clang association” [241].
▪ Impulse control disorders (ICDs) and related psycho-behavioural anomalies
Impulse control disorders (ICDs) refer to common psychiatric conditions which typically result in impaired occupational and social functioning. According to the DSM-IV [242], these primarily include: kleptomania, pyromania, trichotillomania (TTM), pathological gambling (PG), and intermittent explosive disorder. The DSM-IV-TR (DSM-IV-Text Revision) recognizes auxiliary disorders categorized as ICDs not otherwise specified (NOS) such as: compulsive shopping (CS), compulsive eating (CE), hypersexuality (HS), and pathological skin picking (PSP). ICDs are primarily characterized by repetitive behaviour and inhibition of these impulsive behaviours [243]. Key defining criteria for ICDs are the failure to resist the impulse to engage in the act, an impending sense of anticipation prior to an act, and a sense of pleasure, gratification, or relaxation while doing the act [244].
Pathological gambling (PG) is characterized by recurrent and persistent gambling behaviour. This condition has also been referred to as chronic, relapsing condition. PG is estimated to affect an estimated 0.9% to 1.6% of the United States population [245]. Men tend to suffer more from PG and begin gambling at an earlier age as compared to women. On the contrary, women are known to progress towards problematic gambling more rapidly than men. Various neurotransmitters have been hypothesized to be associated with different aspects of PG such as high levels of noradrenaline and low levels of serotonin signalling [246].
Kleptomania refers to the uncontrollable impulse to steal. It is characterized by an anxious urge to perform an act which provides pleasure in the moment but in the long-run, causes significant distress and dysfunction. It is estimated that 6 out of every 1000 people in the US suffer from this condition [247]. The etiology underlying this condition is yet unclear. However, serotonergic dysfunction has been hypothesized to be responsible for the poor decision-making in these individuals. It has been reported that any damage to the orbitofrontal-subcortical circuits in the brain might result in kleptomania. Kleptomaniacs have decreased white matter microstructural integrity in the ventro-medial frontal regions of their brains [248].
Pyromania refers to an impulsive, deliberate, and repetitive act of fire setting without the expectation of an external reward. More than 90% of the individuals with pyromania have been diagnosed with comorbid Axis I disorders. It was revealed that a left inferior frontal perfusion deficit might be associated with pyromania [249].
Trichotillomania (TTM) is a chronic ICD characterized by repetitive hair pulling, causing significant distress and/or functional impairment. Deregulation of serotonin, dopamine, and GABA levels is associated with TTM. The frontal-basal ganglia circuitry is thought to be of particular importance in TTM. Some reports suggest that TTM is associated with elevated grey matter densities in the left amygdalo-hippocampal formation, left striatum, and multiple cortical regions [250].
Intermittent Explosive Disorder (IED) is characterized by recurrent impulsive aggressive behaviour which is disproportionate to any resulting psychosocial stress. Traditionally, the neurobiological underpinnings of aggression are associated with deficiencies in the serotonergic system. For instance, reduced availability of the 5-HT transporter in the anterior cingulate cortex is known to be associated with IED. Researchers observed a stronger response to angry faces in the amygdala of IED patients as compared to controls. IED patients have lower amygdala– orbitofrontal cortex connectivity as compared to controls [251].
Compulsive shopping (CS) disorder is a condition that is ubiquitous around the world. The disorder has prevalence of 5.8% in the US [252]. Though, most of the subjects studied are women (~80%), this gender difference may be arbitrary. Subjects with CS manifest a preoccupation with shopping, pre-purchase anxiety, and a feeling of relief following the purchase. The brains of the CS individuals show increased activity in the nucleus accumbens (NAc) and the anterior cingulate cortex (ACC) during the initial presentation phase and the decision making phase of the shopping exercise, respectively [253].
Compulsive eating (CE) disorders refer to disruptions in the eating behaviour. According to DSM-IV, binge eating in the absence of compensatory behaviour, is provided a clinical diagnosis of eating disorder not otherwise specified (EDNOS). Individuals with CE disorder exhibit decreased reward sensitivities, greater cognitive attention bias towards food, and altered activity in regions of the brain associated with impulsivity and compulsivity. In humans, reduction in 5-HT activity is known to support compulsive eating. Neuroimaging represents alterations in the cortico-striatal circuitry in CE similar to those observed in substance abuse [254,255].
Hypersexual Disorder or Compulsive sexual behaviour (CSB) has been proposed as a new psychiatric disorder under the Sexual Disorders section in DSM-V. Repeated engagement in sexual activities inspite of the negative consequences is the major hallmark of CSB [256-271]. Hypersexuality can manifest itself in a variety of ways with differing degrees of severity and further divided into paraphilic and nonparaphilic types. Paraphilic sexual behaviours refer to behaviours outside the bounds of the conventional range of sexual behaviours. Nonparaphilic behaviours refer to engagement in common sexual practices. Clinical signs of CSB might include anxiety, depression, alcohol or drug use/dependency, somatic complaints, relationship problems [256,257].
PSP or Excoriation is a disabling condition characterized by compulsive picking of the skin resulting in noticeable tissue damage. The face is the most commonly picked area, but any part of the body can be affected. Experiments in animal models indicate that glutamate based neurotransmission might have an important role in the pathology of PSPD [258].
Nowadays, ICDs are increasingly emerging in many PD patients as side-effects of dopaminergic therapies, thus, becoming key concerns in the treatment of PD [259]. The ICARUS (Impulse Control disorders and the association of neuRopsychiatric symptoms, cognition and qUality of life in ParkinSon disease) study reported that the prevalence of the primary ICDs in PD is having broad range, from 3.5% to 42.8% in different cases. This difference might be due to the under diagnosis of ICDs in clinical practises. Repetitive behaviour such as: walkabouts, hoarding, kleptomania, impulsive smoking, reckless generosity and reckless driving are other recognized ICDs in PD patients. ICDs have also been reported in other movement disorders such as restless legs syndrome (RLS), progressive supranuclear palsy (PSP), and multiple system atrophy, which require treatment with dopaminergic medication [259].
▪ Obsessive-compulsive disorder and related psycho-behavioural anomalies
Obsessive-compulsive disorder (OCD) is a heterogeneous condition that makes the patient feeble [260]. The disease is characterized by sudden mixed feelings and repetitive behaviour. Presently, structural and functional neuroimaging based analyses are being considered for OCD [260]. It is believed that neurochemical functioning mediates OCD symptoms. However, recent psychopharmacologic studies have revealed the critical role of serotonin (5-HT) neurotransmitter system in OCD symptoms [261]. Selective serotonin reuptake inhibitors (SSRIs) were found to be more beneficial in the long-term as compared to noradrenergic reuptake inhibitors in OCD treatment. Successful treatment of OCD using antipsychotic and psychostimulants was reported. OCD is experimentally found to cooccur with ADHD. Psychostimulants are being used for treating both the conditions. Another interesting compulsive habit or disorder is Onychophagia (OP) which leads to destruction of nails. Nail biting has received little attention in the psychiatric and dermatological literature in the past. Nonetheless, OP has received much attention in recent days on account of co-occurring psychopathological symptoms that show relevance and close relationship with OCD and mood disorders. Frequency and severity of OP specifies the degree of OCD pathology. Neurotransmitter based studies have revealed imbalance in serotonin, dopamine and norepinephrine levels in OCD [262]. Increased dopamine levels induce compulsion and obsession, leading to anxiety in the subject. Similarly, glutamate is another important neurotransmitter in relation to OCDs. Antiglutamatergic drugs like lamotrigine, topiramate, and gabapentin have shown profound therapeutic effect in OCDs [263]. Further research on these therapeutic paradigms is essential for better OCD treatment. Owing to its heterogeneous function and receptor activity in Cortico-Striato-Thalamo-Cortical (CSTC) circuit, dopamine serves as the principal neurotransmitter involved in the pathophysiology of OCD [264]. Clinical translational studies have confirmed the continuous participation of the dopaminergic system causing obsession and perseverations. Stimulants like amphetamines which function as dopamine antagonist indeed show pro-OCD behaviour resulting in the development of persistent behaviour, sudden muscle movements, and perseverations in case of brain injury [260].
▪ Recent trends and possible therapeutics for symptomatic rectification
The spectrum of neurological disorders is so vast and overlapping that they implicit the occurrence of multiple diseases viz. AD, PD, Frontotemporal Dementia (FTD), Pick’s disease, spinocerebellar ataxias, progressive supranuclear palsy, brain trauma, Huntington’s disease, ALS, etc. These diseases are known to have extremely high morbidity rates, reinforcing the importance of their timely diagnosis and treatment. Unfortunately, none of these diseases are known to be completely curable [265]. Hence, the only strategy is symptomatic treatment. The management of symptoms largely depends upon the mode of neurodegeneration as well as other associated physiological changes [265,266].
As a thumb rule, progressive neuronal cell death is the chief contributing factor to the inception and progression of most neurodegenerative diseases [267]. Symptomatic manifestation of the disease is largely dependent upon the specific cell death pathways associated with the condition. Elucidating these pathways may pave the way for disease specific symptomatic treatment [268]. Some of the most conventionally applied treatment methods and strategies for the treatment of neurological diseases are discussed earlier [269].
▪ Dopaminergic treatments
Neurons located in the substantia nigra are responsible for an extensive network of axonal processes. These neurons communicate with the basal ganglia via the transmission of the neurotransmitter dopamine (DA). The associated neuronal pathways are responsible for regulating the body movements [270]. Neurodegenerative diseases, such as PD involve the degeneration of these dopamine producing neurons of substantia nigra. As a result, the concentration of dopamine decreases substantially and thus obstructing all decreases substantially and thus obstructing all downstream neurological pathways.
Such a condition leads to dysfunctional motor coordination, the characteristic feature of PD. Thus, administration of dopamine precursors (Levodopa) can help in replenishing dopamine, and improving the functioning of the dopamine signalling pathways and their associated motor functions [270].
Currently, the therapeutic approach for the treatment of PD involves administration of Levodopa, along with a peripheral decarboxylase inhibitor. Levodopa is known to be converted into dopamine in dopaminergic neurons, thus, replenishing the deficiency of this essential endogenous neurotransmitter [271]. Though it is well tolerated and cost effective, long term administration may cause fluctuating motor responses and dyskinesia in the patient. Success of this treatment promoted the development of dopamine agonist based drugs that can directly stimulate the dopamine receptors. These agonists mimic the dopamine function in activating dopamine receptors and the subsequent cascade of neurotransmitter pathways. Ergoline and non-ergoline are two distinct classes of dopamine agonists that target the dopamine D2- type receptors. Most commonly administered Ergoline dopamine agonists are bromocriptine, pergolide, lisuride, and cabergoline. Most common non-ergoline agonists include ropinirole and pramipexole [272].
▪ Antipsychotic drugs
The signalling pathways related to dopamine essentially depend on the crucial step of dopamine binding to its receptor. However, the functional excess of DA or oversensitivity of DA receptors may lead to the dysfunction of dopamine based neurosignaling pathways. In fact, these factors form the basis of psychosis in many clinical cases. Similarly, delusions and hallucinations due to dopamine imbalance may eventually lead to schizophrenia. Such patients are prescribed antipsychotic drugs that can block and/or reduce oversensitivity of the DA receptors. Chlorpromazine was the first antipsychotic drug discovered way back in the early 1950s [273]. Haloperidol is an anti-psychotic that is predominantly used for the treatment of AD [274]. These drugs are typical of first generation anti-psychotic drugs. Their relatively recent counterparts, often referred to as second generation or atypical antipsychotic drugs (AAPDs) have similar therapeutic efficacy but with significantly reduced adverse effects. Some of the most commonly administered AAPDs are clozapine, risperidone, and aripiprazole [275]. They are also considered to be the most preferable mode of treatment/management of dementia associated cognitive impairment as well as non-cognitive behavioural and psychiatric changes [276]. Administration of anti-psychotics is often accompanied with many side effects that may have fatal consequences. Development of Parkinsonian symptoms, increased feeling of sedation, dizziness, unsteadiness as well as chest infections, are some of the most common side effects of ant-psychotics. Clinical trials have confirmed that administration of these drugs in elderly AD patients increases the risk of mortality [277]. A similar trend has been observed in PD patients as well. Administration of these drugs even worsened the motor symptoms of the patients in some cases [278]. Therefore, anti-psychotic drug based treatments must be carefully monitored with the help of periodic haematological parameter evaluation.
▪ Cholinesterase inhibitors
Multiple in vitro and in vivo studies demonstrated the presence of a close association between cholinergic activation and metabolic implications of APP. It is evident that presence of cholinergic lesions influences the ratio between cortical amyloid precursor protein (APP) and cerebrospinal fluid (CSF) levels. It reduces cholinergic neurotransmission and promotes amyloidogenic metabolism in the brain. This symptomatic manifestation of such changes is usually represented as cognitive dysfunction associated with AD, HD, and many other cognitive disorders [279]. It is verified that application of cholinesterase inhibitors (ChEI) can help in averting such neuro-pathological changes, stabilizing cognitive dysfunction, and symptomatic management of dementia in the early stages of AD. Donepezil hydrochloride, galantamine hydrobromide, and rivastigmine tartrate are some of the conventionally administered ChEIs that are currently being used as part of the primary treatment for cognitive improvement in AD patients [280]. Recent clinical trials where AD patients were administered ChEIs also demonstrated their benefit for neuropsychiatric and behavioural symptoms [281]. Similar patterns are observed in the management of dementia with Lewy bodies as well as cognitive impairment associated with PD [282].
It has also been suggested that ChEIs can be administered with antipsychotic drugs as a method of treatment of cognitive dysfunctions in Schizophrenia patients [283]. It has been proposed that ChEIs may also have beneficial effects on Huntington’s disease (HD). However, such treatment regimens are yet to be standardized for specific clinical settings [284].
▪ Analgesic and Anti-inflammatory drugs
Recent neuroimaging studies have revealed that brain function is effectively correlated with the occurrence of chronic pain, which leads to alterations in the emotional, cognitive, and modulatory processes. Peripheral nerve damage is one of the most common causes of chronic pain (neuropathic pain). Observation suggests, occurrence of chronic neurodegenerative diseases also form crucial part of the complex etiological basis of such pain symptoms. Around 40–60% of PD patients are known to suffer from chronic pain [285]. Similar results were observed in studies conducted on AD patients. It was further postulated that the neurodegeneration associated with such diseases may affect brain areas responsible for pain perception. Hence, the choice of treatment in such patients is quite complex. Most often, such patients are prescribed analgesics for pain management [286]. Non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen are the most commonly administered analgesics that may be administered either alone or in combination with opioids viz. codeine [287].
It has been proposed that the process of amyloid β peptide induced neuro-degeneration in AD involves inflammatory responses. Therefore, suppression of such inflammatory responses can help in mitigating the disease progression [288]. However, clinical trials estimating the efficacy of glucocorticoid therapy, hydroxychloroquine, and non-steroidal anti-inflammatory drugs (NSAIDs), especially in the treatment of AD indicated mixed results [111]. Such responses may be attributed to non-selective inhibition of cyclooxygenase (COX) as well as insufficient penetration of the drug through the blood-brain barrier (BBB). Current studies are focused on sorting out such issues. It has been proposed that development of combination therapies based on antioxidants or glutamate antagonists may serve the purpose [289,290]. Some of the most commonly used drugs that are used for the symptomatic management of neurological diseases by manipulating the neurotransmitter levels are listed in Table 3.
| Sl.No | Disease | Symptoms | Neurotransmitters involved | Drugs used for treatment | Reference |
|---|---|---|---|---|---|
| 1 | Parkinson’s disease | Tremor Bradykinesia Rigid muscles Impaired posture & balance Speech changes Writing changes Loss of automatic movements |
Dopamine, Acetylcholine, Serotonin, GABA, Dynorphin, Neurotensin, Substance P |
Levodopa and carbidopa Dopamine agonists Tolcapone and entacapone Selegiline Trihexyphenidyl Benztropine dopaminergic treatments caffein A2A receptor antagonists and CERE-120 (adeno-associated virus serotype 2-neurturin) |
Mizuno Y: Recent research progress in and future perspective on treatment of Parkinson's disease. Integr Med Int 2014;1:67-79. Schwarzschild MA, Xu K, Oztas E, Petzer JP: Neuroprotection by caffeine and more specific A2A receptor antagonists in animal models of Parkinson's disease. Neurology 2003;61(suppl 6):S55-S61. Marks WJ Jr, Ostrem JL, Verhagen L, et al: Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol 2008;7:400-408. |
| 2 | Alzheimer’s disease | Memory loss Impaired problem solving Obsessive, repetitive or impulsive behaviour, Disturbed sleep, Mood swings, Depression, Hallucinations |
Acetylcholine (Ach) | Cholinesterase inhibitors, NSAIDs (non-steroidal anti-inflammatory drugs), Memantine (Namenda), sleep medications (zolpidem, eszopiclone), Anti-anxiety medications (clonazepam and lorazepam) | Stewart WF, Kawas C, Corrada M, Metter EJ: Risk of Alzheimer's disease and duration of NSAID use. Neurology 1997;48:626-632. |
| 3 | Schizophrenia | Hallucinations, Delusions Thought disorders, Movement disorders, ‘Flat affect’, Poor ‘executive functioning’ |
Dopamine | Antipsychotic Drugs | Tamminga CA, Medoff DR. The biology of schizophrenia. Dialogues in Clinical Neuroscience. 2000;2(4):339-348. |
| 4 | ALS | Progressive muscle weakness (painless) | GABA | Riluzole, edaravone | Foerster, Bradley R. et al. “3T MR Spectroscopy Reveals an Imbalance between Excitatory and Inhibitory Neurotransmitters in Amyotrophic Lateral Sclerosis.” JAMA neurology 70.8 (2013): 1009–1016. PMC. Web. 7 Nov. 2017. |
| 5 | Multiple sclerosis | Fatigue, Impaired vision, Numbness, tingling, Muscle spasms, Depression and anxiety, Speech and swallowing difficulties |
noradrenaline, excitatory amino acid (glutamate and aspartate), | Ampyra (dalfampridine), Imuran (azathioprine), Cytoxan, Novantrone (mitoxantrone IV), Natalizumab (Tysabri), Potent IV steroids, Interferon beta drugs | Barkhatova, V. P., Zavalishin, I. A., Askarova, L. S., Shavratskii, V. K., & Demina, E. G. (1998). Changes in neurotransmitters in multiple sclerosis. Neuroscience and behavioral physiology, 28(4), 341-344. |
| 6 | OCD | Fear of germs or contamination, Aggressive thoughts, Excessive cleaning and/or handwashing, Repeatedly ordering and arranging things, Compulsive counting | Serotonin, glutamate, dopamine | Serotonin reuptake inhibitors (SRIs), | Cooney JW, Stacy M. Neuropsychiatric Issues in Parkinson’s Disease. Current Neurology and Neuroscience Reports. 2016. doi:10.1007/s11910-016-0647-4 |
| 7 | ICD | pathological gambling, pyromania, intermittent explosive disorder, trichotillomania, kleptomania | Noradrenaline, Serotonin | Selective serotonin reuptake inhibitors (SSRIs) | Brewer JA, Potenza MN. The Neurobiology and Genetics of Impulse Control Disorders: Relationships to Drug Addictions. Biochemical pharmacology. 2008;75(1):63-75. doi:10.1016/j.bcp.2007.06.043. |
Table 3: Description of considered diseases along with their respective symptoms, involved neurotransmitters and applied drugs.
Available reports are in-line with the statement that, psycho-behavioural anomalies are the determining criteria for the diagnosis of neurological diseases. Behavioural anomalies indicate the specific neurochemical dysregulation in brain and also help postulate the measures for symptomatic rectification through proper medications.
Conclusion
The present review highlighted the interlinking relationship between neurochemical dysfunctioninduced psycho-behavioural anomalies in various neurological complications. We have emphasized the role of psycho-behavioural study as an essential tool for better understanding of neurological disorders. Available literature has shown that neurodegenerative diseases are unidirectional, and cardinal motoric symptoms arise when majority of the neurons have died. Psycho-behavioural anomalies have been shown to occur in parallel to the degree of neurodegeneration and expressions of such psycho-behavioural anomalies are observed in the early stages of the disease. Therefore, there are possibilities of identifying a set of psychobehavioural anomalies at the very onset of the disease. Such treatment paradigm could change or limit the disease progression. It is noteworthy that the pattern of neurodegeneration varies from patient to patient. Specific neurochemical abnormalities influence the drastic changes in behaviour pattern in neurological patients. Hence, by tracing the behavioural roots, researchers could find the key cause underlying such anomalies. Most of the times, in the absence of motor dysfunction, patients and physicians ignore the minor psychological anomalies. Hence, awareness and careful diagnosis of psycho-behavioural anomalies for a particular disease is the urgent need of time. Future research could consider specific set of psychological anomalies as the hallmark of early diagnostic parameters for neurological diseases.
Acknowledgement
Our sincere thanks are due to Mr. Venkata Varun for his graphical and illustrative support for this manuscript. Neelima Arora thanks UGC for Postdoctoral fellowship.
References
- Boyd CAR. Chemical neurotransmission, an hypothesis concerning the evolution of neurotransmitter substances. J. Theor. Biol 76(4), 415-417 (1979).
- Süudhof TC. Neurotransmitter release. Hand. Exp. Pharmacol 184, 1-21 (2008).
- Spitzer NC. Activity-dependent neurotransmitter respecification. Nat. Rev. Neurosci (2012)
- Richard S, Aguilera N, Thévenet M, et al. Neuronal expression of a thyroid hormone receptor α mutation alters mouse behaviour. Behav. Brain. Res 321, 18-27 (2017).
- Masson J, Sagné C, Hamon M, et al. Neurotransmitter transporters in the central nervous system. Pharmacol. Rev 51(3), 439-464 (1999).
- Branco T, Staras K. The probability of neurotransmitter release, variability and feedback control at single synapses. Nat. Rev. Neurosci 10, 373-383 (2009).
- Morris J, Marzano M, Dandy N, et al. Theories and models of behaviour and behaviour change. For Sustain Behav. Behav. Chang. Theor 1-27 (2012).
- Haywood J. Regional differences in neurotransmitter enzymes during the development of the chick brain. J. Neurochem 30, 1195-1197 (1978).
- Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults, general trends, individual differences and modifiers. Cereb. Cortex 15(11), 1676-1689 (2005).
- Garris PA. Advancing neurochemical monitoring. Nat. Methods 7, 106-108 (2010).
- Diehl DJ, Gershon S. The role of dopamine in mood disorders. Compr. Psychiatry 33(2), 115-120 (1992).
- Wise RA. Dopamine, learning and motivation. Nat. Rev. Neurosci 5, 483-494 (2004).
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin, news from mouse molecular genetics. Nat. Rev. Neurosci 4, 1002-1012 (2003).
- Brown DA. Acetylcholine. British. J. Pharmacol 147, S1 (2006).
- Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers. Archiv 460(2), 525-542 (2010).
- Zhou Y, Danbolt NC. Glutamate as a neurotransmitter in the healthy brain. J. Neural. Trans 121(8), 799-817 (2014).
- Martorana A, Koch G. Is dopamine involved in Alzheimer’s disease? Front. Aging. Neurosci 6 (2014).
- Morrison JH. Life and Death of Neurons in the Aging Brain. Science 238(5337), 412-419 (1997).
- Gao HM, Hong JS. Why neurodegenerative diseases are progressive, uncontrolled inflammation drives disease progression. Trends. Immunol 29(8),357-65 (2008).
- Altamura C, Maes M, Dai J, et al. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur. Neuropsychopharmacol 5(1), 71-75 (1995).
- Yoshiyama Y, Lee VMY, Trojanowski JQ. Therapeutic strategies for tau mediated neurodegeneration. J. Neurol. Neurosurg. Psychiatry 84(7), 784-795 (2013).
- Lazarini F, Gabellec MM, Moigneu C, et al. Adult neurogenesis restores dopaminergic neuronal loss in the olfactory bulb. J. Neurosci 34(43), 14430–14442 (2014).
- Südhof TC. Neurotransmitter release, The last millisecond in the life of a synaptic vesicle. Neuron 80(3), 675-690 (2013).
- Alkema MJ, Hunter-Ensor M, Ringstad N, et al. Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron 46(2), 247-260 (2005).
- Spitzer NC. Neurotransmitter Switching in the Developing and Adult Brain. Annu. Rev. Neurosci 40, (2017).
- Newberg AB, Iversen J. The neural basis of the complex mental task of meditation, Neurotransmitter and neurochemical considerations. Med. Hypotheses 61(2), 282-291 (2003).
- Segura-Aguilar J, Paris I, Muñoz P, et al. Protective and toxic roles of dopamine in Parkinson’s disease. J. Neurochem 129(6), 898-915 (2014).
- Rangel-Barajas C, Coronel I, Florán B. Dopamine Receptors and Neurodegeneration. Aging. Dis 6, 349 (2015).
- Tye KM, Mirzabekov JJ, Warden MR, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537-541 (2013).
- Wanat MJ, Willuhn I, Clark JJ, et al. Phasic dopamine release in appetitive behaviours and drug addiction. Curr. Drug. Abuse. Rev 2(2), 195-213 (2009).
- Sipetic SB, Vlajinac HD, Maksimovic JM, et al. Cigarette smoking, coffee intake and alcohol consumption preceding Parkinson’s disease, A case-control study. Acta. Neuropsychiatr 24(2), 109-114 (2012).
- Migály P. Hallucination, dopamine and hypnotizability. Biol Psychiatry. 53(2), 207-208 (1992).
- Kurniawan IT, Guitart-Masip M, Dolan RJ. Dopamine and effort-based decision making. Front. Neurosci 5(8), 0081 (2011).
- Gorwood P. Neurobiological mechanisms of anhedonia. Dialogues Clin. Neurosci 10(3), 291-299 (2008).
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure, Reward in humans and animals. Psychopharmacology 199(3), 457-480 (2008).
- Davis KL, Kahn RS, Ko G, et al. Dopamine in schizophrenia, A review and reconceptualization. Am. J. Psychiatry 48(11), 1474-1486 (1991).
- Tunik E, Feldman AG, Poizner H. Dopamine replacement therapy does not restore the ability of Parkinsonian patients to make rapid adjustments in motor strategies according to changing sensorimotor contexts. Park. Relat. Disord 13(7), 425-433 (2007).
- Mosienko V, Beis D, Pasqualetti M, et al. Life without brain serotonin, Reevaluation of serotonin function with mice deficient in brain serotonin synthesis. Behav. Brain. Res 277, 78-88 (2015).
- Martinowich K, Lu B. Interaction between BDNF and serotonin, Role in mood disorders. Neuropsychopharmacology 33(1),73-83 (2008).
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology 38(8), 1083-1152 (1999).
- Tierney AJ. Structure and function of invertebrate 5-HT receptors, A review. Comp Biochem Physiol A. Mol. Integr. Physiol 128(4), 791-804 (2001).
- Meneses A. 5-HT system and cognition. Neurosci. Biobehaviour. Rev 23(8), 1111-1125 (1999).
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav. Brain. Res 195(1), 198-213 (2008).
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol. Rev 72(1), 165-229 (1992)
- Bernhardt PC. Influences of serotonin and testosterone in aggression and dominance, Convergence with social psychology. Curr. Dir. Psychol. Sci 6(2), 44-48 (1997).
- Schildkraut JJ. The catecholamine hypothesis of affective disorders, a review of supporting evidence. J. Neuropsychiatry. Clin. Neurosci 7(4),524-533 (1965).
- Kaufman J, Yang BZ, Douglas-Palumberi H, et al. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol. Psychiatry 59(8), 673-680 (2006).
- Drachmann Bukh J, Bock C, Vinberg M, et al. Interaction between genetic polymorphisms and stressful life events in first episode depression. J. Affect. Disord 119(1), 107-115 (2009).
- Kohen R, Cain KC, Mitchell PH, et al. Association of Serotonin Transporter Gene Polymorphisms With Poststroke Depression. Arch. Gen. Psychiatry 65(11), 1296-1302 (2008).
- Deary IJ, Battersby S, Whiteman MC, et al. Neuroticism and polymorphisms in the serotonin transporter gene. Psychol. Med 29(3), 735-739 (1999).
- Lesch K-P, Bengel D, Heils A, et al. Association of Anxiety-Related Traits with a Polymorphism in the Serotonin Transporter Gene Regulatory Region. Science 274(5292), 1527-1531 (1996).
- Klauck SM, Münstermann E, Bieber-Martig B, et al. Molecular genetic analysis of the FMR-1 gene in a large collection of autistic patients. Hum Genet 100(2), 224-229 (1997).
- Ogilvie AD, Battersby S, Bubb VJ, et al. Polymorphism in serotonin transporter gene associated with susceptibility to major depression. Lancet 347(9003), 731-733 (1996).
- Sander T, Harms H, Dufeu P, et al. Serotonin transporter gene variants in alcohol-dependent subjects with dissocial personality disorder. Biol. Psychiatry 43(12), 908-912 (1998).
- Malhotra AK, Goldman D, Mazzanti C, et al. A functional serotonin transporter (5-HTT) polymorphism is associated with psychosis in neuroleptic-free schizophrenics. Mol. Psychiatry 3(4), 328-332 (1998).
- Konneker T, Barnes T, Furberg H, et al. A searchable database of genetic evidence for psychiatric disorders. Am. J. Med. Genet. Neuropsychiatr. Genet 147B(6), 671-675 (2008).
- Serretti A, Lilli R, Lorenzi C, et al. Serotonin transporter gene (5-HTTLPR) and major psychoses. Mol. Psychiatry 7(1), 95-99 (2002).
- Hsiung SC, Adlersberg M, Arango V, et al. Attenuated 5-HT1A receptor signaling in brains of suicide victims, Involvement of adenylyl cyclase, phosphatidylinositol 3-kinase, Akt and mitogen-activated protein kinase. J. Neurochem 87(1), 182-194 (2003).
- Lin SH, Lee LT, Yang YK. Serotonin and mental disorders, a concise review on molecular neuroimaging evidence. Clin. Psychopharmacol. Neurosci 12(3), 196-202 (2014).
- Malm H, Brown AS, Gissler M, et al. Gestational Exposure to Selective Serotonin Reuptake Inhibitors and Offspring Psychiatric Disorders, A National Register-Based Study. J. Am. Acad. Child. Adolesc. Psychiatry 55(5), 359-366 (2016).
- Lucki I. The spectrum of behaviours influenced by serotonin. Biol. Psychiatr 44(3) 151-162 (1998).
- Patrick RP, Ames BN. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2, Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behaviour. FASEB. J 29(6), 2207-2222 (2015).
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function, adaptive gain and optimal performance. Annu. Rev. Neurosci 28, 403-450 (2005).
- Tang NKY, Harvey AG. Effects of cognitive arousal and physiological arousal on sleep perception. Sleep 27(1), 69-78 (2004).
- Takahashi Y, Kipnis DM, Daughaday WH. Growth hormone secretion during sleep. J. Clin. Invest 47(9), 2079-2090 (1968).
- Zimmerman TJ, Boger WP. The beta-adrenergic blocking agents and the treatment of glaucoma. Surv. Ophthalmol 23(6), 347-62(1979).
- Mizuyama R, Uno L, Matsushima T. Food variance and temporal discounting in socially foraging chicks. Anim. Behav 120, 143-151 (2016).
- Schnell C, Negm M, Driehaus J, et al. Norepinephrine-induced calcium signaling in astrocytes in the respiratory network of the ventrolateral medulla. Respir. Physiol. Neurobio 226, 18-23 (2016).
- Esler MD, Hasking GJ, Willett IR, et al. Noradrenaline release and sympathetic nervous system activity. J. Hypertens 3(2), 117-129 (1985).
- Chrousos GP. Stress and disorders of the stress system. Nat. Rev. Endocrinol 5(7), 374-381 (2009).
- Del Campo N, Chamberlain SR, Sahakian BJ, et al. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol. Psychiatr 69(12), e145-e157 (2011).
- Faraone S V, Biederman J, Spencer T, et al. Efficacy of atomoxetine in adult attention-deficit/hyperactivity disorder, a drug-placebo response curve analysis. Behav. Brain. Funct 1(1), 16(2005).
- Bello NT. Clinical utility of guanfacine extended release in the treatment of ADHD in children and adolescents. Patient. Prefer. Adherence 9(1), 877–85 (2015).
- Herrmann N, Lanctôt KL, Khan LR. The role of norepinephrine in the behavioural and psychological symptoms of dementia. J. Neuropsychiatry. Clin. Neurosci 16(3), 261–76 (2004).
- Matthews KL, Chen CPLH, Esiri MM, et al. Noradrenergic changes, aggressive behaviour, and cognition in patients with dementia. Biol. Psychiatry 51(5), 407–416 (2002).
- Head A, Kendall MJ, Ferner R, et al. Acute effects of beta blockade and exercise on mood and anxiety. Br. J. Sports. Med 30(3), 238–42 (1996).
- Opie LH, White DA. Adverse interaction between nifedipine and beta-blockade. British. Med. J 281(6253), 1462 (1980).
- Centorrino F, Goren JL, Hennen J, et al. Multiple Versus Single Antipsychotic Agents for Hospitalized Psychiatric Patients, Case-Control Study of Risks Versus Benefits. Am. J. Psychiatry 161(4), 700–706 (2004).
- Hasselmo ME. The role of acetylcholine in learning and memory. Curr. Opinion. Neurobiol 16(6), 710–715 (2006).
- Kihara T, Shimohama S. Alzheimer’ s disease and acetylcholine receptors. Acta. Neurobiol. Exp (Wars). 64(1), 99–105 (2004).
- Rand JB. Acetylcholine. WormBook 131(1), 1–21 (2007).
- Ohmura Y, Tsutsui-Kimura I, Yoshioka M. Impulsive Behaviour and Nicotinic Acetylcholine Receptors. J. Pharmacol. Sci 118(4), 413–422 (2012).
- He D, Wu H, Wei Y, et al. Effects of harmine, an acetylcholinesterase inhibitor, on spatial learning and memory of APP/PS1 transgenic mice and scopolamine-induced memory impairment mice. Eur. J. Pharmacol 768 (12), 96–107(2015).
- Fu A-L, Zhang X-M, Sun M-J. Antisense inhibition of acetylcholinesterase gene expression for treating cognition deficit in Alzheimer’s disease model mice. Brain. Res 1066(1), 10–5 (2005).
- Lo DC. Neurotrophic factors and synaptic plasticity. Neuron 15(5), 979-81(1995).
- Ericsson KA, Kintsch W. Long-term working memory. Psychol. Rev 102(2), 211–245 (1995).
- Markus A, Patel TD, Snider WD. Neurotrophic factors and axonal growth. Curr. Opinion. Neurobiol 12(5), 523–531(2002).
- Skaper SD. The neurotrophin family of neurotrophic factors, An Overview. Meth. Molecul. Biol 1(1), 1–12 (2012).
- Pruunsild P, Kazantseval A, Aid T, et al. Dissecting the human BDNF locus, Bidirectional transcription, complex splicing, and multiple promoters. Genomics 90(3), 397–406(2007).
- Rossi C, Angelucci A, Costantin L, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur. J. Neurosci 24(7), 1850–1856 (2006).
- Budni J, Bellettini-Santos T, Mina F, et al. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging. Dis 6(5), 331 (2015).
- Whitehouse PJ, Price DL, Struble RG, et al. Alzheimer’s disease and senile dementia, loss of neurons in the basal forebrain. Science 215(4537), 1237–1249(1982).
- Auld DS, Kornecook TJ, Bastianetto S, Quirion R. Alzheimer’s disease and the basal forebrain cholinergic system, relations to beta-amyloid peptides, cognition, and treatment strategies. Prog. Neurobiol 68(3), 209–245 (2002).
- Weissmiller AM, Wu C. Current advances in using neurotrophic factors to treat neurodegenerative disorders. Transl. Neurodegener 1(1), 14(2012).
- Zhang F, Kang Z, Li W, et al. Roles of brain-derived neurotrophic factor/tropomyosin-related kinase B (BDNF/TrkB) signalling in Alzheimer’s disease. J. Clinical. Neurosci 19(7), 946–949 (2012).
- Roizen NJ, Patterson D. Down’s syndrome. Lancet. 361(9365), 1281–1289(2003).
- Ypsilanti AR, Girão da Cruz MT, Burgess A, et al. The length of hippocampal cholinergic fibers is reduced in the aging brain. Neurobiol. Aging 29(11), 1666–1679(2008).
- Howells DW, Porritt MJ, Wong JYF, et al. Reduced BDNF mRNA Expression in the Parkinson’s Disease Substantia Nigra. Exp. Neurol 166(1), 127–135(2000).
- Borrell-Pagès M, Canals JM, Cordelières FP, et al. Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. J Clin Invest. 116(5), 1410–1424(2006).
- Mitchelmore C, Gede L. Brain derived neurotrophic factor, Epigenetic regulation in psychiatric disorders. Brain. Res 1586(1), 162–172(2014).
- Allen SJ, Watson JJ, Shoemark DK, et al. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Therapeutics 138(2), 155–175 (2013).
- Koskela M, Bäck S, Võikar V, et al. Update of neurotrophic factors in neurobiology of addiction and future directions. Neurobiol. Dis 97(1), 189–200 (2017).
- Sönmez MB, Görgülü Y, Çinar RK, et al. Alterations of BDNF and GDNF serum levels in alcohol-addicted patients during alcohol withdrawal. Eur. J. Psychiatry 30(2), 109-118(2016).
- Lee DA, Gross L, Wittrock DA, et al. Localization and expression of ciliary neurotrophic factor (CNTF) in postmortem sciatic nerve from patients with motor neuron disease and diabetic neuropathy. J. Neuropathol. Exp. Neurol 55(8), 915–923(1996).
- Pasquin S, Sharma M, Gauchat JF. Ciliary neurotrophic factor (CNTF), New facets of an old molecule for treating neurodegenerative and metabolic syndrome pathologies. Cytokine. Growth. Factor. Rev 26(5), 507–515 (2015).
- Emerich DF, Winn SR, Hantraye PM, et al. Protective effect of encapsulated cells producing neurotrophic factor CNTF in a monkey model of Huntington’s disease. Nature 386(6623), 395–399 (1997).
- Henderson CE. Role of neurotrophic factors in neuronal development. Curr. Opinion. Neurobiol 6(1), 64–70(1996).
- Zheleznyakova GY, Cao H, Schiöth HB. BDNF DNA methylation changes as a biomarker of psychiatric disorders, literature review and open access database analysis. Behav. Brain. Funct 12(1), 17(2016).
- Dong E, Dzitoyeva SG, Matrisciano F, et al. Brain-derived neurotrophic factor epigenetic modifications associated with schizophrenia-like phenotype induced by prenatal stress in mice. Biol. Psychiatry 77(6), 589–596(2015).
- Satoh JI, Kawana N, Yamamoto Y. Pathway Analysis of ChIP-Seq-Based NRF1 Target Genes Suggests a Logical Hypothesis of their Involvement in the pathogenesis of Neurodegenerative Diseases. Gene. Regul. Syst. Bio 2013(7), 139–152 (2013).
- Lee CS, Lee C, Hu T, et al. Loss of nuclear factor E2-related factor 1 in the brain leads to dysregulation of proteasome gene expression and neurodegeneration. Proc. Natl. Acad. Sci. USA 108(20), 8408–8413 (2011).
- Martins IJ. Anti-Aging Genes Improve Appetite Regulation and Reverse Cell Senescence and Apoptosis in Global Populations. Adv. Aging. Res 5(1), 9-26(2016).
- Martins IJ. Magnesium Therapy Prevents Senescence with the Reversal of Diabetes and Alzheimer's Disease. Health 8(5), 694-710 (2016).
- Appiah-Kusi E, Leyden E, Parmar S, et al. Abnormalities in neuroendocrine stress response in psychosis, the role of endocannabinoids. Psychol. Med 46(1), 27–45(2016).
- Rubino Ti, Zamberletti E, Parolaro D. Endocannabinoids and mental disorders. Endocannabinoids 2015(1), 261–283(2015).
- Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur. J. Pharmacol 359(1), 1–18 (1998).
- Di Marzo V, Bisogno T, De Petrocellis L. Anandamide, Some like it hot. Trends. Pharmacol. Sci 22(7), 346–349(2001).
- Berry EM, Mechoulam R. Tetrahydrocannabinol and endocannabinoids in feeding and appetite. Pharmacol. Ther 95(2), 185–190 (2002).
- Marsicano G, Chaouloff F. Moving bliss, A new anandamide transporter. Nat. Neurosci 15(1), 5–6(2012).
- Marquez P, Baliram R, Gajawada N, et al. Differential involvement of enkephalins in analgesic tolerance, locomotor sensitization, and conditioned place preference induced by morphine. Behav. Neurosci 120(1), 10–15(2006).
- Marzo V Di. Anandamide serves two masters in the brain. Nat. Neurosci 13(12), 1446–1448(2010).
- Self DW. Anandamide, A candidate neurotransmitter heads for the big leagues. Nat. Neurosci 2(4), 303–304(1999).
- Cryan JF, Dinan TG. Mind-altering microorganisms, The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci 13(10), 701–712(2012).
- Cryan JF, O’Mahony SM. The microbiome-gut-brain axis, From bowel to behaviour. Neurogastroenterol. Motil 23(3), 187–192(2011).
- Burokas A, Moloney RD, Dinan TG, et al. Microbiota regulation of the mammalian gut-brain axis. Adv. Appl. Microbiol 91(12), 1–62(2015).
- Wang HX, Wang YP. Gut microbiota-brain axis. Chinese. Med. J 129(19), 2373–2380(2016).
- Quigley EMM. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Cur. Neurol. Neurosci. Rep 17(12), 94(2017).
- Bauer P V, Hamr SC, Duca FA. Regulation of energy balance by a gut-brain axis and involvement of the gut microbiota. Cellular. Molecul. Life. Sci 73(4), 737–755(2016).
- Chen X, D’Souza R, Hong ST. The role of gut microbiota in the gut-brain axis, current challenges and perspectives. Prot. Cell 4(6), 403–414(2013).
- Behan PO. Neurological Differential Diagnosis. J. Neurol. Neurosur. Psych 41(6), 579(1978).
- Jensen M, Cox AP, Chaudhry N, et al. The neurological disease ontology. J. Biomed. Semantics 4(1), 42(2013).
- Moll JWB, Vecht CJ. Immune diagnosis of paraneoplastic neurological disease. Clin. Neurol. Neurosurg 97(1), 71–81(1995).
- Kumar A, Chanana P. Sleep reduction, A link to other neurobiological diseases. Sleep. Biol. Rhythms 12(3), 150–161(2014).
- Tang W, Su D. Locomotion analysis and its applications in neurological disorders detection, state-of-art review. Netw. Model. Anal. Heal. Informatics. Bioinforma 2(1), 1–12 (2013).
- Li X-L, Hu N, Tan M-S, et al. Behavioural and Psychological Symptoms in Alzheimer’s Disease. Biomed. Res. Int 2014(7), 1–9 (2014).
- Liu M, Zhang D, Shen D. Hierarchical fusion of features and classifier decisions for Alzheimer’s disease diagnosis. Hum. Brain. Mapp 35(4), 1305–1319 (2014).
- Mega MS, Cummings JL, Fiorello T, et al. The spectrum of behavioural changes in Alzheimer’s disease. Neurology 46(1), 130–135 (1996).
- Andrews S, Ismail Z, Anstey KJ, et al. Alzheimer’s Genetic Risk Score linked to Incident Mild Behavioural Impairment. BioRxiv 2017(1), 200840 (2017).
- Godefroy O, Martinaud O, Verny M, et al. The dysexecutive syndrome of alzheimer’s disease, The GREFEX study. J. Alzheimer. Dis 42(4), 1203–1208 (2014).
- Ossenkoppele R, Pijnenburg YAL, Perry DC, et al. The behavioural/dysexecutive variant of Alzheimer’s disease, clinical, neuroimaging and pathological features. Brain 138(9), 2732–2749 (2015).
- Anderson KE. Behavioural disturbances in Parkinson’s disease. Dialogues. Clin. Neurosci 6(3), 323–332(2004).
- Miwa H. Stereotyped behaviour or punding in Parkinson’s disease. J. Neurology 6(3), 61–67(2007).
- Voon V, Hassan K, Zurowski M, et al. Prevalence of repetitive and reward-seeking behaviours in Parkinson disease. Neurology 67(7), 1254–1257(2006).
- Boeve BF, Silber MH, Ferman TJ. REM Sleep Behaviour Disorder in Parkinson’s Disease and Dementia with Lewy Bodies. J. Geriatr. Psychiatry. Neurol 17(3), 146–157(2004).
- Lyons BE, Austin D, Seelye A, et al. Pervasive computing technologies to continuously assess Alzheimer’s disease progression and intervention efficacy. Front. Aging. Neurosci 7, 102 (2015).
- Ryd C, Nygård L, Malinowsky C, et al. Can the everyday technology use questionnaire predict overall functional level among older adults with mild cognitive impairment or mild-stage alzheimer’s disease? – a pilot study. Scand. J. Caring. Sci 31(1), 201-209 (2017).
- Strac DS, Muck-Seler D, Pivac N. Neurotransmitter measures in the cerebrospinal fluid of patients with Alzheimer’s disease, A review. Psychiatr. Danub 27(1), 14-24 (2015).
- Rudy CC, Hunsberger HC, Weitzner DS, et al. The Role of the Tripartite Glutamatergic Synapse in the Pathophysiology of Alzheimer’s Disease. Aging. Dis 6(2), 131-148 (2015).
- Jahn H. Memory loss in alzheimer’s disease. Dialogues. Clin. Neurosci 15(4), 445-454 (2013).
- Arbor SC, Lafontaine M, Cumbay M. Amyloid-beta Alzheimer targets — protein processing, lipid rafts, and amyloid-beta pores. Yale. J. Biol. Med 89(1), 5-21 (2016).
- Nobili A, Latagliata EC, Viscomi MT, et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat. Commun 8, 14727.
- Mather M, Harley CW. The Locus Coeruleus, Essential for Maintaining Cognitive Function and the Aging Brain. Trends. Cogn. Sci 20(3), 214-226 (2016).
- Szot P. Common factors among Alzheimer’s disease, Parkinson’s disease, and epilepsy, Possible role of the noradrenergic nervous system. Epilepsia 53(1), 61-66 (2012).
- Kihara T, Shimohama S. Alzheimer’s disease and acetylcholine receptors. Acta. Neurobiol. Exp 64(1), 99-105 (2004).
- Kolisnyk B, Al-Onaizi M, Soreq L, et al. Cholinergic Surveillance over Hippocampal RNA Metabolism and Alzheimer’s-Like Pathology. Cereb. Cortex 27(7), 3553-3567 (2017).
- Yue W, Li Y, Zhang T, et al. ESC-derived basal forebrain cholinergic neurons ameliorate the cognitive symptoms associated with Alzheimer’s disease in mouse models. Stem. Cell. Reports 5(5), 776-790 (2015).
- Talantova M, Sanz-Blasco S, Zhang X, et al. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc. Natl. Acad. Sci. USA 110(27), E2518–E2527 (2013).
- Rupsingh R, Borrie M, Smith M, et al. Reduced hippocampal glutamate in Alzheimer disease. Neurobiol. Aging 32(5), 802-810 (2011).
- Institute of Medicine (US) Forum on Neuroscience and Nervous System Disorders. Glutamate-Related Biomarkers in Drug Development for Disorders of the Nervous System, National Academies Press, Washington, USA (2011).
- Bechtholt-Gompf AJ, Walther H V, Adams MA, et al. Cohen BM. Blockade of Astrocytic Glutamate Uptake in Rats Induces Signs of Anhedonia and Impaired Spatial Memory. Neuropsychopharmacology 35(10), 2049-2059 (2010).
- Itkin A, Dupres V, Dufrêne YF, et al. Calcium ions promote formation of amyloid β-peptide (1-40) oligomers causally implicated in neuronal toxicity of Alzheimer’s disease. PLoS. One 6(3), e18250 (2011).
- Danysz W, Parsons CG. Alzheimer's disease, β-amyloid, glutamate, NMDA receptors and memantine--searching for the connections. Br. J. Pharmacol 167(2), 324-352 (2012).
- Vaarmann A, Kovac S, Holmström KM, et al. Dopamine protects neurons against glutamate-induced excitotoxicity. Cell. Death. Dis 4, e455 (2013).
- Storga D, Vrecko K, Birkmayer JGD, et al. Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci. Lett 203(1), 29-32 (1996).
- Kempadoo KA, Mosharov E V, Choi SJ, et al. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc. Natl. Acad. Sci. USA 113(51), 14835-14840 (2016).SSSSSSSSSSS
- Rossato JI, Bevilaqua LRM, Izquierdo I, et al. Dopamine Controls Persistence of Long-Term Memory Storage. Science 325(5943), 1017-1020 (2009).
- Russo SJ, Nestler EJ. The Brain Reward Circuitry in Mood Disorders. Nat. Rev. Neurosci 14(9), 609-625 (2014).
- Moreno-Castilla P, Rodriguez-Duran LF, et al. Dopaminergic neurotransmission dysfunction induced by amyloid-β transforms cortical long-term potentiation into long-term depression and produces memory impairment. Neurobiol. Aging 41, 187-199 (2016).
- Nowrangi MA, Lyketsos CG, Rosenberg PB. Principles and management of neuropsychiatric symptoms in Alzheimer’s dementia. Alzheimers. Res. Ther 7(1), 12 (2015).
- Borgkvist A, Malmlöf T, Feltmann K, et al. Dopamine in the hippocampus is cleared by the norepinephrine transporter. Int. J. Neuropsychopharmacol 15(4), 531-540 (2011).
- Chalermpalanupap T, Kinkead B, Hu WT, et al. Targeting norepinephrine in mild cognitive impairment and Alzheimer’s disease. Alzheimers. Res. Ther 5(2), 21 (2013).
- Heneka MT, Kummer MP, Stutz A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493(7434), 674-678 (2013).
- Jardanhazi-Kurutz D, Kummer MP, Terwel D, et al. Induced LC degeneration in APP/PS1 transgenic mice accelerates early cerebral amyloidosis and cognitive deficits. Neurochem. Int 57(4), 375-382 (2010).
- Oikawa N, Kimura N, Yanagisawa K. Alzheimer-type tau pathology in advanced aged nonhuman primate brains harboring substantial amyloid deposition. Brain. Res 1315, 137-149 (2010).
- Hammerschmidt T, Kummer MP, Terwel D, et al. Selective loss of noradrenaline exacerbates early cognitive dysfunction and synaptic deficits in APP/PS1 mice. Biol. Psychiatry 73(5), 454-463 (2013).
- Gannon M, Che P, Chen Y, et al. Noradrenergic dysfunction in Alzheimer’s disease. Front. Neurosci 9, 220 (2015).
- Elrod R, Peskind ER, DiGiacomo L, et al. Effects of Alzheimer’s disease severity on cerebrospinal fluid norepinephrine concentration. Am. J. Psychiatry 154(1), 25-30 (1997).
- Palmer AM, Wilcock GK, Esiri MM, et al. Monoaminergic innervation of the frontal and temporal lobes in Alzheimer’s disease. Brain. Res 401(2), 231-238 (1987).
- Fitzgerald PJ. Is elevated norepinephrine an etiological factor in some cases of schizophrenia? Psychiatry. Res 215(3), 497-504 (2014).
- Heneka MT, Nadrigny F, Regen T, et al. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc. Natl. Acad. Sci USA 107(13), 6058-6063 (2010).
- Amor S, Puentes F, Baker D, et al. Inflammation in neurodegenerative diseases. Immunology 129(2), 154-69 (2010).
- Lidow MS, Goldman‐Rakic PS, Gallager DW, et al. Quantitative autoradiographic mapping of serotonin 5‐HT1 and 5‐HT2 receptors and uptake sites in the neocortex of the rhesus monkey. J. Comp. Neurol 280(1), 27-42 (1989).
- Xu Y, Yan J, Zhou P, et al. Neurotransmitter receptors and cognitive dysfunction in Alzheimer’s disease and Parkinson’s disease. Prog. Neurobiol 9(1) 1-13 (2012).
- Cirrito JR, Disabato BM, Restivo JL, et al. Serotonin signaling is associated with lower amyloid-b levels and plaques in transgenic mice and humans. Proc. Natl. Acad. Sci USA 108(36), 14968–14973 (2011).
- Claeysen S, Bockaert J, Giannoni P. Serotonin, A New Hope in Alzheimer’s Disease? ACS. Chem. Neurosci 6(1), 940–943 (2015).
- Smith GS, Yilong M, Dhawan V, et al. Selective serotonin reuptake inhibitor (SSRI) modulation of striatal dopamine measured with [11C]-raclopride and positron emission tomography. Synapse 63(1), 1–6 (2009).
- Dunn RW, Corbett R, Fielding S. Effects of 5-HT1A receptor agonists and NMDA receptor antagonists in the social interaction test and the elevated plus maze. Eur. J. Pharmacol 169(1), 1–10 (1989).
- Vlcek K, Laczo J. Neural correlates of spatial navigation changes in mild cognitive impairment and Alzheimer’s disease. Front. Behav. Neurosci 8(1), 89 (2014).
- Ma SH, Zhang LL JQ. Protective effect of bioflavonoid morin on Cadmium induced oxidative neuropathy. Biomed. Res 28(3), 1148–54 (2017).
- Geetha C, Pugazhenthi D. Classification of alzheimer’s disease subjects from MRI using fuzzy neural network with feature extraction using discrete wavelet transform. Biomed. Res 1(1), 234 (2017).
- Wakabayashi K, Tanji K, Mori F, et al. The Lewy body in Parkinson’s disease, Molecules implicated in the formation and degradation of α-synuclein aggregates. Neuropathology 27(5), 494–506 (2007).
- Caballol N, Martí MJ, Tolosa E. Cognitive dysfunction and dementia in Parkinson disease. Movement. Disord 22(S17), S01-S23 (2007).
- Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat. Rev. Dis. Prim 2017(3), 17013 (2017).
- Titova N, Padmakumar C, Lewis SJG, et al. Parkinson’s, a syndrome rather than a disease? J. Neural. Transmission 124(8), 907–914 (2017).
- Nafisi S, Shadaloi A, Feizbakhsh A, et al. RNA binding to antioxidant flavonoids. J. Photochem. Photobiol. B. Biol 94(1), 1–7 (2009).
- Priya R, Kumar U, Saran A, et al. Oxidative stress in psoriasis. Biomed. Res 25(1), 132–134 (2013).
- Chang MY, Liu CM, Shieh DE CC. Evaluation and analysis of phytochemical antioxidant capacity. Biomed. Res 28(14), 6431–6434 (2017).
- Nikcevic L, Savic M, Lazovic M, et al. The influence of early thrombolysis on C-reactive protein values and functional outcome after acute ischemic stroke. Biomed. Res 27(4), 1183–1187 (2016).
- Han CL, Fu R LW. Synthesis and antidementia effects of a new Zn (II) coordination polymer. Biomed. Res 27(4), 1237–1239 (2016).
- Zhang Y, Zhang C, Cheng Z, et al. Serum levels of brain-derived neurotrophic factor and clinical efficacy of mirtazapine in geriatric patients with major depression. Biomed. Res 26(2), 231–236 (2015).
- Werner FMb, Coveñas R. Classical neurotransmitters and neuropeptides involved in parkinson’s disease, Focus on anti-parkinsonian drugs. Curr. Drug ther 10(2), 66–81 (2015).
- Pathak A, Senard J-M. Blood pressure disorders during Parkinson’s disease, epidemiology, pathophysiology and management. Expert. Rev. Neurother 6(8), 1173–1180 (2006).
- Videnovic A, Lazar AS, Barker RA, et al. ‘The clocks that time us’-circadian rhythms in neurodegenerative disorders. Nat Rev Neuro. 10(12), 683–693(2014).
- Yu J, Feng Y, Xiang Y, et al. Retinal Nerve Fiber Layer Thickness Changes in Parkinson Disease, A Meta-Analysis. PLoS. One 9(1), e85718 (2014).
- Videnovic A, Golombek D. Circadian and sleep disorders in Parkinson's disease. Experimen. neurology 243(5), 45-56 (2013).
- Burbulla LF, Song P, Mazzulli JR, et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 357(6357), 1255–1261 (2017).
- Roeper J. Dissecting the diversity of midbrain dopamine neurons. Trends. Neurosci 36(6), 336–342 (2013).
- Ilango A, Kesner AJ, Keller KL, et al. Similar Roles of Substantia Nigra and Ventral Tegmental Dopamine Neurons in Reward and Aversion. J. Neurosci 34(3), 817–822 (2014).
- Halliday GM, Leverenz JB, Schneider JS, et al. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov. Disord 29(5), 634–50 (2014).
- Tong J, Hornykiewicz O, Kish SJ. Inverse relationship between brain noradrenaline level and dopamine loss in Parkinson disease, a possible neuroprotective role for noradrenaline. Arch. Neurol 63(12), 1724–1728 (2006).
- Aosaki T, Miura M, Suzuki T, et al. Acetylcholine-dopamine balance hypothesis in the striatum, An update. Geriat. Geront. Internat 10(S1), 1-112 (2010).
- Navailles S, Bioulac B, Gross C, et al. Serotonergic neurons mediate ectopic release of dopamine induced by l-DOPA in a rat model of Parkinson’s disease. Neurobiol. Dis 38(1), 136–143 (2010).
- Gerfen CR, Surmeier DJ. Modulation of Striatal Projection Systems by Dopamine. Annu. Rev. Neurosci 34(7), 441–466 (2011).
- Muller MLTM, Bohnen NI, Kotagal V, et al. Clinical markers for identifying cholinergic deficits in Parkinson’s disease. Mov. Disord 30(2), 269–273 (2015).
- Perez-Lloret S, Barrantes FJ. Deficits in cholinergic neurotransmission and their clinical correlates in Parkinson’s disease. NPJ. Park. Dis 2(2), 16001 (2016).
- Factor SA, McDonald WM, Goldstein FC. The role of neurotransmitters in the development of Parkinson’s disease-related psychosis. Euro. J. Neurol 2017(11), 1244–1254 (2017).
- Politis M, Niccolini F. Serotonin in Parkinson’s disease. Behav. Brain. Res 277(1), 136–145(2015).
- Waldron EJ, Barrash J, Swenson A, et al. Personality Disturbances in Amyotrophic Lateral Sclerosis, A Case Study Demonstrating Changes in Personality Without Cognitive Deficits. J. Int. Neuropsychol. Soc 20(7), 764–771 (2014).
- Grace J. Frontal systems behaviour scale. In Encyclopedia of clinical neuropsychology. Springer. New York 1090-1093 (2011).
- Lillo P, Garcin B, Hornberger M, et al. Neurobehavioural Features in Frontotemporal Dementia With Amyotrophic Lateral Sclerosis. Arch. Neurol 67(7), 826-830 (2010).
- Woolley SC, York MK, Moore DH, et al. Detecting frontotemporal dysfunction in ALS, utility of the ALS Cognitive Behavioural Screen (ALS-CBS). Amyotroph. Lateral. Scler 11(3),303-11 (2010).
- Rabkin J, Goetz R, Murphy JM, et al. Cognitive impairment, behavioural impairment, depression, and wish to die in an ALS cohort. Neurology 87(13), 1320–1328 (2016).
- Grande I, Berk M, Birmaher B, et al. Bipolar disorder. Lancet 387(10027), 1561–1572 (2016).
- Rihmer Z, Döme P. Suicide and bipolar disorder. Mileston. Drug. Therapy 2016(1), 53–69 (2016).
- Deveney CM, Connolly ME, Jenkins SE, et al. Neural recruitment during failed motor inhibition differentiates youths with bipolar disorder and severe mood dysregulation. Biol. Psychol 89(1), 148–155 (2012).
- Johnson SL, Murray G, Fredrickson B, et al. Creativity and bipolar disorder, Touched by fire or burning with questions? Clinic. Psychol. Review. 32(1), 1–12 (2012).
- Cerullo MA, Adler CM, Delbello MP, et al. The functional neuroanatomy of bipolar disorder. Int. Rev. Psychiatry 21(4), 314-22 (2009).
- Cousins DA, Butts K, Young AH. The role of dopamine in bipolar disorder. Bipol. Disorders 11(8), 787–806 (2009).
- Stahl SM. Neurotransmission of cognition, part 1. Dopamine is a hitchhiker in frontal cortex, Norepinephrine transporters regulate dopamine. J Clinical Psychiatry 64(1), 4–5 (2002).
- Berk M, Dodd S, Kauer-Sant’Anna M, et al. Dopamine dysregulation syndrome, Implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr Scand 116(S434), 41–49 (2007).
- Mahmood T, Silverstone T. Serotonin and bipolar disorder. J. Affectiv. Disorders 66(1), 1–11 (2001).
- Jablensky A. The diagnostic concept of schizophrenia, its history, evolution, and future prospects. Dialog. Clin. Neurosci 12(3), 271–287 (2010).
- Harvey PD, Koren D, Reichenberg A, et al. Negative symptoms and cognitive deficits, What is the nature of their relationship? Schizophr. Bulletin 32(2),250–258 (2006).
- Reeder C, Harris V, Pickles A, et al. Does change in cognitive function predict change in costs of care for people with a schizophrenia diagnosis following cognitive remediation therapy? Schizophr. Bull 40(6), 1472–1481 (2014).
- Arsova S, Bajraktarov S, Barbov I, et al. Patients with schizophrenia and self-care. Maced. J. Med Sci 7(2), 285–288 (2014).
- Gur RE, Kohler CG, Ragland JD, et al. Flat affect in schizophrenia, Relation to emotion processing and neurocognitive measures. Schizophr. Bulletin 32(2), 279–287 (2006).
- Tron T, Peled A, Grinsphoon A, et al. Facial expressions and flat affect in schizophrenia, automatic analysis from depth camera data. In Biomedical and Health Informatics (BHI), 2016 IEEE-EMBS International Conference. 220-223 (2016).
- Wang P, Kohler C, Barrett F, et al. Quantifying facial expression abnormality in schizophrenia by combining 2D and 3D features. In Computer Vision and Pattern Recognition, 2007. CVPR'07. IEEE Conference 1-8 (2007).
- Keefe RSE, Harvey PD. Cognitive impairment in schizophrenia. In Novel antischizophrenia treatments. Springer Berl. Heidelberg 213(1), 11–37 (2012).
- Carbon M, Correll CU. Thinking and acting beyond the positive, the role of the cognitive and negative symptoms in schizophrenia. CNS. Spectr 19(S1), 35–53 (2014).
- Chalasani PP, Krishnamurthy KK, David HH. Catatonia, Schizophrenia, and Affective Disorders - Diagnostic Associations in Different Cultural Settings. Audio., Trans. IRE. Prof Gr 53(1), 49–52 (2011).
- Edition F. Diagnostic and statistical manual of mental disorders. Washington, Amer. Psycholog. Association (1994).
- Simeon D, Berlin H, First MB. Impulse-Control Disorders. (3rd Edtn.) Psychiatry 1658–1701 (2008).
- Williams W a, Potenza MN. The neurobiology of impulse control disorders. Rev. Bras. Psiquiatr 30 (Supp. 1), S24–S30 (2008).
- Calado F, Griffiths MD. Problem gambling worldwide, An update and systematic review of empirical research (2000–2015). J. Behav. Addict 5(4), 592–613 (2016).
- Raylu N, Oei TPS. Pathological gambling, A comprehensive review. Clin. Psychol. Rev 22(7), 1009–1061 (2002).
- Aboujaoude E, Gamel N, Koran L. Overview of kleptomania and phenomenological description of 40 patients. Clin. Psychiatry 6(6), 244–247 (2004).
- Murray JB. Kleptomania, A review of the research. J. Psychol. Interdiscip Appl 126(2), 131–137 (1992).
- Grant JE, Kim SW. Clinical characteristics and psychiatric comorbidity of pyromania. J. Clin. Psychiatry 68(11), 1717–1722 (2007).
- Diefenbach GJ, Mouton-Odum S, Stanley MA. Affective correlates of trichotillomania. Behav. Res. Ther 40(11), 1305-15 (2002).
- Ferguson KE. Intermittent explosive disorder. Practitioner’s Guide to Evidence-Based Psychotherapy 2006(1), 335–351 (2006).
- Koran LM, Faber RJ, Aboujaoude E, et al. Estimated prevalence of compulsive buying behaviour in the United States. Americ. J. Psychiatry 163(10), 1806–1812 (2006).
- Mitchell JE, Burgard M, Faber R, et al.. Cognitive behavioural therapy for compulsive buying disorder. Behav. Res. Ther 44(12), 1859–1865 (2006).
- Davis C, Carter JC. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite 53(1), 1–8 (2009).
- Altman SE, Shankman SA. What is the association between obsessive-compulsive disorder and eating disorders? Clinic. Psychol. Review 29(7), 638–646 (2009).
- Campbell MM, Stein DJ. Hypersexual disorder. Behavioural addictions, DSM-5 and beyond. 104(6), 101–123 (2016).
- Womack SD, Hook JN, Ramos M, et al.. Measuring Hypersexual Behaviour. Sex. Addict. Compulsivity. 20(1-2), 65–78 (2013).
- Grant JE, Odlaug BL. Update on pathological skin picking. Curr. Psych. Reports. 2009(8), 283–288 (2009).
- Cooney JW, Stacy M. Neuropsychiatric Issues in Parkinson’s Disease. Curr. Neuro. Neurosci. Reports 16(5), 49 (2016).
- Lapidus KAB, Stern ER, Berlin HA, et al. Neuromodulation for Obsessive-Compulsive Disorder. Neurotherapeutics 11(3), 485–495 (2014).
- Barr LC, Goodman WK, Price LH, et al. The serotonin hypothesis of obsessive compulsive disorder, Implications of pharmacologic challenge studies. J. Clin. Psych 1992(4), 17–28 (1992).
- Pacan P, Grzesiak M, Reich A, et al. Onychophagia as a spectrum of obsessive-compulsive disorder. Acta. Derm. Venereol 89(3), 278–280 (2009).
- Pittenger C, Krystal JH, Coric V. Glutamate-modulating drugs as novel pharmacotherapeutic agents in the treatment of obsessive-compulsive disorder. NeuroRx 3(1), 69–81 (2006).
- Brennan BP, Rauch SL, Jensen JE, et al. A critical review of magnetic resonance spectroscopy studies of obsessive-compulsive disorder. Bio. Psych 73(1), 24–31 (2013).
- O’Callaghan C, Bertoux M, Hornberger M. Beyond and below the cortex, the contribution of striatal dysfunction to cognition and behaviour in neurodegeneration. J. Neurol. Neurosurg. Psychiatry 85(4), 371–378 (2014).
- Ballard C, Aarsland D, Francis P, et al. Neuropsychiatric symptoms in patients with dementias associated with cortical lewy bodies, Pathophysiology, clinical features, and pharmacological management. Drugs. Aging 30(8), 603–611 (2013).
- Sastry PS, Rao KS. Apoptosis and the nervous system. J. Neurochem 74(1), 1–20 (2000).
- Hengartner MO. The biochemistry of apoptosis. Nature 407(6805), 770–6(2000).
- Chen Y-J, Cheng F-C, Sheu M-L, et al. Detection of subtle neurological alterations by the Catwalk XT gait analysis system. J. Neuroeng. Rehabil 11(1), 62 (2014).
- Lang AE, Lozano AM. Parkinson's disease. N. Eng. J. Med 339(15), 1044-53 (1998).
- Kishore A, Popa T, Velayudhan B, et al. Long and short duration response of dopaminergic treatment on motor cortex plasticity in Parkinson’s disease. Mov. Disord 27(6), S208 (2012).
- Stocchi F. Dopamine receptor agonists in the treatment of advanced Parkinson’s disease. Parkins. Relat. Disord 15(12), S54–S57 (2009).
- Miller R. Mechanisms of Action of Antipsychotic Drugs of Different Classes, Refractoriness to Therapeutic Effects of Classical Neuroleptics, and Individual Variation in Sensitivity to their Actions, PART I. Curr. Neuropharmacol 7(4), 302–314 (2009).
- Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of Atypical Antipsychotic Drugs in Patients with Alzheimer’s Disease. N. Engl. J. Med 355(15), 1525–1538 (2006).
- Cai HL, Jiang P, Tan QY, et al. Therapeutic efficacy of atypical antipsychotic drugs by targeting multiple stress-related metabolic pathways. Transl. Psychiatry 7(5), e1130 (2017).
- Howland RH. Risks and benefits of antipsychotic drugs in elderly patients with dementia. J Psychosoc. Nurs .Ment. Health. Serv 46(11), 19–23 (2008).
- Edge L. Antipsychotic drugs for dementia, a balancing act. Lancet Neurology 2009(8), 1-125 (2009).
- Divac N, Stojanović R, Savić Vujović K, et al. The Efficacy and Safety of Antipsychotic Medications in the Treatment of Psychosis in Patients with Parkinson’s Disease. Behav. Neurol. 2016(7), 8154 (2016).
- Giacobini E. Cholinesterases, New roles in brain function and in Alzheimer’s disease. Neurochem. Res 28(3), 515–522 (2003).
- Ellis JM. Cholinesterase inhibitors in the treatment of dementia. J. Am. Osteopath. Assoc 105(3), 145–158 (2005).
- Masterman DL. Role of Cholinesterase Inhibitors in Managing Behavioural Problems in Alzheimer’s Disease. Prim. Care Comp. J. Clin. Psychiatry 6(3), 126–131 (2004).
- Rolinski M, Fox C, Maidment I, et al. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane database Syst. Rev 2012(3), CD006504 (2012).
- Pae C-U. Role of the cholinesterase inhibitors in the treatment of schizophrenia. Expert. Opin. Investig. Drugs 2013(22), 293–298 (2013).
- Vattakatuchery JJ, Kurien R. Acetylcholinesterase inhibitors in cognitive impairment in Huntington’s disease, A brief review. World J. Psychiatry 3(3), 62–64 (2013).
- Borsook D. Neurological diseases and pain. Brain 2012(1), 320–344 (2012).
- Gallini A, Gardette V, Vellas B, Lapeyre-Mestre M, Andrieu S, Brefel-Courbon C. Persistent use of analgesic medications in mild-to-moderate Alzheimer’s disease. Drugs. Aging 30(6), 439–445 (2013).
- Ryder SA, Stannard CF. Treatment of chronic pain, Antidepressant, antiepileptic and antiarrhythmic drugs. Cont. Edu. Anaesthes., Critical Care. and Pain 5(1), 18–21 (2005).
- Aisen PS. The potential of anti-inflammatory drugs for the treatment of Alzheimer’s disease. Lancet Neurol 1(5), 279–284 (2002).
- Gilgun-Sherki Y, Melamed E, Offen D. Anti-inflammatory drugs in the treatment of neurodegenerative diseases, current state. Curr. Pharm. Des 12(27), 3509–3519 (2006).
- Krause DL, Müller N. Neuroinflammation, Microglia and Implications for Anti-Inflammatory Treatment in Alzheimer’s Disease. Int. J. Alzheimers. Dis 2010(6), 1-110, 1–9 (2010).
- Mizuno Y. Recent research progress in and future perspective on treatment of Parkinson’s disease. Integr. Med. Int 1(1), 67-79 (2014).
- Schwarzschild MA, Xu K, Oztas E, Petzer JP. Neuroprotection by caffeine and more specific A2A receptor antagonists in animal models of Parkinson’s disease. Neurology 61(6), S55-S61 (2003).
- Marks WJ Jr, Ostrem JL, Verhagen L, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet. Neurol 7(1), 400-408 (2008).
- Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer’s disease and duration of NSAID use. Neurology 48(1), 626-632 (1997).
- Tamminga CA, Medoff DR. The biology of schizophrenia. Dialogues. Clin. Neurosci 2(4), 339-348 (2000).
- Foerster, Bradley R. et al. 3T MR Spectroscopy Reveals an Imbalance between Excitatory and Inhibitory Neurotransmitters in Amyotrophic Lateral Sclerosis. JAMA. Neurol 70(8), 1009-101 (2013).
- Barkhatova VP, Zavalishin IA, Askarova LS, et al. Changes in neurotransmitters in multiple sclerosis. Neurosci. Behav. Physiol 28(4), 341-344 (1998).
- Cooney JW, Stacy M. Neuropsychiatric Issues in Parkinson’s Disease. Curr. Neurol. Neurosci. Rep 16(1), 49 (2016).
- Brewer JA, Potenza MN. The Neurobiology and Genetics of Impulse Control Disorders: Relationships to Drug Addictions. Biochem. Pharmacol 75(1), 63-75 (2008).