Research Article - Neuropsychiatry (2018) Volume 8, Issue 1
Neural Correlates of Emotional Ambiguity in Patients with Schizophrenia – Relationship with Expressive Deficits
- *Corresponding Author:
- Jozarni J Dlabac-de Lange
University Medical Center Groningen
Department of Psychiatry, Postbox 30.001
9700 RB Groningen, The Netherlands
Abstract
Abstract
Objective: Negative symptoms can be grouped into two factors, expressive deficits and social-emotional withdrawal. We aimed to examine the neural correlates of the two negative symptom factors during a social cognition task, which measures emotional ambiguity in a social context by presenting an array of faces with varying degrees of consistency in emotional expressions.
Methods: Patients with schizophrenia (N=38) and healthy controls (N=20) performed a social cognition task during fMRI that probed both affect and ambiguity. Differences in brain activation between the healthy controls and patients were non-parametrically tested. Subsequently, the expressive deficits and social-emotional withdrawal factors were regressed against task-related brain activation.
Results: Severity of expressive deficits was negatively correlated with activation of the ventromedial prefrontal cortex when comparing ambiguous emotional decisions to ambiguous gender decisions. During emotional ambiguity, severity of expressive deficits was negatively correlated with activation in thalamic, prefrontal, precentral, parietal and temporal brain areas. No associations between social-emotional withdrawal and brain activation were observed.
Conclusion: Hypoactivation of the fronto-thalamic circuitry during ambiguous social appraisal may imply a reduced action readiness in social situations, underlying expressive deficits but not social-emotional withdrawal. The findings provide further evidence for different neurobiological bases of the two factors of negative symptoms.
Keywords
Schizophrenia, Negative symptoms, Imaging, Social cognition, Expressive deficits
Introduction
many patients with schizophrenia, substantial and persistent deficits in social functioning and social cognition have been reported [1,2]. There is evidence for a relationship between impaired social cognition and aspects of functional outcome in patients with schizophrenia, such as community functioning, social skills, and social behavior [3]. Emotion perception is a key component of social cognition. It can be described as the ability to infer emotional information from facial expressions, vocal inflections or a combination of these [3]. In schizophrenia, there is substantial evidence for deficits in facial emotion perception, which may negatively affect psychosocial functioning and quality of life [4]. Furthermore, negative symptoms of schizophrenia may be associated with these deficits could contribute to negative symptoms, as they hamper effective social interaction and may enhance the likelihood of abstaining from social interaction. On the other hand, negative symptoms could enhance social cognitive deficits, as social withdrawal may negatively affect one’s proficiency in understanding others.
Regarding the neurocircuitry of negative symptoms in patients with schizophrenia, neuroimaging studies on reward and motivation found abnormalities in information processing of the prefrontal cortex (PFC), the anterior cingulate cortex (ACC) and the striatum [7- 9]. Five parallel fronto-subcortical circuits link regions of the frontal cortex to the striatum, globus pallidus/substantia nigra, and thalamus. These circuits mediate motivation, social behavior, executive functions, motor and oculomotor functions [10]. Thus, alterations in the fronto-striato-thalamo-cortical circuitry may underlie or contribute to the development of negative symptoms of schizophrenia.
Recently, there has been a shift in the approach of negative symptoms. Originally, negative symptoms were thought to constitute one dimension. However, recent studies have found two or more dimensions of negative symptoms [11-13]. Most of these studies have found two factors of negative symptoms, namely expressive deficits and avolition/apathy/social emotional withdrawal [11,13,14]. These two factors may have different underlying neural working mechanisms [15-17]. These findings emphasize the importance of acknowledging the two factors of negative symptoms.
In this study, differences in brain activation between patients with schizophrenia and healthy controls during a social cognition task were explored. Furthermore, we explored the differential correlates of the two negative symptom dimensions, namely expressive deficits and social-emotional withdrawal, with brain activation during the task. The aim of this paper was to examine possible differences in the underlying neural working mechanisms of expressive deficits and social-emotional withdrawal during a social cognition task that measures emotional ambiguity [18].
Materials and Methods
▪ Subjects
Our analyses are performed with the baseline data from two trials conducted at our center in recent years, investigating the effects of treatment with antipsychotics (EUDRA-CT: 2007-002748-79) or transcranial magnetic stimulation (Dutch Trial Registry: NTR1261) on negative symptoms of schizophrenia [15,19]. The fMRI data on the Wall of Faces task have not been published as yet. Patients (N=38) were recruited from three regional mental health care institutions (Lentis, GGz Drenthe and GGz Friesland) and the University Medical Center Groningen (UMCG). All patients that were included in the current study were 18 years or older and met the DSM-IV criteria for schizophrenia, which was confirmed by a Schedules for Clinical Assessment (SCAN 2.1) trained rater [20]. Severities of symptoms were assessed with the Positive and Negative Syndrome Scale (PANSS) [21] and the Montgomery Åsberg Depression Rating Scale (MADRS) [22]. Exclusion criteria were age <18 or >60 years, rTMS and MRI contraindications, neurological disorders, head injury with loss of consciousness in the past, substance dependency within the previous 6 months, previous treatment with rTMS, severe behavioral disorders, inability to provide informed consent and pregnancy. Participants gave oral and written consent after the procedure had been fully explained. The studies were executed in accordance with the declaration of Helsinki and approved by a licensed local medical ethical committee (METCUMCG).
Healthy controls (N=20) were matched to the patients based on age, gender, education level and handedness. Level of education was defined according to the scoring system of Verhage [23]. The healthy controls did not have a psychiatric history or any current psychiatric problems as measured by the mini-SCAN [24]. Differences in demographic characteristics between patients and controls were tested with independent t-tests, except for gender and handedness, which were tested with a Chi-square test for independence.
▪ Task design
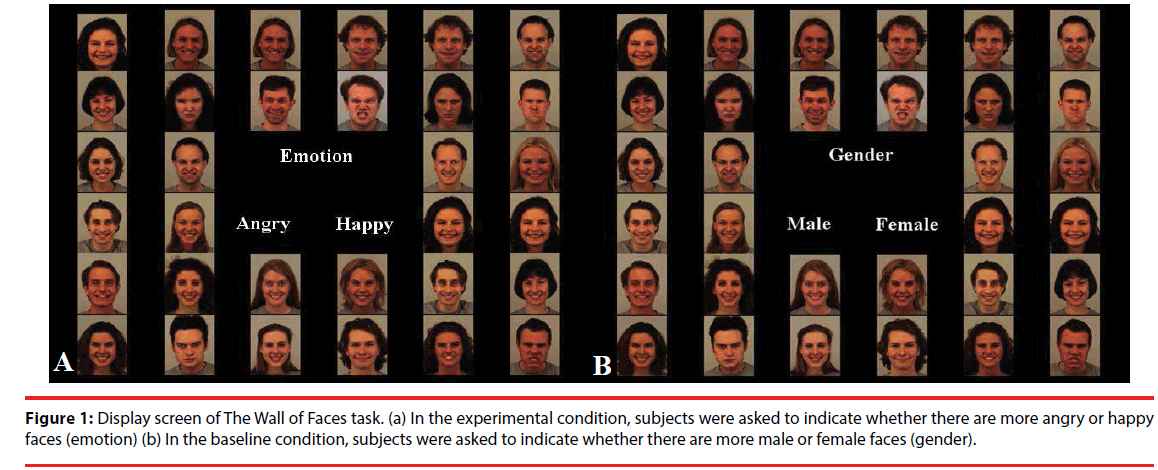
The Wall of Faces (WoF) task examines the neural substrates underlying emotional ambiguity [18]. During this task, a group of faces is presented at once, and the dominant emotion or gender has to be identified, in both ambiguous and unambiguous situations. In healthy subjects, ambiguous emotional trials relative to ambiguous gender trials have been shown to activate the ventromedial prefrontal cortex (VMPFC) and the ventral ACC [18]. Ambiguous relative to unambiguous trials activated the dorsal part of the ACC, the dorsolateral prefrontal cortex (DLPFC), the visual cortex and the posterior parietal cortex (PPC) [18].
Figure 1 shows a display screen of The Wall of Faces task. In each trial, an array of 32 emotional faces (i.e., angry or happy) was presented to a subject. The ratio of angry to happy faces (emotional trials, experimental condition) and male to female faces (gender trials, control condition) varied and could be equal (ambiguous, 16:16) or unequal (unambiguous, 26:6 or 6:26). In each trial, the array of faces was presented for the duration of 3 s with an additional 1.5 s response time. Participants were asked to identify the predominant emotion (experimental condition) or the predominant gender (control condition) intuitively, and not by counting of the number of faces. During face presentation and additional response time, the options “Angry - Happy” or “Female - Male” were displayed on the screen. Blocks of 8 trials (48 s) started with an instruction (“emotion” or “gender”) and were interleaved with a rest condition (24 s). Emotion and gender blocks alternated. The task was presented using E-prime 1.2, which logged timing of the task and responses of the subjects. Subjects responded by button presses on an MR-compatible button box using the index and middle finger of their right hand.
▪ Behavioral measures
The PANSS was used to assess the two factors of negative symptoms, expressive deficits and social-emotional withdrawal. These two factors are based on an extensive factor analysis of the PANSS, which revealed a two-factor structure of negative symptoms [13]. The factor expressive deficits consisted of PANSS items Flat affect (N1), Poor rapport (N3), Lack of spontaneity (N6), Mannerisms and posturing (G5), Motor retardation (G7), and Avolition (G13). The social-emotional withdrawal factor consisted of PANSS items Emotional withdrawal (N2), Passive/apathetic social withdrawal (N4), and Active social avoidance (G16) [13].
Task performance was measured by comparing reaction times (in seconds) and accuracy of the unambiguous trials (% correct). As there were no correct responses in the ambiguous trials, since the distribution of faces were equal (i.e., 16 versus 16), the response percentage of male faces for the gender trials and angry faces for the emotional trials were measured. Missing entries were not included in the analysis. Performance was compared between patients and controls using an independent samples t-test.
Within the patient group, expressive deficits and social-emotional withdrawal were correlated with performance, controlling for level of education. Also, correlation coefficients between expressive deficits and social-emotional withdrawal on the one hand and education level, MADRS, PANSS positive subscale and percentage of estimated D2 receptor occupancy [25] on the other hand were calculated.
▪ Image acquisition
MRI scans were acquired with a Philips 3 Tesla MRI scanner (Achieva Intera, Best, The Netherlands), equipped with an 8-channel SENSE head coil. Movement was restricted by foam pads fixating the head, and noise was reduced by earplugs and head phones. The task was presented on a screen visible via a mirror on top of the head coil.
During the task, a pseudo-continuous Arterial Spin Labeling (PCASL) sequence was acquired. As mentioned earlier, baseline data of two trials were used in this study. ASL was used because in these two trials a second scan was made after several weeks to assess treatment effect, and ASL is more suitable to compare measurements when they are repeated over time. In addition, ASL provides reliable absolute quantification of cerebral blood flow, and it has higher spatial and temporal resolution than other techniques [26]. In comparison to a BOLD sequence, ASL can examine baseline activity of the brain instead of relative changes as measured with BOLD. Furthermore, ASL has lower inter-subject variability and allows superior functional localization [27,28]. Control and labeled scans (4 s; 127 of both) were alternated. Labeling time was 1650 ms, delay time 1525 ms and acquisition time was 825 ms. Further parameters: flip angle 90º, 14 slices, FOV (ap, fh, rl)=224 × 98 × 224 mm, voxel size 1.75 × 1.75 × 7 mm.
▪ Data analysis
Data were analyzed using in-home scripts based on Statistical Parametric Mapping (SPM8; FIL Wellcome Department of Imaging Neuroscience, London, UK) routines and functions. First, raw PAR files were converted to NIFTI format. Next, labeled and control images were realigned separately, because intensity differences between both image modalities may cause spurious motion correction. Mean images of both realignments were created. The mean labeled image was co-registered to the mean control image and the same parameters were applied to all labeled images. Images were then smoothed with an 8 mm FWHM Gaussian isotropic kernel.
Due of the low signal-to-noise ratio of ASL data, nuisance factors were filtered from the data by regression, including the motion parameters, white matter (WM) signals, and cerebrospinal fluid (CSF) signal. For the CSF and WM signal, masks were created by co-registering the anatomy to the mean control image, which was then segmented. The first principle components of the CSF and WM signals were extracted from the functional image series by using these WM and CSF masks. Regression of the ASL data with nuisance factors was done separately for control and labeled images. After this, labeled images were subtracted from control images using spline interpolation of subsequent scans in both image types separately [27,28]. The subtracted ASL images were entered in a first level analysis. The four task conditions and an instruction condition (notifying task and resting blocks) were modeled in a block design convolved with a Hemodynamic Response Function. Implicit masking and high-pass filtering were not applied in the first-level analysis, which are standard procedures for BOLD fMRI, but not for ASL. Instead, an explicit mask was used consisting of the gray and white matter of the segmented brain. Since the ASL images contained artifacts in the highest and lowest planes (i.e., in the cranium, not in the brain tissue), these parts were excluded from the explicit mask. Contrasts were created of the emotional versus the gender trials, the ambiguous versus the unambiguous trials, for emotion-gender in ambiguity, gender ambiguity, emotional ambiguity and emotion-gender in unambiguity.
Since the ASL sequence changed during the study due to a scanner upgrade, the histogram (i.e., image intensity range) of the contrast images was found to be different. Equalization of the intensity distribution of contrast-images was applied by taking the 25% and 75% values of the cumulative histogram of the baseline betaimage (last column design matrix) and using these values for histogram normalization of the contrast images. Fourteen controls and 16 patients were scanned before the update and 6 controls and 22 patients were scanned after the update. The mean control image created during realignment was co-registered to the anatomy, and the anatomy and histogram-normalized contrast-images were normalized to the T1 template of SPM.
The normalized contrast images were entered in a second level analysis using Statistical non- Parametric Mapping (SnPM) [29]. Nonparametric analyses were used since ASL data has a non-normal distribution. Analyses were done with a variance smoothing of 8 mm FWHM, 5000 iterations, and no additional scaling. Out of brain voxels were removed by masking with the brain mask of SPM. Resulting pseudo-T maps were inspected at a threshold of p<0.001, k>20, pseudo-T>3 (pseudo-T threshold to control for type-I errors), as is used in other ASL functional MRI studies [15,30]. This pseudo-T threshold was used to correct for multiple comparison, which has been suggested in case of non-homogeneous smoothness of the data (Tom Nichols, SPM mailing list Item #7573 (26 Nov 2001 17:56) - “Re: Cluster level statistics in SnPM, https://www.jiscmail.ac.uk/cgi-bin/ webadmin?A0=spm)”. Contrasts of interest in the final analyses of the Wall of Faces tasks were: 1) ambiguous emotion relative to the unambiguous emotion, 2) ambiguous emotion relative to ambiguous gender and 3) ambiguous relative to unambiguous regardless of valence.
First, activity of all contrasts was investigated in the healthy control sample and patient sample using the one sample T-test option of SnPM. Next, patients and controls were compared using the two sample T-test of SnPM. The effect of the two factors of negative symptoms on brain activation during emotional appraisal in patients with schizophrenia was investigated by regression of the PANSS expressive deficits and the PANSS social-emotional withdrawal [13] to all contrasts of interest, using the “MultiSub: Simple Regression; 1 covariate of interest” function of SnPM. As negative symptoms may be secondary to depressive symptoms, positive symptoms and medication side effects, we repeated the analyses with the PANSS positive symptom scale, the estimated percentage of D2 receptor occupancy [25] and the Montgomery Åsberg Depression Rating Scale (MADRS) total score to control for potential confounding variables. The MADRS data of one patient was missing.
Results
▪ Demographics
Data from 38 patients and 20 control subjects were included in the analyses. Demographic characteristics are shown in Table 1. The subject groups did not differ significantly in age, gender, handedness or education level.
| Schizophrenia patients | Healthy Controls | P-value | |
|---|---|---|---|
| Age (years) | 32.42 (11.05) | 31.10 (11.69) | 0.67 |
| Gender (% male) | 87 | 70 | 0.12 |
| Handedness (% right)* | 97 | 90 | 0.32 |
| Education (Verhage) | 4.89 (1.87) | 5.7 (1.34) | 0.09 |
| Illness duration (years) | 9.02 (9.57) | ||
| Current antipsychotics (%) | |||
| None | 18.42 | ||
| Antipsychotic polypharmacy | 26.32 | ||
| Aripiprazole | 15.79 | ||
| Bromperidol | 2.63 | ||
| Clozapine | 23.68 | ||
| Flupentixol | 5.26 | ||
| Haloperidol | 2.63 | ||
| Olanzapine | 28.95 | ||
| Paliperidone | 2.63 | ||
| Quetiapine | 5.26 | ||
| Risperidone | 13.16 | ||
| Zuclopentixole | 7.89 | ||
| PANSS scores | |||
| Positive subscale | 14.03 (4.77) | ||
| Negative Subscale | 18.66 (4.35) | ||
| General pathology subscale | 31.68 (7.99) | ||
| Depression subscale (items G1, G2, G3, G6) | 9.63 (4.08) | ||
| Expressive deficits (items N1, N3, N6, G5, G7 and G13)¹ | 14.34 (4.04) | ||
| Social-emotional withdrawal (items N2, N4, G16)¹ | 8.34 (2.99) | ||
| MADRS score | 17 (9.92) |
Data are means (+/- SD) or percentage; PANSS, Positive and Negative Syndrome Scale; MADRS, Montgomery Asberg Depression Rating Scale. *some data is missing.
1) Liemburg, E., Castelein, S., Stewart, R., van der Gaag, M., Aleman, A., Knegtering, H., et al. (2013) Two subdomains of negative symptoms in psychotic disorders: established and confirmed in two large cohorts. Journal of psychiatric research, 47, 718-725.
Table 1: Demographic and baseline clinical characteristics.
▪ Behavioral results
In general, patients were less accurate and needed more time to respond, but these differences were not statistically significant, except for accuracy on gender unambiguous trials (p=0.02, effect size Cohen’s d=0.61). Additionally, a larger percentage of patients did not press the button box after a face presentation as compared to controls and thus the percentage of missing data in the patient group was larger, but again these differences were not statistically significant. The percentage missing data for the unambiguous emotion trials was 2.6% for the control group and 5.9% for the patient group (p=0.08), for the ambiguous emotion trial 10.5% for the control group and 13.7% for the patient group (p=0.35), for the unambiguous gender trial 2.3% for the control group and 6.4% for the patient group (p=0.23) and for the ambiguous gender trial 4.9% for the control group and 11% for the patient group (p=0.13). Table 2 shows accuracy for unambiguous trials and the response selection for ambiguous trials.
| Groups | Mean | SD | P | |
|---|---|---|---|---|
| Accuracy in percentage for emotion unambiguous trials (6 angry and 26 happy or 6 happy and 26 angry faces) | Healthy controls | 92.5 | 8.3 | 0.64 |
| Patients | 91.4 | 8.0 | ||
| Accuracy in percentage for gender unambiguous trials (6 male and 26 female or 6 female and 26 male faces) | Healthy controls | 98.0 | 4.3 | 0.02 |
| Patients | 93.4 | 9.6 | ||
| Percentage angry faces for emotion ambiguous trials (16 angry and 16 happy faces) | Healthy controls | 52.7 | 8.5 | 0.66 |
| Patients | 51.0 | 15.3 | ||
| Percentage male faces for gender ambiguous trials (16 male and 16 female faces) | Healthy controls | 46.3 | 13.0 | 0.43 |
| Patients | 43.2 | 14.5 | ||
| Data are means (+/- SD), presented for the two groups. | ||||
Table 2: Accuracy for unambiguous trials, response selection for ambiguous trials of both patients and healthy controls during the task.
There were significant positive correlations between expressive deficits and reaction time on all trials; gender ambiguous trials (r=0.4, p=0.016), gender unambiguous trials (r=0.39, p=0.018), emotional ambiguous trials (r=0.36, p=0.027) and emotional unambiguous trials (r=0.37, p=0.023). Also, there were significant positive correlations between expressive deficits and accuracy on both unambiguous gender (r=0.36, p=0.031) and unambiguous emotional (r=0.34, p=0.038) trials. There was no significant association between expressive deficits and level of education.
There were no significant correlations between social-emotional withdrawal on the one hand and performance measures or level of education on the other hand.
Imaging results
▪ One sample t-test control group
Ambiguous emotion relative to unambiguous emotion showed more brain activation in the left DLPFC, the left postcentral gyrus and the right middle occipital gyrus. Ambiguous emotion relative to ambiguous gender showed lower brain activation in the left middle occipital gyrus. The ambiguous relative to unambiguous contrast (across gender and emotion decision trials) demonstrated more brain activation in the left cuneus and the right middle occipital gyrus.
▪ One sample t-test patient group
The ambiguous relative to unambiguous contrast, regardless of valence, demonstrated higher levels of brain activation in the right superior frontal gyrus. The other contrasts did not show significant differences in activation.
„ Brain activation in patients with schizophrenia as compared to healthy controls
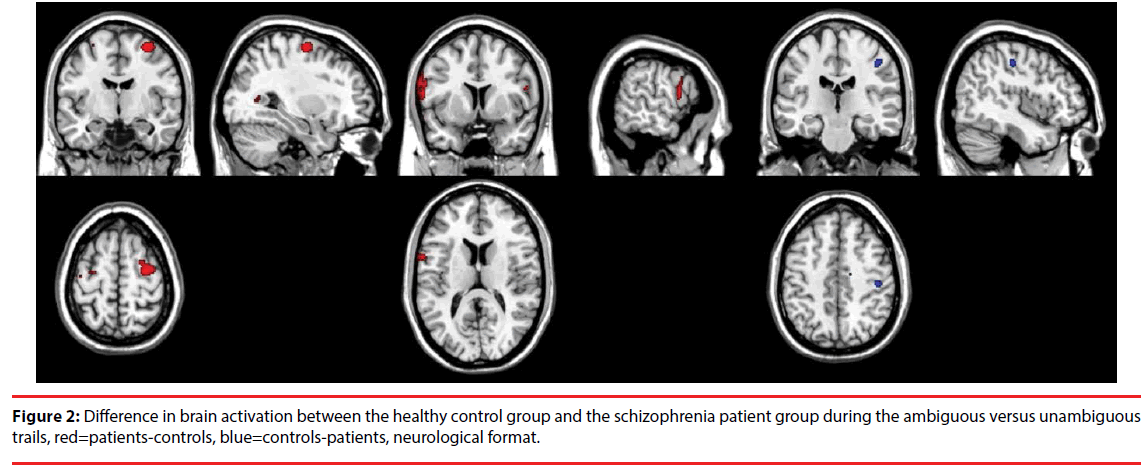
Patients with schizophrenia as compared to controls showed higher activation in the right DLPFC and the left precentral gyrus, and lower activation in the right postcentral gyrus of the parietal lobe during the ambiguous relative to unambiguous contrast, see Table 3 and Figure 2. The other contrasts did not result in significant differences.
| Contrast | K | Pseudo-T | X | Y | Z | Area |
|---|---|---|---|---|---|---|
| Ambiguous versus unambiguous | 143 | 3,78 | 30 | -8 | 62 | right middle frontal gyrus |
| 159 | 3,59 | -60 | 8 | 14 | left precentral gyrus | |
| 28 | 3,36 | 44 | -22 | 46 | right postcentral gyrus |
Table 3: Areas (MNI coordinates) that show significant differences in activation between patients with schizophrenia and healthy controls (p<0.001, k>20, pseudo-T>3).
▪ Regression analysis with the PANSS expressive deficits
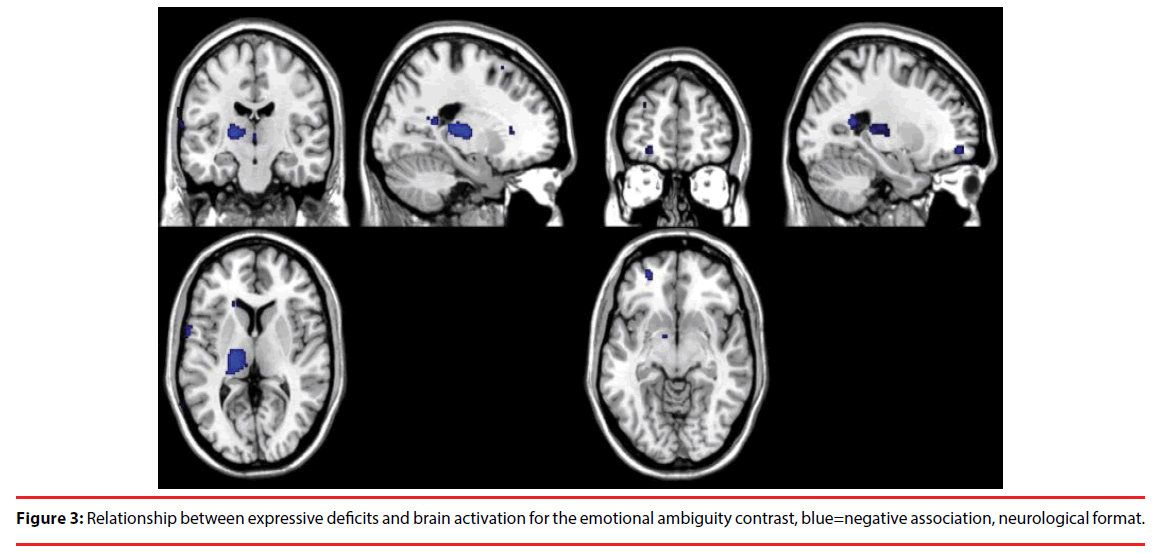
For the ambiguous emotion relative to unambiguous emotion contrast, severity of expressive deficits was negatively correlated with brain activation in the left thalamus, the bilateral precentral gyrus, the bilateral precuneus, the right superior temporal gyrus and the left middle frontal gyrus. For the same contrast, there was a positive correlation between levels of expressive deficits and brain activation in the right postcentral gyrus. Figure 3 illustrates the effect of expressive deficits on brain activation during the emotional ambiguity contrast.
In the ambiguous emotion relative to the ambiguous gender contrast, severity of expressive deficits was negatively correlated with activation of the VMPFC (ventral anterior cingulate and the medial prefrontal gyrus) and the left middle and superior temporal gyrus. Figure 4 illustrates the effect of expressive deficits on brain activation during the ambiguous emotion versus ambiguous gender contrast.
Regression analysis of the ambiguous versus unambiguous contrast revealed a negative correlation between expressive deficits and brain activation in the right superior temporal gyrus and left cuneus, and a positive correlation between expressive deficits and brain activation in the right postcentral gyrus. For an overview of the MNI coordinates and areas see Table 4.
| Contrast | K | pseudo-t | x | y | z | Area |
|---|---|---|---|---|---|---|
| Ambiguous versus unambiguous | 45 | 3,73 | 54 | -20 | 58 | Right Postcentral Gyrus |
| 28 | 3,64 | 46 | -46 | 16 | Right Superior Temporal Gyrus | |
| 73 | 3,33 | -4 | -98 | 12 | Left Cuneus | |
| Affect versus gender in ambiguity | 189 | 4,36 | 22 | 32 | -4 | Ventromedial Prefrontal Cortex |
| 36 | 3,58 | -56 | -68 | 28 | Left Middle Temporal Gyrus | |
| 20 | 3,57 | -62 | 4 | 6 | Left Superior Temporal Gyrus | |
| Ambiguous emotion-unambiguous emotion | 62 | 4,59 | -62 | 4 | 8 | Left Precentral Gyrus |
| 490 | 4,21 | -20 | -16 | 6 | Left Thalamus | |
| 193 | 3,83 | -36 | -74 | 42 | Left Precuneus | |
| 61 | 3,82 | 46 | -48 | 14 | Right Superior Temporal Gyrus | |
| 26 | 3,73 | -26 | 52 | -8 | Left Middle Frontal Gyrus | |
| 29 | 3,53 | 68 | 2 | 12 | Right Precentral Gyrus | |
| 114 | 3,4 | 6 | -74 | 46 | Right Precuneus | |
| 26 | 3,61 | 52 | -18 | 58 | Right Postcentral Gyrus |
Table 4: Areas (MNI coordinates) showing a significant association with expressive deficits of schizophrenia (p<0.001, k>20, pseudo-T>3).
▪ Regression analysis with the PANSS social-emotional withdrawal
No significant association of social-emotional withdrawal with brain activation was found.
▪ Depressive symptoms, positive symptoms and D2 receptor occupancy
There was no significant correlation between the MADRS and the PANSS expressive deficits (r=0.05, p=0.76), the PANSS positive subscale and the PANSS expressive deficits (r=0.084, p=0.62) and the estimated D2 receptor occupancy and the PANSS expressive deficits (r=0.092, p=0.58).
There was a significant correlation between the MADRS and the PANSS social-emotional withdrawal items (r=0.54, p=0.001) and the PANSS positive subscale and the PANSS socialemotional withdrawal items (r=0.34, p=0.037). There was no significant association between the estimated D2 receptor occupancy and the PANSS social-emotional withdrawal (r=0.18, p=0.29).
No significant association on the MADRS was found for the ambiguous emotion relative to unambiguous emotion contrast. In the ambiguous emotion relative to ambiguous gender contrast, regression analysis showed a positive relation between level of depressive symptoms and brain activation in the right anterior cingulate, and a negative association between depressive symptoms and brain activation in the left precuneus, the left posterior cingulate and the right superior frontal gyrus. Regression analysis of the ambiguous versus unambiguous contrast found a negative association between depressive symptoms and brain activation in the right caudate.
Regression analysis of percentage of estimated D2 receptor occupancy found a negative relation between levels of D2 receptor occupancy and brain activation in the right supramarginal gyrus of parietal lobe and right inferior frontal gyrus during the ambiguous emotion relative to unambiguous emotion contrast. No significant association was found for the ambiguous emotion relative to ambiguous gender contrast. For the ambiguous versus unambiguous contrast a negative relation between estimated D2 receptor occupancy and brain activation was found in the right inferior frontal lobe and the right temporal lobe.
Regression analysis of the PANSS positive subscale revealed a positive relation between higher levels of positive symptoms and brain activation in the right middle frontal gyrus and right posterior cingulate during the ambiguous emotion relative to unambiguous emotion contrast. In the ambiguous emotion relative to ambiguous gender contrast, regression analysis found a negative relation between positive symptoms and brain activation in the right superior temporal gyrus. Finally, the ambiguous versus unambiguous contrast revealed a positive relation between positive symptoms and brain activation in the left superior temporal gyrus.
Discussion
In this study, we investigated whether the two factors of negative symptoms of schizophrenia, namely expressive deficits and social-emotional withdrawal, were related to abnormalities in the neurocircuitry of emotion appraisal, using a task that probed emotional ambiguity in a group of different faces. To our knowledge, this is the first study to investigate the neural correlates of emotional decision making under uncertainty in schizophrenia. We compared brain activation of healthy controls with that of patients with schizophrenia, and related levels of expressive deficits and social-emotional withdrawal with brain activation. Ambiguity, regardless of valence, activated the fronto-parietal circuitry differently in patients with schizophrenia as compared to healthy controls. Severity of expressive deficits was associated with hypoactivation of the frontothalamic pathway for the emotional ambiguity contrast. In addition, severity of expressive deficits was also related to hypoactivation of the VMPFC, including the ventral ACC, in the ambiguous emotion versus ambiguous gender contrast. Moreover, these findings appear to be independent of severity of depressive symptoms, positive symptoms and percentage of estimated D2 receptor occupancy, as the regression analysis of the MADRS, PANSS positive subscale and percentage of estimated D2 receptor occupancy showed a distinctive pattern of brain activation that differed from the areas found for expressive deficits. Furthermore, there was no significant correlation between the MADRS, PANSS positive subscale, the estimated D2 receptor occupancy on the one hand and the PANSS expressive deficits items on the other hand.
A key finding of our study concerns the association of expressive deficits, but not socialemotional withdrawal, with lower prefrontal and thalamic activation during ambiguous emotion perception. The association between higher levels of expressive deficits and hypoactivation of the PFC is in line with earlier studies that report on dysfunctioning of the PFC in patients with negative symptoms of schizophrenia [7]. Interestingly, a large study on patients with neurodegenerative diseases, such as Alzheimer and frontotemporal dementia, found a poorer performance on an emotion expression tasks to be correlated with volume loss in prefrontal and thalamic regions in the brain [31]. Hypoactivation of the fronto-thalamic circuit during ambiguous social appraisal may imply a reduced action readiness in social situations, underlying expressive deficits. Indeed, behavioral data showed a significant positive correlation between expressive deficits and reaction times, implicating that patients with more expressive deficits needed more time to respond than patients with lower levels of expressive deficits. In contrast, no significant correlations between the behavioral data and levels of socialemotional withdrawal were found. Earlier studies have found social-emotional withdrawal to be associated with reduced frontoparietal activation during a planning task [15] and apathy/avolition/emotional withdrawal but not expressive deficits to be associated with reduced ventral striatal activation during a monetary reward task [32]. It is possible that socialemotional withdrawal is related to anticipatory pleasure deficits [33], which cannot be detected with the Wall of Faces task, as it is not designed for this purpose. In conclusion, the present task may be more sensitive for brain circuits involved in expressive deficits, which further supports the neuroanatomical differentiation of the two factors of negative symptoms.
A previous study conducted among healthy volunteers found that ambiguous emotion relative to ambiguous gender contrast activated brain areas that seem to be involved in emotional processing and emotion recognition, such as the VMPFC (ventral ACC and the ventral medial PFC), the right superior temporal gyrus and the right supramarginal gyrus [18]. We did not replicate these findings in our healthy control group. This may imply that the contrast does not reliably isolate brain regions associated with emotional processing, maybe because both contrasts contain human faces that contain social-emotional cues or because the task instruction was geared towards a group-level judgment. Alternatively, statistical power could play a role as well as signal loss in ventral prefrontal areas. However, regression analysis of the same contrast in the patient group revealed higher levels of expressive deficits to be associated with hypoactivation of the VMPFC. Thus, impairment in the neurocircuitry of emotional processing may specifically emerge in association with impairments in social cognition in schizophrenia with expressive deficits.
Interestingly, the correlation analysis within the patient group found patients with higher levels of expressive deficits to have greater reaction times, but also greater accuracy. This is in line with a previous study that found severity of symptoms in schizophrenia contributed relatively more to the variance in speed of emotion processing than to the variance in accuracy [34]. Indeed, emotional expressions bring forth quick responses and often this includes imitation of the emotion in the observed face [35]. Earlier studies have found impairments in emotional face imitation in patients with schizophrenia [36,37] and this impairment was not accompanied by impairment in the identification of emotional expressions [37]. In other words, impairment in emotional processing may not necessarily be accompanied by impairment in accuracy during identification of emotional faces. The ability to quickly process social stimuli is essential for social interactions, and it has been suggested that decreased speed in processing social stimuli may lead to deficits in social functioning [3]. Thus, the compromised emotional processing speed in patients with more expressive deficits may negatively affect interactions.
A limitation of this study could be the artificial nature of the stimulus display (thirty-two faces on one screen), as people are not presented with an array of individual pictures of faces in daily life. Indeed, impairments may become more apparent when using stimuli that better approximate realworld contexts [38]. Future studies could use more dynamic and realistic images of real-life social situations to further elucidate the neural circuitry of emotion processing in social contexts. Another limitation includes the chronic use of antipsychotic medication in the patient group as compared to controls. Antipsychotics alter neuronal function, and up to date it is unclear how chronic use of antipsychotic medication influences brain function [39]. A final limitation is the use of ASL, which is not used as frequently as BOLD fMRI, and thus replication of these findings may be relatively more difficult.
Prefronto-striato-thalamic functional dysconnectivity seems to be implicated in the pathophysiology of schizophrenia [40]. However, there may be intercultural differences in social processes and it would be interesting to examine these intercultural differences in social cognitive processes in patients with schizophrenia. Also, environmental factors [41,42], dietary influence [43,44] or stress-induced lifestyle condition may influence brain function and future studies may want to investigate this.
In conclusion, fronto-thalamic dysfunctioning during an ambiguous emotional appraisal task was associated with expressive deficits but not with social-emotional withdrawal. Thus, the two factors of negative symptoms seem to have different underlying neural mechanisms. The alterations found could contribute to increase our understanding of the neural basis of social dysfunctioning as a characteristic of expressive deficits, typically seen in patients with schizophrenia.
Acknowledgement
We would like to thank all the participants of this study.
Funding
The study on treatment of negative symptoms of schizophrenia with transcranial magnetic stimulation (Dutch Trial Registry: NTR1261) was supported by means of an unconditional research grant from AstraZeneca and an unconditional research grant from Stichting Roos. Author A.A. was supported by a VICI grant from N.W.O., grant number 435-11-004
Conflict of Interest
Henderikus Knegtering, MD, PhD, is on the speakers’ list of and/or has received unconditional grants from Janssen, Eli Lilly, Bristol Meyers Squibb and Astra Zeneca. André Aleman received speaker fees from Lundbeck. All other authors declare that there are no conflicts of interests.
Compliance with Ethical Standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: An overview. Schizophr. Bull 34(3): 408-411 (2008).
- Aleman A, Kahn RS. Strange feelings: Do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog. Neurobiol 77(5), 283-298 (2005).
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: A review. Schizophr. Bull 32 Suppl 1: S44-63 (2006).
- Kohler CG, Walker JB, Martin EA, et al. Facial emotion perception in schizophrenia: A meta-analytic review. Schizophr. Bull 36(5), 1009-1019 (2010).
- Gur RE, Loughead J, Kohler CG, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch. Gen. Psychiatry 64(12),1356-1366 (2007).
- Ventura J, Wood RC, Jimenez AM, et al. Neurocognition and symptoms identify links between facial recognition and emotion processing in schizophrenia: Meta-analytic findings. Schizophr. Res 151(1-3), 78-84 (2013).
- Goghari VM, Sponheim SR, MacDonald AW. The functional neuroanatomy of symptom dimensions in schizophrenia: A qualitative and quantitative review of a persistent question. Neurosci. Biobehav. Rev 34(3), 468-486 (2010).
- Harvey PO, Armony J, Malla A, et al. Functional neural substrates of self-reported physical anhedonia in non-clinical individuals and in patients with schizophrenia. J. Psychiatr. Res 44(11), 707-716 (2010).
- Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage 29(2), 409-416 (2006).
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update. J Psychosom. Res 53(2), 647-654 (2002).
- Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: Implications for assessment. Schizophr. Bull 32(2), 238-245 (2006).
- Peralta V, Cuesta MJ. Negative symptoms in schizophrenia: A confirmatory factor analysis of competing models. Am. J. Psychiatry 152(10), 1450-1457 (1995).
- Liemburg E, Castelein S, Stewart R, et al. Two subdomains of negative symptoms in psychotic disorders: Established and confirmed in two large cohorts. J. Psychiatr. Res 47(6), 718-725 (2013).
- Messinger JW, Tremeau F, Antonius D, et al. Avolition and expressive deficits capture negative symptom phenomenology: Implications for DSM-5 and schizophrenia research. Clin. Psychol. Rev 31(1), 161-168 (2011).
- Liemburg EJ, Dlabac-De Lange JJ, Bais L, et al. Neural correlates of planning performance in patients with schizophrenia - relationship with apathy. Schizophr. Res 161(2-3), 367-375 (2015).
- Kirschner M, Hager OM, Bischof M, et al. Ventral striatal hypoactivation is associated with apathy but not diminished expression in patients with schizophrenia. J. Psychiatry. Neurosci 40(5), 140383 (2015).
- Mucci A, Dima D, Soricelli A, et al. Is avolition in schizophrenia associated with a deficit of dorsal caudate activity? A functional magnetic resonance imaging study during reward anticipation and feedback. Psychol. Med 45(8), 1765-1778 (2015).
- Simmons A, Stein MB, Matthews SC, et al. Affective ambiguity for a group recruits ventromedial prefrontal cortex. Neuroimage 29(2), 655-661 (2006).
- Dlabac-de Lange JJ, Bais L, van Es FD, et al. Efficacy of bilateral repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: Results of a multicenter double-blind randomized controlled trial. Psychol. Med 1-13 (2014).
- Giel R, Nienhuis FJ. SCAN-2.1: Schedules for clinical assessment in neuropsychiatry (in dutch).Geneva/Groningen: WHO (1996).
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull 13(2):261-276 (1987).
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 134(1), 382-389 (1979).
- Verhage F. Revised scoring method. (1983).
- Nienhuis FJ, van de Willige G, Rijnders CA, et al. Validity of a short clinical interview for psychiatric diagnosis: The mini-SCAN. Br. J. Psychiatry 196(1), 64-68 (2010).
- Lako IM, van den Heuvel ER, Knegtering H, et al. Estimating dopamine D(2) receptor occupancy for doses of 8 antipsychotics: A meta-analysis. J. Clin. Psychopharmacol 33(5), 675-681 (2013).
- Borogovac A, Asllani I. Arterial spin labeling (ASL) fMRI: Advantages, theoretical constrains and experimental challenges in neurosciences. Int. J. Biomed. Imaging 2012(1), 818456 (2012).
- Liu TT, Wong EC. A signal processing model for arterial spin labeling functional MRI. Neuroimage 24(1), 207-215 (2005).
- Wang Z, Aguirre GK, Rao H, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn. Reson. Imaging 26(2), 261-269 (2008).
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain. Mapp 15(1), 1-25 (2002).
- Henriksen OM, Jensen LT, Krabbe K, et al. Resting brain perfusion and selected vascular risk factors in healthy elderly subjects. PLoS. One 9(5), e97363 (2014).
- Gola KA, Shany-Ur T, Pressman P, et al. A neural network underlying intentional emotional facial expression in neurodegenerative disease. Neuroimage. Clin 14(1), 672-678 (2017).
- Hartmann MN, Hager OM, Reimann AV, et al. Apathy but not diminished expression in schizophrenia is associated with discounting of monetary rewards by physical effort. Schizophr. Bull 41(2), 503-512 (2015).
- Gard DE, Kring AM, Gard MG, et al. Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophr. Res 93(1-3), 253-260 (2007).
- Barkhof E, de Sonneville LM, Meijer CJ, et al. Processing of facial and nonsocial information is differentially associated with severity of symptoms in patients with multiepisode schizophrenia. J. Nerv. Ment. Dis 203(2), 112-119 (2015).
- Frith C. Role of facial expressions in social interactions. Philos. Trans. R. Soc. Lond. B. Biol. Sci 364(1535), 3453-3458 (2009).
- Schwartz BL, Mastropaolo J, Rosse RB, et al. Imitation of facial expressions in schizophrenia. Psychiatry. Res 145(2-3), 87-94 (2006).
- Park S, Matthews N, Gibson C. Imitation, simulation and schizophrenia. Schizophr. Bull 34(4),698-707 (2008).
- Sasson NJ, Pinkham AE, Weittenhiller LP, et al. Context effects on facial affect recognition in schizophrenia and autism: Behavioral and eye-tracking evidence. Schizophr. Bull (2015).
- Handley R, Zelaya FO, Reinders AA, et al. Acute effects of single-dose aripiprazole and haloperidol on resting cerebral blood flow (rCBF) in the human brain. Hum. Brain. Mapp 34(2), 272-282 (2013).
- Vandevelde A, Leroux E, Delcroix N, et al. Fronto-subcortical functional connectivity in patients with schizophrenia and bipolar disorder during a verbal fluency task. World. J. Biol. Psychiatry 2017(1):1-9.
- Mucci A, Galderisi S, Green MF, et al. Familial aggregation of MATRICS consensus cognitive battery scores in a large sample of outpatients with schizophrenia and their unaffected relatives. Psychol. Med 1-10 (2017).
- Martins I. Increased risk for obesity and diabetes with neurodegeneration in developing countries. J. Mol. Genet. Med S1, 001 (2013).
- Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations and mental diseases. Am. J. Med. Genet. B. Neuropsychiatr. Genet 174(6), 651-660 (2017).
- Martins IJ. Magnesium therapy prevents senescence with the reversal of diabetes and Alzheimer’s disease. Health 8, 694-710 (2016).