Research Article - (2019) Volume 9, Issue 4
Microstructural Abnormalities of the Secondary Motor Area Coordinating Sensory and Motor Functions in Patients with Complex Regional Pain Syndrome
- Corresponding Author:
- Do-Hyung Kang
Emotional Information and Communication Technology Association
508, Samseongro, Gangnam-gu, Seoul, Republic of Korea
Tel: +82-10-2642-2072
Fax: +82-50-7083-6323
Abstract
ABSTRACT
Objective Complex regional pain syndrome (CRPS), which is characterized by persistent pain with related sensorimotor symptoms, involves structural and functional changes in the brain cortices. Further study on the microstructure of the brain using diffusion kurtosis imaging (DKI) is needed in patients with CRPS. This cross-sectional study investigated the neural underpinnings of the microstructure of the brains of patients with CRPS.
Methods DKI was performed on 25 patients with CRPS and 23 healthy controls. The mean kurtosis was determined by voxel-based analyses.
Results
Compared with healthy controls, patients had a significantly higher mean kurtosis in the bilateral supplementary motor area (SMA), right dorsal premotor area, left precuneus, and cerebellar vermis. Higher pain intensity in patients according to the McGill Pain Questionnaire
was associated with a lower mean kurtosis of the left SMA (r=–0.469, P=0.024). The significant association between the mean kurtosis of the left SMA and precuneus observed in healthy controls was not observed in patients.
Conclusion
Our findings suggest that the wide variety of symptoms encompassing the sensory and motor systems of patients with CRPS are associated with microstructural impairment of the secondary motor area, which receives sensory information and projects motor information.
This measure could potentially help clinicians to understand microstructural abnormalities of the secondary motor area coordinating sensory and motor functions.
Keywords
Complex Regional Pain Syndrome (CRPS), Diffusion Kurtosis Imaging (DKI), Microstructural impairment, Secondary motor area
Abbreviations
CRPS: Complex Regional Pain Syndrome; DKI: Diffusion Kurtosis Imaging; SMA: Supplementary Motor Area; CNS: Central Nervous System; MRI: Magnetic Resonance Imaging; DTI: Diffusion Tensor Imaging; MPQ: McGill Pain Questionnaire; FA: Fractional Anisotropy.
Introduction
There are two main types, complex regional pain syndrome (CRPS) I with no definite nerve lesion and CRPS II with an identified nerve lesion. CRPS type I which is formerly known as reflex sympathetic dystrophy (RSD) is a disorder of a portion of the body, usually starting in a limb, which manifests as extreme pain, swelling, limited range of motion, and changes to the skin [1]. Currently, the disease is understood as result of a complex interplay between altered somatosensory, motor, autonomic and inflammatory systems, and peripheral and central sensitization [2]. Complex regional pain syndrome (CRPS), which involves multi-system dysfunction and severe, chronic pain, has a complex pathogenesis that is characterized by the involvement of both the peripheral nervous system and the central nervous system (CNS) [3,4]. The clinical features of CRPS involve neurogenic inflammation, nociceptive sensitization, vasomotor dysfunction, and maladaptive neuroplasticity, such as allodynia and hyperalgesia [1,5,6]. The CNS undergoes functional and structural changes in individuals with persistent pain, and the altered brain structure and function of patients with CRPS have been studied extensively [7-9]. However, because of the limits of conventional magnetic resonance imaging (MRI) technology, far too little attention has focused on the microstructural changes associated with the pathophysiology of CRPS.
Diffusion kurtosis imaging (DKI), a relatively new diffusion technique, has been used to elucidate brain microstructural impairment in neurological and systemic diseases [10-13]. Compared with the traditional diffusion tensor imaging (DTI) technique, DKI characterizes the non-Gaussian water distribution, whereas conventional DTI characterizes the Gaussian diffusion in neural tissue [14-16]. DKI can provide more information about the microstructural abnormalities of brain tissue than traditional DTI [17,18]. Given that the water diffusion in biological tissues is non-Gaussian due to the effects of the cellular microstructure and positive kurtosis means that the distribution is more strongly peaked, a higher mean kurtosis indicates a reduction in or loss of cellular microstructures such as cell membranes and organelles [19]. For instance, an increase in the diffusion parameters in DKI is correlated with white and gray matter reduction in Alzheimer’s disease [20,21].
Continuous pain in CRPS is associated with attenuated motor cortex reactivity, suggesting that abnormal motor cortex reactivity may be linked with motor dysfunction of the affected hand in CRPS [22]. And, a bilateral disinhibition of the motor cortex was found in patients with complex regional pain syndrome [23]. Altered central sensorimotor processing and changes of brain structure in prefrontal and motor cortex were found in CRPS patients [24,25]. Substantial adaptive changes within the central nervous system may contribute to motor symptoms in CRPS [26], and the presence of pain and other CRPS symptoms may induce lasting changes in motor cortical plasticity, which could be of importance in rehabilitative strategies for the sensory motor system in CRPS I patients [27]. In addition, repetitive motor cortex stimulation can affect pain perception such as decreased pain intensities or pain relief as the analgesic efficiency [28], and is effective not only to treat pain, but also improve the sympathetic changes in CPRS [29]. Along with the supplementary motor area (SMA), the premotor cortex constitutes the secondary motor cortex [30], and this region is critical for goal-directed actions [31]. The secondary motor area receives sensory information from reciprocal connections with the sensory, parietal, and retrosplenial cortices and exerts control on actions by projecting to various motor-related subcortical regions [32]. Secondary motor cortex can be defined by a distinct set of afferent and efferent connections, suggested that a major function for secondary motor cortex is to flexibly map antecedent signals such as sensory cues to motor actions, thereby enabling adaptive choice behavior [30]. The SMA participates in the initiation or coordination of learned, voluntary movements [32]. The SMA also appears crucial in the temporal organization of movement and in the performance of motor tasks that demand the retrieval of motor memory [33].
The precuneus is part of the anterior sensorimotor sub region. As the precuneus is responsible for visuomotor learning [34], microstructural impairment of this region can play a role in the motor impairment of patients with CRPS, which is consistent with the altered integration of visual and proprioceptive inputs in the posterior parietal cortex [35]. Moreover, this sensorimotor subregion of the precuneus has strong connections with medial sensorimotor regions, including the SMA, premotor cortex, and superior parietal cortex [36]. CRPS patients had experienced physical trauma such as traffic accidents, physical injury or bad fall. The effect of traumatic experience was associated with precuneus region in altered restingstate brain function of healthy adults and PTSD patients [37,38].
We investigated whole brain including primary somatosensory area, primary motor area, secondary somatosensory area, secondary motor area, and SMA in both CRPS patients and healthy controls using DKI. This was an exploratory study. Thus, the aim of the present study was to find out microstructural features and abnormality of brain in patients with CRPS comparing with healthy controls using DKI.
Materials and Methods
Participants
Twenty-five patients with CRPS diagnosed by Budapest criteria from a board-certified anesthesiologist were recruited from the Pain Clinic. Patients were asked not to change their medications before undergoing MRI. Additionally, 25 healthy control subjects with no lifetime history of psychiatric disorders or hospitalization who were not taking any medications were recruited via Internet advertisements. However, two of the control subjects were excluded due to technical problems during DKI acquisition, and data from 23 controls were analyzed. The exclusion criteria included a history of substance abuse or dependence or neurological disease or brain injury or evidence of a medical illness that could cause psychiatric symptoms. Our study was approved by the Institutional Review Board, and written informed consent was obtained from all participants. This study was conducted in accordance with the Declaration of the World Medical Association.
Pain severity was assessed using the short-form McGill Pain Questionnaire (MPQ), including the MPQ-Present Pain Intensity scale (PPI, 0=no pain, 5=excruciating pain) [39]. The MPQ consists of 15-word descriptors of pain (11 sensory and 4 affective), which are rated on an intensity scale of 0–3. The pain scores are derived from the sum of the sensory and affective intensity ratings.
Image acquisition
All data were acquired using a 3.0-Tesla MRI scanner (Siemens Magnetom Trio, Erlangen, Germany). A spin-echo echo-planar imaging sequence was used to acquire DKI images, with six b values (0, 500, 1000, 1500, 2000, and 2500 s/mm2) and diffusion-encoding vectors along 30 nonparallel directions for each nonzero b value. Other parameters were as follows: TR/ TE=5900/109 ms; number of averages=1; slice thickness=3.5 mm; FOV=240 × 240 mm2; matrix=128 × 128; parallel imaging factor=2; number of axial slices=40. A high-resolution sagittal T1-weighted anatomical image was acquired using a three-dimensional MPRAGE sequence (TR/TE=1670/1.89 ms; flip angle=9°; FOV=240 × 240 mm2; acquisition voxel size=0.98 × 0.98 × 1.0 mm3; 208 slices).
Image processing
The DKI data were corrected for eddy current distortion and for head motion with the B0 image using the fMRIB software library (FSL) (www. fmrib.ox.ac.uk). Mean kurtosis was calculated using the Diffusional Kurtosis Estimator tool (ver. 2.6.0, Neuroimaging Informatics Tools and Resources Clearinghouse) [40]. For voxel-based analysis, a fractional anisotropy (FA) map was created from the DKI data. Each subject’s FA image was spatially registered into the standard FA template in FSL (FMRIB58_FA_1mii.gz) using affine transformation. For spatial normalization, the obtained transformation matrix was applied to the mean kurtosis map of each subject. The spatially normalized parametric maps were smoothed with a 6-mm full-width at halfmaximum (FWHM) Gaussian kernel and used for within- and between-group analyses. The statistical threshold was set at P<0.001, with a spatial extent threshold of a cluster size 50 mm3 (about four voxels in the original space).
Statistical analysis
The statistical analyses were performed using SPSS Statistics 21.0 (Chicago, IL, USA). Two-sample t- and Chi-square tests were used to compare between-group differences in demographic and clinical characteristics. Pearson’s correlation coefficients were used to investigate relationships between the mean kurtosis and pain severity, and to examine associations of mean kurtosis values between regions that showed significant group differences. The statistical analyses were twotailed, with a significance level of 0.05.
Results
Table 1 shows the demographic and clinical data of all study participants. There were no significant differences between the groups in age, gender, or handedness.
Table 1: Demographic and clinical characteristics of the study participants.
| Patients with CRPS (N=25) |
Healthy controls (N=23) |
χ2 or t | P | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Male | 12 | 48.0 | 13 | 56.5 | 0.349 | .555 |
| Handedness, right | 23 | 92.0 | 20 | 87.0 | 0.327 | .568 |
| Initial pain location | ||||||
| Upper limb | 6 | 24 | ||||
| Lower limb | 6 | 24 | ||||
| Multiple sites | 13 | 52 | ||||
| Psychiatric comorbidity | ||||||
| Depressive disorder | 11 | 44 | ||||
| Anxiety disorder | 1 | 4 | ||||
| Other mood disorders | 7 | 28 | ||||
| Mean | SD | Mean | SD | |||
| Age | 36.1 | 11.4 | 31.3 | 6.8 | 1.814 | .077 |
| McGill Pain Questionnaire | ||||||
| Present pain intensity | 3.2 | 1.3 | ||||
| Sensory | 19.4 | 9.9 | ||||
| Affect | 6.8 | 3.7 | ||||
| Duration of illness (years) | 2.8 | 3.0 | ||||
ABB: CRPS: Complex Regional Pain Syndrome
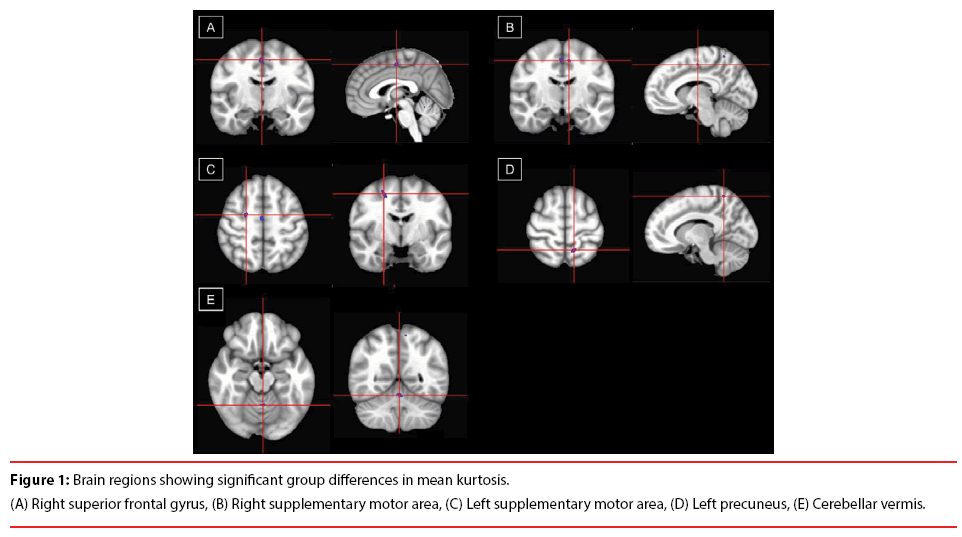
Compared with healthy controls, the CRPS patients had significantly higher mean kurtosis in the SMA bilaterally (Figure 1A and 1B), right dorsal premotor area (Figure 1C), left precuneus (Figure 1D), and cerebellar vermis (Figure 1E and Table 2). There was a negative correlation between the mean kurtosis of the left SMA and the pain severity of the patients (measured on the MPQ-PPI; r=–0.469, P=0.024), indicating that higher pain intensity was associated with lower mean kurtosis of the left SMA.
Table 2: Brain regions showing significant group differences in mean kurtosis.
| Region, Brodmann area | Talairach coordinates | Voxels, n | Mean kurtosis | ||
|---|---|---|---|---|---|
| x | y | Z | |||
| R Supplementary motor area, 6 | 2 | -13 | 55 | 158 | |
| Patients with CRPS | 0.692 ± 0.041 | ||||
| Healthy controls | 0.633 ± 0.048 | ||||
| P-value | < 0.001 | ||||
| L Supplementary motor area, 6 | -8 | -15 | 54 | 33 | |
| Patients with CRPS | 0.972 ± 0.031 | ||||
| Healthy controls | 0.925 ± 0.031 | ||||
| P-value | < 0.001 | ||||
| R Dorsal premotor area, 6 | 23 | -9 | 57 | 205 | |
| Patients with CRPS | 0.801 ± 0.041 | ||||
| Healthy controls | 0.732 ± 0.040 | ||||
| P-value | < 0.001 | ||||
| L Precuneus, 7 | -11 | -57 | 64 | 92 | |
| Patients with CRPS | 0.770 ± 0.026 | ||||
| Healthy controls | 0.714 ± 0.033 | ||||
| P-value | < 0.001 | ||||
| Cerebellar vermis | -1 | -56 | -23 | 63 | |
| Patients with CRPS | 1.002 ± 0.033 | ||||
| Healthy controls | 0.960 ± 0.045 | ||||
| P-value | < 0.001 | ||||
ABB: CRPS: Complex Regional Pain Syndrome; R: Right; L: Left
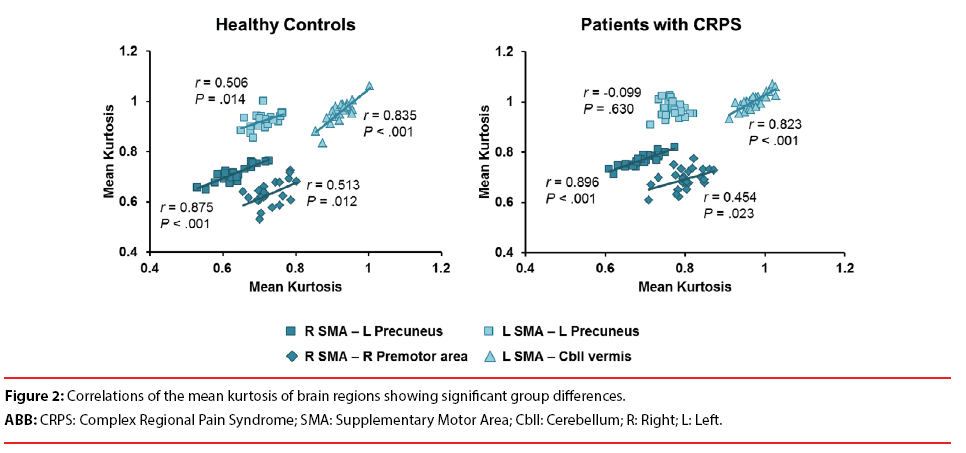
Among the means of kurtosis in the brain regions showing group differences, there were significant positive correlations in between the right SMA and the left precuneus (r=0.875, P<0.001), between right SMA and right premotor area (r=0.513, P=0.12), between the left SMA and the left precuneus (r=0.506, P=0.014) and between left SMA and cerebellar vermis (r=0.835, P<0.001) in healthy controls (Figure 2). On the other hand, there were significant positive correlations in CRPS patients between the right SMA and the left precuneus (r=0.896, P<0.001), between right SMA and right premotor area (r=0.454, P=0.023), between the left SMA and cerebellar vermis (r=0.823, P<0.001). However, the any significant correlation between the mean kurtosis of the left SMA and the left precuneus was not found (r=-0.099, P=0.630) in patients with CRPS (Figure 2).
Discussion
This is the first study to investigate the microstructure of the brain using DKI in patients with CRPS. Patients with CRPS mainly showed microstructural impairment in the secondary motor area in this study, where the sensory and motor systems meet [30]. Specifically, the mean kurtosis of the left SMA was associated with pain severity and showed a pattern of association with the other regions in patients that differed from the pattern observed in the controls. Given the mixed results regarding altered brain structure and function in patients with CRPS [41], the new DKI imaging method can add new, beneficial findings that will help to understand the pathological microstructure of the brain in CRPS.
Previously, CRPS was found to be associated with altered brain structures in the prefrontal and motor cortices [42] as well as altered function in pain-related motor systems [35]. Patients with CRPS showed significant reorganization of the central motor circuits, with increased activation of the primary and secondary (e.g., the SMA) motor areas, correlated with the extent of motor dysfunction [35]. Our results indicate that the impaired microstructure of the sensorimotor system requires reorganization of this area. Interestingly, our results also indicate that the structural impairment is located mainly in the medial aspect of the secondary motor area rather than in the sensory system. Thus, the wide variety of symptoms in CRPS may stem from microstructural changes in the secondary motor system such as SMA and premotor cortex. Supplementary motor complex and motor cortex stimulation in patients with phantom limb pain or chronic central pain significantly reduced the intensity of pain [43]. Thus, microstructural impairment in the SMA of CRPS patients could mediate dysfunction in pain processing and may affect the intensity of pain. Central sensitization from sensory pain signals [7] and dysfunctional coordination in sensory to motor pain signals related to the SMA may cause pathologically chronic pain symptoms in CRPS. Along with the SMA, the premotor cortex constitutes the secondary motor cortex [30], and this region is critical for goal-directed actions [31]. This secondary motor system is responsible for flexibly mapping antecedent signals, such as sensory cues to motor actions [30]. As neurons in these regions are associated with direct projection to the spinal cord and control of movement [44,45], dysfunction in the control of movement to the spinal cord may result from the microstructural abnormalities in these regions.
On the other hand, traumatic accident experience in CRPS patients may be associated with impairments in left precuneus. Brain of adult rats exposed to traumatic brain injury showed long-term upregulation of inflammation and suppression of cell proliferation [46]. As a result, trauma-related CNS sensitization, NMDA receptor-mediated neurotoxicity [47], neuroinflammation [6] and hyperalgesia in cerebral pain processing may contribute to neuronal cell death and gray matter reduction, which may mediate microstructural abnormalities in cortical pain processing area from sensory-motor system to pain regulation in CRPS patients.
Given that SMA was associated with coordination of learned, voluntary movements [32] and retrieval of motor memory [33], higher intensity of pain signals in SMA and dysfunctional coordination of internally voluntary pain signals in left SMA may increase pain intensity in CRPS patients. Considering that neurons in the SMA project directly to the spinal cord and may play a role in the direct control of movement [44,45], relatively less cell death of SMA with internally stronger pain signaling in CRPS patients may increase the neuronal nociceptive signaling to the spinal cord, resulting in higher pain intensity. In controls, the mean kurtosis in left precuneus showed significant associations with the mean kurtosis in left SMA. However, the association between the mean kurtosis of left precuneus and left SMA was lacking in patients with CRPS. This suggests that the microstructural covariance of these regions is decreased, which might make patients vulnerable to persistent regional pain [48,49]. The inverse association between the mean kurtosis of the left SMA and pain severity in patients can be interpreted in a similar context. In patients, the dissimilar patterns of the left and right SMAs with regard to their association with other regions suggests that the pain-related role of the left SMA might be distinct from that of the right SMA. A study on the heterogeneity of the SMA reported that right SMA activation was observed during motor and sensory tasks, whereas left SMA activation occurred during word-generation and memory tasks [50]. Therefore, the left SMA may play a role in the cognitive control of pain in CRPS.
Together with the SMA, the cerebellum plays an important role in motor control; in particular, the cerebellar vermis is included within the spinocerebellum and receives somatic sensory input from the head and proximal body via ascending spinal pathways and is a target of projections from the motor areas in the cerebral cortex [51]. The cerebellum, including the vermis, is involved in various aspects of nociception, reflecting pain perception and sensorimotor structures [52]. In addition, cerebellum was associated with physical trauma, and cerebellar vermis showed particularly relative hypermetabolism in traumatic brain injured patients [53]. Thus, we speculate that the association between impairment of the cerebellar vermis and higher mean kurtosis is related to a dysfunction in pain perception and motor control in CRPS patients.
The recent paper [54] reported that the early stage group showed reduced gray matter (GM) volume and perfusion in areas associated with spatial body perception, somatosensory cortex, and the limbic system, whereas the late stage group exhibited increased perfusion in the motor cortex but no changes in GM volume. The late stage group in the paper had suffered from chronic pain at least over 2 years with average of about 7 years of chronic pain [54]. And in our present study CRPS patients had suffered from chronic pain during average 3.8 years. Thus, CRPS patients in our study are fit to the late stage of chronic pain. In addition, our results seem to be coincide with the results of late stage group of CRPS [54], considering that late stage group of CRPS showed dysfunction in motor cortex [54], while early stage group showed dysfunction in spatial body perception, and somatosensory cortex [54]. Thus, we can infer that early stage of CRPS may be associated with afferent and sensory-related impairments in pain pathways, while late stage of CRPS may be involved in efferent and motor-related impairments in pain pathways. In particular, late stage of CRPS may be associated with the impairment in inhibitory systems of pain signals in secondary motor area which can coordinate sensory and motor functions and exert control on actions by projecting to various motor-related subcortical regions [30].
There are several limitations to this study. First, as this study was cross-sectional, further research must confirm whether the identified microstructural brain changes are the cause or the result of CRPS. Second, medications for CRPS patients may also influence brain activity and hence cortical microstructure, since most patients in this study were taking opioids, antidepressants, and/or anxiolytics. Finally, although the patients were diagnosed with CRPS, they had experienced different accidents or injuries and different parts were injured. Consequently, additional studies with more patients are needed to classify the effects of different kinds of accident and injury in more detail.
Conclusions
This study examined the differences in the brain microstructure of patients with CRPS and healthy controls using DKI. Patients with CRPS exhibited microstructural abnormalities in the secondary motor area, including the bilateral SMA, right dorsal premotor area, left precuneus, and cerebellar vermis. Because this secondary motor area receives sensory information and projects motor information, microstructural impairment in these regions could constitute the pathophysiology for the persistent experience of regional pain that is not confined to a specific nerve territory in CRPS. DKI studies should be useful and play a beneficial role in baseline and follow-up evaluations of CRPS patients.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Acknowledgments
The authors wish to thank all participants for their valuable time engaging with this research. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2018R1A2B6001806).
References
- Bussa M, Guttilla D, Lucia M, et al. Complex regional pain syndrome type I: A comprehensive review. Acta. Anaesthesiol. Scand 59(6), 685-697 (2015).
- Nickel FT, Maihöfner C. Current concepts in pathophysiology of CRPS I. Handchir. Mikrochir. Plast. Chir 42(1), 8-14(2010).
- Birklein F, O'Neill D, Schlereth T. Complex regional pain syndrome: An optimistic perspective. Neurology 84(1), 89-96 (2015).
- Bussa M, Mascaro A, Cuffaro L, et al. Adult complex regional pain syndrome type I: A Narrative Review. PM. R 9(7), 707-719 (2017).
- Marinus J, Moseley GL, Birklein F, et al. Clinical features and pathophysiology of complex regional pain syndrome. Lancet. Neurol 10(7), 637-648 (2011).
- Jeon SY, Seo S, Lee JS, et al. [11C]-(R)-PK11195 positron emission tomography in patients with complex regional pain syndrome: A pilot study. Medicine 96(1), e5735-e5735 (2017).
- Maihöfner C, Forster C, Birklein F, et al. Brain processing during mechanical hyperalgesia in complex regional pain syndrome: a functional MRI study. Pain 114(1-2), 93-103 (2005).
- Barad MJ, Ueno T, Younger J, et al. Complex regional pain syndrome is associated with structural abnormalities in pain-related regions of the human brain. J. Pain 15(2), 197-203 (2014).
- Kim JH, Choi SH, Jang JH, et al. Impaired insula functional connectivity associated with persistent pain perception in patients with complex regional pain syndrome. PLoSOne 12(7), e0180479-e0180479 (2017).
- Helpern JA, Adisetiyo V, Falangola MF, et al. Preliminary evidence of altered gray and white matter microstructural development in the frontal lobe of adolescents with attention-deficit hyperactivity disorder: a diffusional kurtosis imaging study. J. Magn. Reson. Imag 33(1), 17-23 (2011).
- Zhu J, Zhuo C, Qin W, et al. Performances of diffusion kurtosis imaging and diffusion tensor imaging in detecting white matter abnormality in schizophrenia. Neuroimage. Clin 7(1), 170-176 (2014).
- Kazumata K, Tha KK, Narita H, et al. Characteristics of diffusional kurtosis in chronic ischemia of adult moyamoya disease: comparing diffusional kurtosis and diffusion tensor imaging. Am. J. Neuroradiol 37(8), 1432-1439 (2016).
- Xie P, Qin B, Song G, et al. Microstructural abnormalities were found in brain gray matter from patients with chronic myofascial pain. Front. Neuroanat 10(1), 122 (2016).
- Lu H, Jensen JH, Ramani A, et al. Three-dimensional characterization of non-gaussian water diffusion in humans using diffusion kurtosis imaging. NMR. Biomed 19(2), 236-247 (2006).
- Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR. Biomed 23(7), 698-710 (2010).
- Gao Y, Zhang Y, Wong CS, et al. Diffusion abnormalities in temporal lobes of children with temporal lobe epilepsy: a preliminary diffusional kurtosis imaging study and comparison with diffusion tensor imaging. NMR. Biomed 25(12), 1369-1377 (2012).
- Hui ES, Cheung MM, Qi L, et al. Towards better MR characterization of neural tissues using directional diffusion kurtosis analysis. NeuroImage 42(1), 122-134 (2008).
- Jensen JH, Falangola MF, Hu C, et al. Preliminary observations of increased diffusional kurtosis in human brain following recent cerebral infarction. NMR. Biomed 24(5), 452-457 (2011).
- Steven AJ, Zhuo J, Melhem ER. Diffusion kurtosis imaging: An emerging technique for evaluating the microstructural environment of the brain. Am. J. Roentgenol 202(1), W26-W33 (2014).
- Struyfs H, Van Hecke W, Veraart J, et al. Diffusion kurtosis imaging: A possible MRI biomarker for AD diagnosis? J. Alzheimers Dis 48(4), 937-948 (2015).
- Yuan L, Sun M, Chen Y, et al. Non-Gaussian diffusion alterations on diffusion kurtosis imaging in patients with early Alzheimer’s disease. Neurosci. Lett 616(1), 11-18 (2016).
- Kirveskari E, Vartiainen NV, Gockel M, et al. Motor cortex dysfunction in complex regional pain syndrome. Clin. Neurophysiol 121(7), 1085-1091 (2010).
- Schwenkreis P, Janssen F, Rommel O, et al. Bilateral motor cortex disinhibition in complex regional pain syndrome (CRPS) type I of the hand. Neurology 61(4), 515-519 (2003).
- Juottonen K, Gockel M, Silén T, et al. Altered central sensorimotor processing in patients with complex regional pain syndrome. Pain 98(3), 315-323 (2002).
- Pleger B, Draganski B, Schwenkreis P, et al. Complex regional pain syndrome type I affects brain structure in prefrontal and motor cortex. PLoSOne 9(1), e85372 (2014).
- Maihöfner C, Baron R, DeCol R, et al. The motor system shows adaptive changes in complex regional pain syndrome. Brain 130(10), 2671-2687 (2007).
- Krause P, Förderreuther S, Straube A. TMS motor cortical brain mapping in patients with complex regional pain syndrome type I. Clin. Neurophysiol 117(1), 169-176 (2006).
- Pleger B, Janssen F, Schwenkreis P, et al. Repetitive transcranial magnetic stimulation of the motor cortex attenuates pain perception in complex regional pain syndrome type I. Neurosci. Lett 356(2), 87-90 (2004).
- Velasco F, Carrillo-Ruiz JD, Castro G, et al. Motor cortex electrical stimulation applied to patients with complex regional pain syndrome. Pain 147(1-3), 91-98 (2009).
- Barthas F, Kwan AC. Secondary motor cortex: where 'sensory' meets 'motor' in the rodent frontal cortex. Trends. Neurosci 40(3), 181-193 (2017).
- Gremel CM, Costa RM. Premotor cortex is critical for goal-directed actions. Front. Comput. Neurosci 7(1), 110 (2013).
- Thaler DE, Rolls ET, Passingham RE. Neuronal activity of the supplementary motor area (SMA) during internally and externally triggered wrist movements. Neurosci. Lett 93(2-3), 264-269 (1988).
- Tanji J. The supplementary motor area in the cerebral cortex. Neurosci. Res 19(3), 251-268 (1994).
- Kawashima R, Roland PE, O'Sullivan BT. Functional anatomy of reaching and visuomotor learning: a positron emission tomography study. Cereb. Cortex 5(2), 111-122 (1995).
- Maihöfner C, Baron R, DeCol R, et al. The motor system shows adaptive changes in complex regional pain syndrome. Brain 130(10), 2671-2687 (2007).
- Margulies DS, Vincent JL, Kelly C, et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc. Natl. Acad. Sci 106(47), 20069-20074 (2009).
- Lu S, Pan F, Gao W, et al. Neural correlates of childhood trauma with executive function in young healthy adults. Oncotarget 8(45), 79843-79853 (2017).
- Liu Y, Li L, Li B, et al. Decreased triple network connectivity in patients with recent onset post-traumatic stress disorder after a single prolonged trauma exposure. Sci. Rep 7(1), 12625 (2017).
- Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1(3), 277-299 (1975).
- Tabesh A, Jensen JH, Ardekani BA, et al. Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn. Reson. Med 65(3), 823-836 (2011).
- van Velzen GA, Rombouts SA, van Buchem MA, et al. Is the brain of complex regional pain syndrome patients truly different? Eur. J. Pain 20(10), 1622-1633 (2016).
- Pleger B, Draganski B, Schwenkreis P, et al. Complex regional pain syndrome type I affects brain structure in prefrontal and motor cortex. PLoSOne 9(1), e85372-e85372 (2014).
- Sokal P, Harat M, ZieliÃâ¦Ãâski P, et al. Motor cortex stimulation in patients with chronic central pain. Adv. Clin. Exp. Med 24(2), 289-296 (2015).
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J. Neurosci 11(3), 667-689 (1991).
- Galea MP, Darian Smith I. Multiple corticospinal neuron populations in the macaque monkey are specified by their unique cortical origins, spinal terminations, and connections. Cereb. Cortex 4(2), 166-194 (1994).
- Acosta SA, Tajiri N, Shinozuka K, et al. Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PLoSOne 8(8), e53376 (2013).
- Abushik PA, Niittykoski M, Giniatullina R, et al. The role of NMDA and mGluR5 receptors in calcium mobilization and neurotoxicity of homocysteine in trigeminal and cortical neurons and glial cells. J. Neurochem 129(2), 264-274 (2014).
- Khundrakpam BS, Lewis JD, Jeon S, et al. Exploring individual brain variability during development based on patterns of maturational coupling of cortical thickness: A longitudinal MRI study. Cereb. Cortex 29(1), 178-188 (2019).
- Chong CD, Dumkrieger GM, Schwedt TJ. Structural co-variance patterns in migraine: A cross-sectional study exploring the role of the hippocampus. Headache 57(10), 1522-1531 (2017).
- Chung GH, Han YM, Jeong SH, et al. Functional heterogeneity of the supplementary motor area. Am. J. Neuroradiol 26(7), 1819-1823 (2005).
- Coffman KA, Dum RP, Strick PL. Cerebellar vermis is a target of projections from the motor areas in the cerebral cortex. Proc. Natl. Acad. Sci 108(38), 16068-16073 (2011).
- Diano M, D'Agata F, Cauda F, et al. Cerebellar clustering and functional connectivity during pain processing. Cerebellum 15(3), 343-356 (2016).
- Lupi A, Bertagnoni G, Borghero A, et al. Relative hypermetabolism of vermis cerebelli in traumatic brain injured patients studied with 18FDG PET: A descriptor of brain damage and a possible predictor of outcome. Curr. Radiopharm 4(2), 167-175 (2011).
- Shokouhi M, Clarke C, Morley-Forster P, et al. Structural and functional brain changes at early and late stages of complex regional pain syndrome. J. Pain 19(2), 146-157 (2018).