Research Article - (2018) Volume 8, Issue 4
Minimally Invasive Surgery for Intracerebral Hematoma Evacuation followed by Rosiglitazone Infusion Therapy Increased Perihematomal Occludin, Zonula Occludens-1 Expression and Decreased the Blood Brain Barrier Permeability in Rabbits
- Corresponding Authors:
- Guofeng Wu
Emergency Department, Affiliated Hospital of Guizhou Medical University, Guiyang, 550004, China
Tel: +86-1380-9431-723
Fax: +86-0851-8722-143 - Mengzhou Xue
Department of Cerebrovascular Diseases, Second Affiliated Hospital of Zhengzhou University, Zhengzhou, 450000, China
Tel: +86-1361-3783-928
Abstract
Objective:
To observe the effects of minimally invasive surgery (MIS) for hematoma of intracerebral hemorrhage (ICH) evacuation followed by rosiglitazone (RSG) infusion therapy on perihematomal tight junction associated proteins occludin and zonula occludens-1 (ZO-1) expression as well as blood brain barrier (BBB) permeability in rabbits.
Methods:
A total of 50 male rabbits (2.5-3.5 kg) were randomly assigned to a sham group (Sham group, 10 rabbits), an ICH model group (HM group, 10 rabbits), a RSG medication group (RSG group, 10 rabbits), a minimally invasive surgery group (MIS group, 10 rabbits) and a MIS combined with RSG group (MIS+RSG group, 10 rabbits). ICH was induced in all of the rabbits except for those in the Sham group. An MIS was performed to evacuate the hematoma at 6 hours after the successful preparation of the ICH model in the MIS group and the MIS+RSG group. The RSG (0.5mg, dissolved in 0.1 ml of 0.9% sodium chloride solution) was infused into the hematoma area in the RSG group and the MIS+RSG group. All rabbits were sacrificed on day 7 after the relative processes were performed successfully, and the perihematomal brain tissue was removed to determine the occludin and ZO-1 expression and BBB permeability by Evens Blue (EB).
Results:
The occludin and ZO-1 expression were all significantly decreased and the BBB permeability was increased in the HM group compared with the Sham group. The RSG used alone or performing the MIS alone to evacuate the ICH resulted in a marked increase in the occludin and ZO-1 expression and decrease in BBB permeability. The MIS+RSG group displayed a great increase in the occludin and ZO-1 expression and a more significant decrease in BBB permeability.
Conclusions:
The MIS combined with RSG medication could significantly increase the perihematomal occludin and ZO-1 expression and decrease in BBB permeability which plays an important therapeutic role in secondary brain damage following ICH.
Keywords
Intracerebral hemorrhage, Minimally invasive surgery, Rosiglitazone, Tight junction, Occludin, Zonula occludens-1, Blood-brain barrier
Introduction
Spontaneous intracerebral hemorrhage (ICH) is defined as bleeding within the brain parenchyma [1], and it has a higher mortality rate of 35.52% within 30 days after onset. Only 20% of individuals who survive ICH are independent at 6 months [2-4]. ICH has higher rates of mortality, morbidity, and disability than any other type of stroke. However, there were no effective treatment approaches currently available for ICH [5]. The use of medications and conservative treatment of ICH is still lack of effective drugs. Surgical treatment of ICH is invasive during clot removal and may result in an iatrogenic impairment. In recent 10 years, minimally invasive surgery (MIS) for evacuating blood clots (hematoma) appears to be a promising strategy. MIS is a safe and practical technique in treating ICH. Patients with ICH may be benefit more from MIS than other treatment options [6-9]. Although MIS could effectively evacuate blood clot and reduce the brain damage following ICH, but does not completely remove the erythrocytes and cytotoxic substances which extravasate into the adjacent brain [10-13]. The role of the MIS in reducing secondary brain damage remains limited. Performing the MIS for removing blood clot followed by medications to prevent secondary damage might be an optimal method [10,14].
Recently published studies have demonstrated that BBB disruption is a key pathophysiological process of ICH and a hallmark of ICH-induced brain injury. It is probable that prevention of ICH-induced BBB disruption will involve blocking multiple pathways or blocking a common end pathway. For example, by stabilizing tight junction (TJ) structure [15]. An important structural element of the BBB is the endothelial TJ. TJ are elaborate networks of transmembrane and cytosolic proteins that regulate epithelial permeability, localized at the most apical end of the lateral plasma membrane [16,17]. The normal expression of the endothelial TJ associated-proteins are the basis of maintaining BBB structure and functioning [18,19]. Wang Y et al. demonstrated that Rhubarb attenuates BBB disruption via increased ZO-1 expression in a rat model of ICH.The level of TJ associated-proteins ZO-1 are closely associated with the degree of BBB damage, and are indicators of BBB destruction [20]. Chu H et al. suggested that Erythropoietin protects against hemorrhagic BBB disruption through the effects of aquaporin-4.The data revealed that Erythropoietin protects BBB from disruption after ICH, and that the main targets are the TJ proteins occludin and ZO-1 [21]. However, little information is known about the changes of occludin and ZO-1 expressions in perihemotomal brain tissues after MIS for ICH. Angiogenic cerebral edema induced by BBB destruction is associated with the clinical prognosis in patients of ICH [22,23].
Rosiglitazone (RSG), an agonist of peroxisome proliferator-activated receptor-gamma (PPARγ), has shown neuroprotective effects in patients with ischemic stroke and Alzheimer’s disease. It has reported that RSG could significantly reduce brain tissue loss, ameliorated white matter injury, and improved sensorimotor and cognitive functions and increased the numbers of newly generated mature oligodendrocytes after middle cerebral artery occlusion [24]. RSG reversed depressive behaviors in mice, as indicated by the forced swimming test and open field test. RSG was also found to inhibit the inflammatory response, decrease corticosterone levels, promote astrocyte proliferation and neuronal axon plasticity in the prefrontal cortex of mice, and exerts an antidepressive effect in unpredictable chronic mildstress- induced depressive mice by maintaining essential neuron autophagy and inhibiting excessive astrocytic apoptosis [25]. Several studies have shown the beneficial role of RSG in the treatment of ICH [10,26]. RSG promoted hematoma resolution, decreased neuronal damage, and improved functional recovery in a mouse ICH model, believing that RSG may activate microglia/macrophages by PPARγ to promote hematoma resolution, which may be a therapeutic target in ICH treatment [26]. Our previous study has revealed that performing MIS to evacuate the hematoma following ICH by RSG infusion therapy decreased perihematomal matrix metalloproteinase-9 (MMP-9) expression, BBB permeability and brain edema in a rabbit ICH model [27]. TJ proteins such as occludin and ZO-1 et al. could be considered the potential biomarkers reflecting the integrity of the BBB in ICH [28]. However, there is less investigations regarding the effect of the combined use both MIS for hematoma evacuation and RSG infusion for the treatment of ICH, as well as the effect on perihematomal occludin and ZO-1 levels and BBB permeability.
The present study was designed to observe the effect of the combined use both MIS for hematoma evacuation and RSG infusion on perihematomal secondary brain injury in a rabbit model of ICH. The aim of this research was to explore the new therapeutic target for ICH.
Methods and Materials
▪Materials
▪Main reagents and drugs
The following reagents and drugs were used in this study: TRIzol reagent (Tiangeng Biochemical technology Co. Ltd., Beijing, China), chloroform, isopropyl alcohol, 100% ethanol, 75% ethanol, RNase Free H2O, 75% DEPC, formamide (molecular formula:HCONH2, Chongqing Chuanjiang Chemical Reagent Factory, Chongqing, China), urethane (molecular formula:C3H7NO2, Wuxi Yangshan Biochemical, Wuxi, China), Evans Blue (EB, Beijing Hengye Zhongyuan Chemical Co. Ltd., Beijing, China), urokinase (Tianjin Biochemical Co. Ltd., Tianjin, China), Rsiglitazone (sigma, California, USA), 4% paraformaldehyde (Wuhan Boster Biological Technology Co. Ltd., Wuhan, China), PrimeScript™ RT reagent Kit with gDNA Eraser (TaKaRa Bio, Japan), Oligo Synthesis (Invitrogen, California, USA), DL2,000 DNA Marker (TaKaRa Bio, Japan), SYBR® Premix Ex Taq™ II (Tli RNaseH Plus), ROX plus (TaKaRa Bio, Japan).
▪Main instruments
In this study, the following instruments were used: a ZH-Lanxing B-Type rabbit stereotaxic apparatus (Huaibei Zhenghua Biological Instrument & Equipment Co. Huaibei, Anhui, China), electronic scales (Sartorius, Hamburg, Germany), a Rainbow Type-722 grating spectrophotometer (Shandong Gaomi Rainbow Analytical instrument, Gaomi, Shandong, China), a 5415R high-speed centrifuge (Heraeus Company, Hanau, Germany), a digital display thermostat water bath HH-2 (Guohua Electric Appliance, Xi’an, China), a desktop general centrifuge (TGL-16B, Shanghai Anting Scientific Instrument Factory, Shanghai, China), a -80℃ freezer (Forman Scientific Company, Shanghai, China), a computed tomography (CT) provided by the Guizhou Medical University, a high performance liquid chromatography machine (HP-1100: Agilent Technologies, Palo Alto, CA, USA), a G1315 A diode-array detector (DAD, Agilent Technologies, USA), , an Agilent 1313A automatic sampler (Agilent Technologies, USA), a column oven (Agilent Technologies, USA) and scales (Beijing Gangdong Hengye Instrument, Beijing, China), fluorescence quantitative PCR instrument (ABI 7500 Fast, Guangzhou, China), polymerase chain reaction (PCR) amplification instrument (BBI, Markham, Canada), SW-CJ- 1D clean bench (Jiangsu Sue clean the equipment factory, Nanjing, China), DK-8 D type electrical thermostatic sink (Shanghai Senxin Experiment Instrument Co. Ltd., Shanghai, China), YXJ- 2 centrifuge (Hunan Instrument Centrifuge Instrument Co. Ltd., Changsha, China), liquid removing device (range 100~1000, 20~200, 0.5~10 ml) (BBI, Canada), and Primer design software: Primer Premier 5.0.
Methods
▪ Experiment grouping
This study was approved by the Animal Care and Use Committee of Guizhou Medical University. A total of 50 male rabbits (weight 2.5-3.5 kg) were provided by the Animal Center of Guizhou Medical University. The rabbits were randomly divided into a sham group (Sham group, 10 rabbits), a hemorrhage model group (HM group,10 rabbits), a minimally invasive surgery group (MIS group, 10 rabbits), a RSG medication group(RSG group, 10 rabbits)and a MIS combined with RSG group (MIS+RSG group, 10 rabbits). An ICH model was established in all rabbits except for those in the Sham group. All rabbits were sacrificed on day 7 after ICH models were performed successfully.
▪ ICH model preparation
The methods used in this study were same to those in our previously published studies [29,30]. Briefly, the rabbits were anesthetized by injecting 20% urethane (2ml/kg) into the marginal ear vein. They were then fastened to a stereotaxic apparatus, and the skin in the operative area was disinfected with povidone iodine. The skin was incised 3cm to expose the bregma and lambdoid. The head was adjusted to make bregma 1.5 mm higher than the lambdoid suture. The bregma cross-suture junction as a base point, taking 6 mm left along coronal plane and 1 mm parallel to sagittal suture as a puncture point. The skull of the rabbit was drilled with a dental drill (1 mm diameter) and 0.5 ml of autologous arterial blood was taken by an insulin syringe from the central ear artery. The syringe was then connected to a size 7 needle with flat tip. The size 7 needle was then quickly inserted vertically into the skull to a depth of 12 mm, and the 0.3 ml (similar to basal ganglia hematoma 30ml in humans) autologous arterial blood was slowly injected into the basal ganglia. The injection lasted at least 3 minutes.
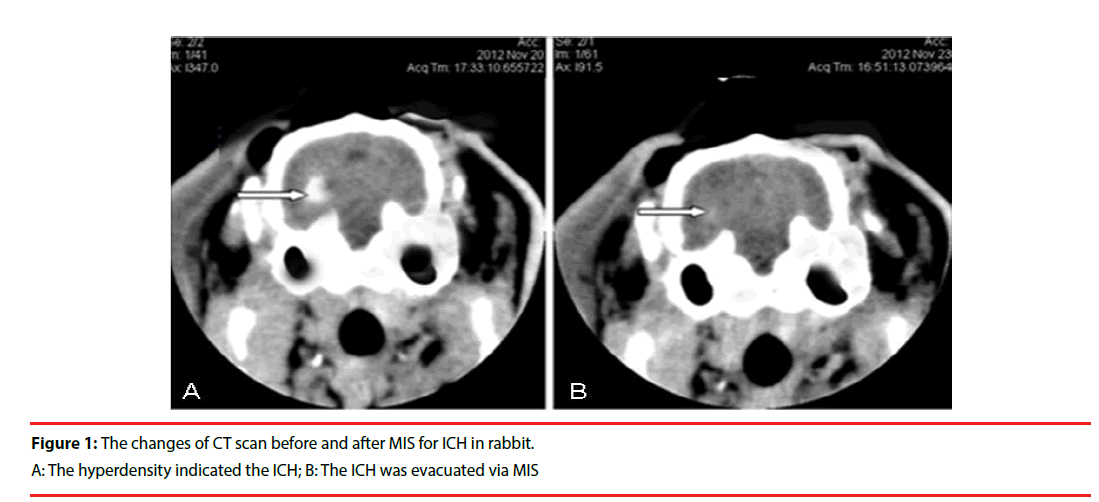
The needle was retained for 8 minutes after blood injection and the needle was then slowly pulled out. The drill hole was sealed with bone wax to prevent pneumocephalus. A CT scan was performed 3 hours later. High-density in the basal ganglia region, with no shadow in the lateral ventricle, confirmed that the ICH model had been successfully established (Figure 1).
The rabbits were sent back to the animal room and housed under a normal condition after successful ICH induction had been confirmed by CT scan. All animals recovered from anesthesia within 5 hours after intravenous injection of 20% urethane. The total anesthesia time was 3 to 5 hours. The exclusion criteria included visualization of backflow along the needle track, blood in the ventricle, and death of the rabbit.
▪ Evaluation of the ICH volume
A CT scan was administrated 3 hours before the rabbits were sacrificed to demonstrate the efficacy of the surgical procedures used for ICH evacuation. Using the Tada formula [π/6× length (cm) ×width (cm) ×height (cm)] to reckoned the hematoma volume before MIS and after operation. Histological sectioning was used to determine the hematoma volume after the rabbits were sacrificed.
▪Minimally invasive surgery for ICH evacuation
The MIS procedures were performed to evacuate the ICH at 6 hours after the ICH model was prepared successfully. The rabbits were anesthetized again and placed in the stereotaxic apparatus. Using the same drill hole, a size 7 needle was inserted into the hematoma. The 0.1ml (5000 U) of urokinase (urokinase 100000 U dissolved in 2ml 0.9% sodium chloride solution,0.1ml is equal 5000 U) was injected into the hematoma region. The needle was kept in place for 1hr., followed by slow aspiration while withdrawing the needle. The drill hole was sealed with bone wax to prevent pneumocephalus. The skin was disinfected and sutured. The rabbits were then sent back to the feeding room for 7 days. In the Sham group, we imitated the procedures of ICH, injected 0.3 ml 0.9% sodium chloride solution into the puncture region and then injected 0.1 ml 0.9% sodium chloride solution into the same area again after 6 hours. In the HM group and RSG group, just received a sham minimally invasive procedures at 6 hours after the ICH model was prepared successfully. The HM group received 0.1 ml 0.9% isodium chloride solution infused into the hematoma after 6 hours. The RSG group received the RSG solution (0.5 mg dissolved in 0.1ml 0.9% sodium chloride solution) into the hematoma region. In the MIS+RSG group, the identical surgical procedures were performed to evacuate the blood clot, followed by infusion of the same amount of RSG solution into the hematoma area immediately. All rabbits were sacrificed on day 7 after the ICH models were performed successfully. A repeated CT scan was performed before the animals were sacrificed to demonstrate the efficacy of the MIS.
▪Medical treatment of the rabbits
The rabbits in each group received an intramuscular injection of penicillin (400,000 U) to prevent infection, and they were fed as usual until they were sacrificed. No other medical treatment was performed.
▪ Brain tissue preparation
The rabbits were anesthetized by injection of 20% urethane (2ml/kg). The brain was extracted and placed on ice. Using the needle track as the center to prepare a coronal section and a sagittal section, the brain tissue adjacent hematoma was sliced and divided into four parts: anterior-inner, anterior-outside, posterior-inner, and posterior-outside. A total of 5 mm of brain tissue surrounding the hematoma was collected from each part mentioned previously. The anterior-inner part and the anterior-outside part were used for measure the protein expression of occludin, ZO-1 via Real-time PCR, whereas the posterior-inner part and posterior-outside parts were used for testing the BBB permeability.
▪ Real-time PCR for occludin, ZO-1 mRNA detection
Brain samples were extracted from the anterior-inner part and the anterior-outside part of the hematoma. A total of 5 mm of brain tissue surrounding the hematoma was collected from each part mentioned previously. Using electronic balance weighed the brain samples (approximately 30 mg, with an accuracy of 0.1 mg). Then the brain samples were pulverized, and the total RNA was isolated using Trizol reagent. cDNA was generated from total RNA by PrimeScript™ RT reagent Kit with gDNA Eraser. The following primers for occludin, ZO-1, β-actin, and the PCR protocol were used, β-actin-F: 5’-GGAGATTACTGCCCTGGCTCCTA-3’ and β-actin-R: 5’-GACTCATCGTACTCCTGCTTGCTG-3’ (Tm83.34℃,150bp), Occludin-F: 5’-TGAGCAGCAGCAGTAACTTTGAG-3’, and Occludin-R: 5’-ATGGGCGGATACTCCCTGAT-3’ (Tm75.29℃,166bp). ZO-1-F: 5’-GTACGCTCAAGTAGGACAACCAG-3’, and ZO-1-R: 5’-TCCGACATCATTTCCACCAG-3’ (Tm84.42℃, 169bp). Multi-target PCR was performed by co-amplifying β-actin as an internal standard. The reaction component included 10 μl of SybrGreen qPCR Master Mix, 0.5 μl of primer F, 0.5 μl of primer R, 7μl of ddH2O, and 2μl of cDNA. The reaction was performed at 95℃ for 2 minutes per cycle. Each of the 45 cycles was 95℃ for 10 seconds, 60℃ (β-actin) and 60℃ (occludin, ZO-1) for 30 seconds, and 68℃ for 40 seconds. The △CT value was used to determine the relative quantity of occludin mRNA and ZO-1 mRNA. The △CT value reflected the difference between the value of the sample mRNA expression and the β-actin mRNA expression. The greater the △CT value, the lesser the sample of mRNA.
▪ BBB permeability measurement
Experimental methods: EB was used as a tracer to measure the BBB permeability. Two hours before experiment, 2% EB (2ml/kg) solution was injected into the ear vein. The brain tissue was quickly removed 2hrs after the EB injection. The perihematomal brain tissue was removed and weighed on an electronic scale (with an accuracy of 0.1 mg), then placed into a test tube with 4ml of formamide. The tube was capped and placed into a 54℃ constant-temperature water bath for 24hrs to extract the EB from the brain tissues. The tube was then centrifuged at 2,400rpm for 5 minutes. A spectrophotometer (λ=632 nm) was then used to measure the absorbance of the supernatant and suck it out by a straw for placement into a quartz cuvette. An ultraviolet spectrophotometer was used to compare colors. The light filter had a wavelength of 632 nm. The absorbency was measured, and formamide was used as a blank control.
Setup of the standard curve: EB (4 mg) was placed into a volumetric flask and weighed (with an accuracy of 0.1 mg). A total of 100ml saline was added and stirred meanwhile. 0.3 ml was removed from this solution and placed in 5.7ml of formamide to make the standard buffer solution. A total of 3 ml of this solution was diluted serially in tubes, each of which contained 3 ml of formamide. The amount of EB in each of the seven tubes was 8μg/ml, 4μg/ml, 2μg/ ml, 1μg/ml, 0.5μg/ml, 0.25μg/ml, 0.125μg/ml. The tubes were capped and placed into a 54℃ water bath for 24 hours. The mentioned method above used to measure absorbance was then used. Linear regression equations between the absorbency and EB content were then created: y= 0.0053x+0.0608 (R=0.9833). The formamide method was used to measure the EB content in the brain tissue to judge the severity of BBB damage. The EB content in brain tissue (μg/g wet brain) = B×formamide (ml)/ wet weight (g), where B refers to the sample EB content (μg/ml) from the linear regression equation according to a standard curve.
▪ Statistical analysis
All data were analyzed using SPSS 19.0. Basic data are expressed as the mean±standard deviation (X ±SD). ANOVA was used for comparisons among groups. A repeated measures ANOVA was used for comparisons across the entire time series. When a difference was detected by ANOVA, an LSD test was used for comparisons between pairs of groups. A p value of less than 0.05 was considered statistically significant. Statistical analysis was performed in consultation with the Department of Biostatistics of Guizhou Medical University.
Results
▪ ICH model preparation
Following the infusion of blood into the basal ganglia, the rabbits manifested with contralateral hemiplegia and were unable to walk or crawl. Brain CTs showed hyperdensity in the basal ganglia (Figure 1), which demonstrated that the ICH model in this study was successful. The exactly ICH volume before management should be around 0.3ml (similar to basal ganglia hematoma 30ml in humans). Using the Tada formula [π/6×length (cm) ×width (cm) × height (cm)] to reckoned the hematoma volume before MIS and after operation. One rabbit died due to overdose of anesthetic agents in Sham group. One rabbit died of intracranial infection in MIS group. Two rabbits were unsuccessful in RSG group. Two rabbits failed to the ICH model in HM group, and two rabbits died with unclear reasons in MIS+RSG group. These 8 rabbits were excluded from the study. Forty two rabbits were included in the experiments finally. There were 9 rabbits in the Sham group, 8 in the HM group, 9 in the MIS group, 8 in the RSG group, and 8 in the MIS+RSG group. All of the rabbits that tolerated surgery and displayed successful ICH, they survived until the experiment was terminated.
▪ Changes in perihematomal occludin mRNA and ZO-1mRNA levels
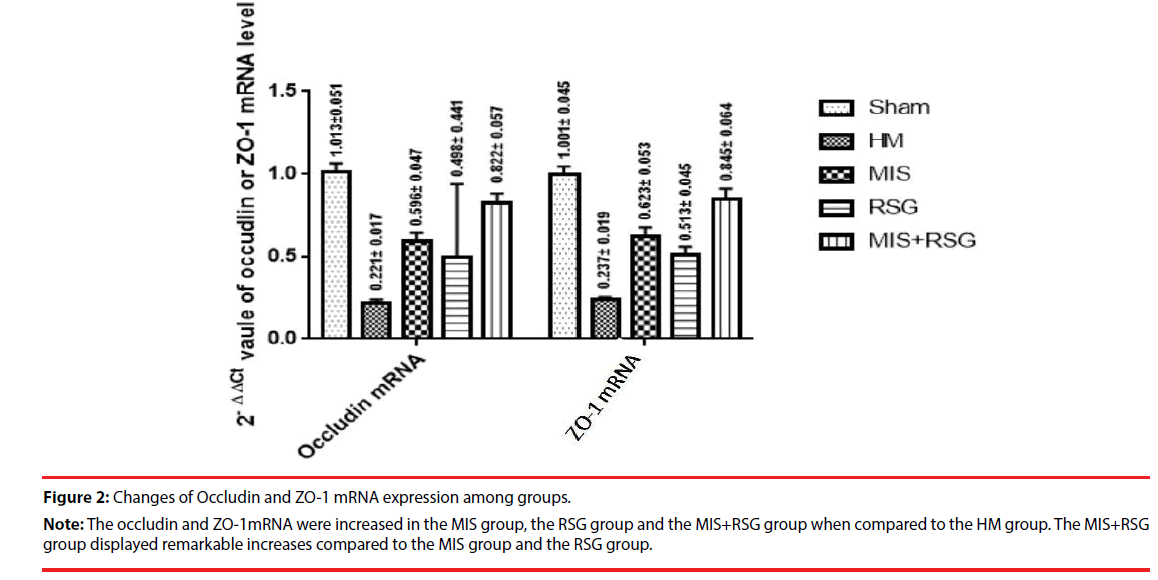
The occludin and ZO-1mRNA significantly decreased in each group compared to the Sham group, which suggested that BBB was severely damaged after ICH. The occludin and ZO-1mRNA were increased in the MIS group, the RSG group and the MIS+RSG group when compared to the HM group (the F value was 443.924, 381.929 respectively, p<0.05). The MIS group was more than the RSG group. The MIS+RSG group displayed remarkable results compared to the MIS group and the RSG group. These results suggested that performing the MIS procedure alone to evacuate the hematoma and using RSG medication alone increased the expression of TJ protein occludin and ZO-1. The MIS was superior to the RSG therapy alone. The MIS+RSG procedure was more effective to treatment the BBB damage after ICH (Figure 2).
▪ Changes in BBB permeability
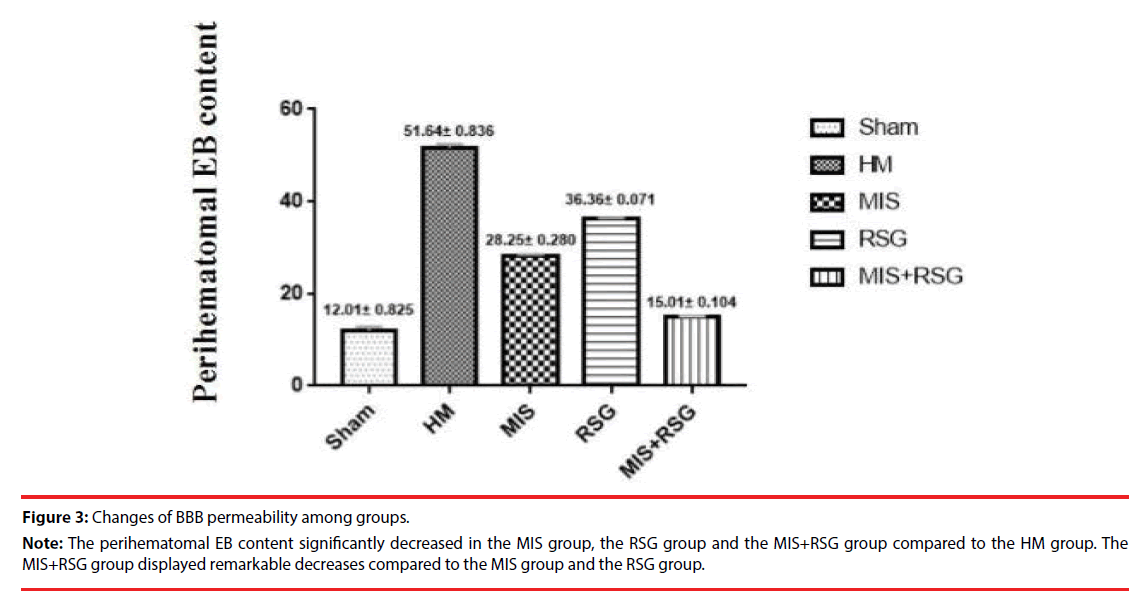
The perihematomal EB content significantly increased in each group compared to the Sham group. The perihematomal EB content were increased in the HM group when compared to the other groups, which indicated that the ICH disrupted BBB permeability. The perihematomal EB content significantly decreased in the RSG group, the MIS group and the MIS+RSG group compared to the HM group (the F value was 2237.516, p<0.05). The MIS+RSG group displayed remarkable results compared to the MIS group and the RSG group. These results suggested that performing the MIS procedure alone to evacuate the intracerebral hematoma and using RSG medication alone decreased BBB disruption, the MIS+RSG procedure was more effective to reduce BBB permeability (Figure 3).
Discussions
ICH is a public-health problem worldwide and is associated with a poor prognosis that is characterized by a high mortality rate and severe neurological dysfunction [31]. In recent years, the minimally invasive surgery has been emerging as an alternative for craniotomy due to its improved survival rate and reduced complication rate [32]. Several clinical and experimental studies have demonstrated minimally invasive techniques to be effective for the treatment of ICH [29,30,32]. However, although MIS can alleviate the hematoma to a large extent by reducing its physical size and the release of neurotoxic substances, it cannot remove the erythrocytes and cytotoxic substances that extravasate into the brain adjacent to the hematoma. Therefore, the role of MIS for reducing secondary brain damage is limited.
The secondary brain damage mainly includes brain edema, BBB destruction and neuron death, may occur in the perihematomal region after ICH. Increasing evidences show that inflammation is the important factor for ICH-induced secondary brain damage. Hemoglobin, heme, and iron are released after red blood cell lysis, detrimental mediators are released by the activated microglia/macrophages, which aggravates ICH-induced inflammatory injury [33]. As a result, performing the MIS to remove the ICH followed by medications to prevent secondary brain damages may be another optimal choice.
Our previous studies have illustrated that MIS for ICH alleviation could reduce the permeability of BBB, but the secondary brain damage remained serious [34,35]. BBB disruption is a key pathophysiological process of ICH [15]. The expressions of occludin and ZO-1 are closely associated with the degree of BBB damage, and are indicator of BBB destruction [20]. Angiogenic cerebral edema induced by BBB disruption is associated with the clinical prognosis in ICH patients [21,22].
RSG is an agonist of PPARγ which may represent a potential treatment strategy for the ICH patients. Using RSG could down regulate MMP-9 expression by upregulating PPARγ expression [36]. It is reasonable to hypothesize that performing MIS for hematoma evacuation following ICH with infusion of RSG into the hematoma region could be more effective for treating ICH-induced secondary brain injury. Our present study supported this hypothesis [27].
The rabbits received minimally invasive procedures at 6 hours after the ICH model was established successfully. That is to say, the time interval between inducement of ICH and evacuation of hematoma is 6hrs. The result came from our previously published studies [27,29,30]. The pathophysiological time window of MIS for hematoma evacuation might be 6-12 hours after hemorrhage [35]. RSG was infused into the hematoma regions at 6hr after the ICH model was established in RSG group, and infused into the hematoma regions after the ICH was removed immediately in MIS+RSG group.
Attempts to evaluate the role of MIS combined with urokinase infusion therapy began with Sun H’s trial in 2010, which showed that the MIS combined with urokinase infusion therapy could reduce the rate of rebleeding after surgery and fatality of patients with basal ganglion hemorrhage by 90 days. It was suggested as a safe and practical technique for the treatment of ICH [8]. Tan Q et al. reported that in comparison with tissue-type plasminogen activator, urokinase-type plasminogen activator (uPA) better ameliorated brain edema and promoted an improved outcome after ICH, uPA therapy more effectively upregulated BBB tight junction protein expression [36].
The formation of blood coagulation after ICH includes three states: liquid, solid and semisolid. Liquid and semisolid hemorrhage was 30-50% of total hematoma volume [36]. In our study, although MIS can effectively reduce liquid and semisolid blood clot,only use of urokinase infusion therapy can dissolve solid blood clot and completely remove the whole blood clot. In addition, clot removal using MIS combing with the infusion of urokinase as soon as possible can attenuate the BBB disruption via clean out the erythrocytes and cytotoxic substances.
In the HM group, the hematoma-occupying effects persisted and the neurotoxic substances extravasated into the perihematomal brain, which manifested as the decreased of the occludin and ZO-1 expressions and severe BBB disruption compared to the Sham group.
In the MIS group, however, the occupying effects of the hematoma were removed, and the levels of neurotoxic substances, such as MMP-9, were reduced. As a result, the levels of occludin and ZO-1 in the MIS group were significantly increased compared with the HM group. Accordingly, the BBB permeability was decreased significantly. The RSG group showed a great increase in occludin and ZO-1 levels and a decrease in EB content, suggesting that RSG used alone reduced secondary brain damage following ICH. Our previously studies have demonstrated that performing MIS to evacuate the ICH reduced MMP-9 levels and secondary brain damage [34,35]. RSG represents a potential target for treatment strategies for ICH [10]. MIS procedures and the RSG used alone reduced BBB disruption after ICH, but the effect was limited.
In the MIS+RSG group, the expressions of occludin and ZO-1 mRNA were increased and the EB content decreased significantly compared to the other groups, showing that following the MIS procedure with RSG could be more efficacious for reducing secondary brain damage. In current study, we achieved favorable results by combining MIS with the infusion of RSG. This strategy was more beneficial than the current method used for the clinical treatment of ICH. In our study, we used RSG solution (0.5mg dissolved in 0.1ml 0.9% sodium chloride solution) into the hematoma region and achieved favorable results on reducing BBB permeability. It would be better to do the doses response effects of RSG on BBB is our future tasks. More detailed study is required to demonstrate the different doses (such as 0.4mg, 0.3mg, 0.2mg, 0.1mg and so on) of using RSG to reduce BBB permeability. In future studies, the effect of RSG on BBB permeability should be confirmed using the PPARγ selective antagonist GW9662.
In this study, the ICH model was established by intracerebral injection of autologous blood in rabbits, and the effects of different treatments on the expression of Occludin and ZO-1 in the brain tissue around the hematoma were observed systematically, some significant results were obtained. However, there are some limitations in our research. Firstly, the number of samples is small, to indicate that MIS combined with RSG therapy has a good effect on secondary brain injury after ICH, an experiment with large samples are required.. Secondly, it would be better to do the doses response effects of RSG on BBB. In future studies, we will focus on the concentration of RSG perfusion. To explore the possible mechanism of the BBB destruction after ICH, we are going to conduct a more detailed study in the near future. Our goals are expected to provide the new strategy for ICH treatment. To reduce the secondary brain damage after ICH is a challenge task of the translational research.
Acknowledgments
We are grateful for the technical help with RTPCR provided by The Guizhou Key Laboratory of Molecular Biology. We are also indebted to the Medical Imaging Department of the Affiliated Wudang Hospital of Guizhou Medical University for the preparation of the ICH model. We thank Professor Qian Tang from the Department of Biostatistics of Guizhou Medical University for the statistical analysis. We also wish to thank all the involved physicians for their hard work during the study.
Source of Funding
This work was supported by the Natural Science Foundation of China (Grants no: 81460185, 81471174 and 81520108011) and the Project of Guizhou Provincial Talent Team for Science and Technology Innovation [(2014) 2040] and the Projects of Guiyang Science and Technology Foundations,grants no: [20161001] 51 and [2017] 5-1. The Funding body did not take part in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
References
- Aguilar MI, Freeman WD. Spontaneous intracerebral hemorrhage. Semin. Neurol 30(5), 555-564 (2010).
- Liu M, Wu B, Wang WZ, et a1. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol 6(5), 456-464 (2007).
- Broderick J, Connolly S, Feldmann E, et a1. Guidelines for the management of spontaneous intracerebral hemorrhagein adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke 38(6), 2001-2023 (2007).
- Adeoye O, Broderick JP. Advances in the management of intracerebral hemorrhage. Nat. Rev. Neurol 6, 593-601 (2010).
- Rincon F, Mayer SA. Intracerebral hemorrhage: getting ready for effective treatments. Curr. Opin. Neurol 23(1), 59-64 (2010).
- Wang WZ, Jiang B, Liu HM, et al. Minimally invasive craniopuncture therapy vs. conservative treatment for spontaneous intracerebral hemorrhage: results from a randomized clinical trial in China. Int. J. Stroke 4, 11-6 (2009).
- Zhou H, Zhang Y, Liu L, et al. A prospective controlled study: minimally invasive stereotactic puncture therapy versus conventional craniotomy in the treatment of acute intracerebral hemorrhage. BMC Neurol 11, 76 (2011).
- Sun H, Liu H, Li D, et al. An effective treatment for cerebral hemorrhage: minimally invasive craniopuncture combined with urokinase infusion therapy. Neurol Res 32(4), 371-377 (2010).
- Zhou XY, Chen JJ, Li Q, et al. Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage a meta-analysis of randomized controlled trials. Stroke 43, 2923-2930 (2013).
- Zhao X, Grotta J, Gonzales N, et al. Hematoma resolution as a therapeutic target: the role of microglia/macrophages. Stroke 40, S92-S94 (2009).
- Moxon-Emrc I, Schliehter LC. Neutrophil deplction reduces blood-brain barrier breakdown, axon injury, and inflammation after intracerebral hemorrhage. J. Neuropathol. Exp. Neurol 70(3), 218-235 (2011).
- Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog. Neurobiol 92(4), 463-477 (2010).
- Loftspring MC, Johnson HL, Feng R, et al. Unconjugated bilirubin contributes to early inflammation and edema after intracerebral hemorrhage. J. Cereb. Blood Flow Metab 31(4), 1133-1142 (2011).
- Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol 11, 720-731 (2012).
- Keep RF, Xiang J, Ennis SR, et al. Blood-brain barrier function in intracerebral hemorrhage. Acta Neurochir. Suppl 105, 73-77 (2008).
- Mariano C, Sasaki H, Brites D, et al. A look at tricellulin and its role in tight junction formation and maintenance. Eur. J. Cell Biol 90(10), 787-796 (2011).
- Ichikawa-Tomikawa N, Sugimoto K, Satohisa S, et al. Possible involvement of tight junctions, extracellular matrix and nuclear receptors in epithelial differentiation. J. Biomed. Biotechnol 2011, 253048 (2011).
- Liebner S, Czupalla CJ, Wolburg H. Current concepts of blood-brain barrier development. Int. J. Dev. Biol 55(5), 467-476 (2011).
- Tajes M, Ramos-Fernández E, Weng-Jiang X, et a1. The blood-brain barrier: structure, function and therapeutic approaches to cross it. Mol. Membr. Biol 31(5), 152-167 (2014).
- Wang Y, Peng F, Xie G, et al. Rhubarb attenuates blood-brain barrier disruption via increased zonula occludens-1 expression in a rat model of intracerebral hemorrhage. Exp Ther Med 12(1), 250-256 (2016).
- Chu H, Ding H, Tang Y, et al. Erythropoietin protects against hemorrhagic blood-brain barrier disruption through the effects of aquaporin-4. Lab Invest 94(9), 1042-1053 (2014).
- Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol 11(8), 720-731 (2012).
- Xu H, Li R, Duan Y, et al. Quantitative assessment on blood-brain barrier permeability of acute spontaneous intracerebral hemorrhage in basal ganglia: a CT perfusion study. Neuroradiology 59(7), 677-684 (2017).
- Han L, Cai W, Mao L, et al. Rosiglitazone promotes white matter integrity and long-term functional recovery after focal cerebral ischemia. Stroke 46(9), 2628-2636 (2015).
- Zhao Z, Zhang L, Guo XD, et al. Rosiglitazone exerts an anti-depressive effect in unpredictable chronic mild-stress-induced depressive mice by maintaining essential neuron autophagy and inhibiting excessive astrocytic apoptosis. Front. Mol. Neurosci 10, 293 (2017).
- Zhao X, Sun G, Zhang J, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann. Neurol 61(4), 352-362 (2007).
- Wu G, Wu J, Jiao Y, et al. Rosiglitazone infusion therapy following minimally invasive surgery for intracerebral hemorrhage evacuation decreases matrix metalloproteinase-9 and blood-brain barrier disruption in rabbits. BMC Neurol 15, 37 (2015).
- Jiao X, He P, Li Y, et al. The role of circulating tight junction proteins in evaluating blood brain barrier disruption following intracranial hemorrhage. Dis. Markers 2015:860120 (2015).
- Wu G, Li C, Wang L, et al. Minimally invasive procedures for evacuation of intracerebral hemorrhage reduces perihematomal glutamate content, blood-brain barrier permeability and brain edema in rabbits. Neurocrit. Care 14, 118-126 (2011).
- Wu G, Wang L, Hong Z, et al. Effects of minimally invasive procedures for removal of intracranial hematoma on matrix metalloproteinase expression and blood-brain barrier permeability in perihematomal brain tissues. Neurol Res 33, 300-306 (2011).
- Adeoye O, Broderick JP. Advances in the management of intracerebral hemorrhage. Nat Rev 6, 593-601 (2010).
- Yang Z, Hong B, Jia Z, et al. Treatment of supratentorial spontaneous intracerebral hemorrhage using image-guided minimally invasive surgery: initial experiences of a flat detector CT-based puncture planning and navigation system in the angiographic suite. AJNR Am. J. Neuroradiol 35(11), 2170-2105 (2014).
- Zhou Y, Wang Y, Wang J, et al. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog. Neurobiol 115, 25-44 (2013).
- Wu G, Wang L, Hong Z, et al. Effects of minimally invasive procedures for removal of intracranial hematoma on matrix metalloproteinase expression and blood-brain barrier permeability in perihematomal brain tissues. Neurol. Res 33, 300-306 (2011).
- Wu G, Sun S, Long X, et al. Early stage minimally invasive procedures reduce perihematomal MMP-9 and blood brain barrier disruption in a rabbit model of intracerebral hemorrhage. Neurol Res 35(6), 649-658 (2013).
- Tan Q, Chen Q, Niu Y, et al. Urokinase, a promising candidate for fibrinolytic therapy for intracerebral hemorrhage. J. Neurosurg 126(2), 548-557 (2017).