Research Article - Neuropsychiatry (2018) Volume 8, Issue 2
Microglial Activation in the Ventral Hippocampus Induced by Acute Social Defeat is Associated with Amygdala Activity
- *Corresponding Author:
- Yu-Hong Jing, MD, PhD
Institute of Anatomy and Histology & Embryology
Neuroscience, Donggang School of Basic Medical Sciences
Lanzhou University, No. 199 of Donggang West Road
Lanzhou City, Gansu province, Postal Code, 730000. Lanzhou, P.R. China
Abstract
ABSTRACT
Objective: Crosstalk between the brain and immune system has been implicated in the dev elo pm e nt ofmental disorders. The hypothalamic pituitary adrenal axis serves as an important bridge forthis process. This study aims to investigate the relationship between the fluctuation of plasmaglucocorticoid (GC) and microglial activation in the ventral hippocampus during social defeat.
Methods: Intruder—residen t paradigm was used to establish the rodent model of acute social de feat.The plasma GC of intruder mice was obtained serially after social aggression. Simultaneously,social behaviors in the novel context, microglial activation, and inflammation in the ventralhippocampus were tested.
Results: Results showed that plasma GC is high during 0.5-8 hours after social aggress io n and thenrecovered. Behavior test showed that social fear and avoidance in the novel context isobserved at day 3 after stimulation, and then social interaction increased at day 7 afterstimulation. High expression of glucocorticoid receptor (GR), NLRP3, and IL-18 accompaniedmicroglial activation in the ventral hippocampus at day 3, and then diminished at day 7
after stimulation. Ventral hippocampal inflammation is correlated with the changes in socialbe havior. Additionally, muscimol, a GABA agonist, was bilaterally microinjected to basolateralamygdala, which alleviated the social avoidance and ventral hippocampal inflammation.
Conclusion:Our results suggested that acu te social defeat that leads to ventral hippocampal microglialactivation and inflammatory cytokine release depend on the activity of basolateral amygdala,but not the fluctuation of the plasma GC.
Keywords
Acute social defeat; Ventral hippocampus, Microglial activation, Basolateral amygdala
Abbreviations
GC, glucocorticoid; GR, glucocorticoid receptor; HPA, hypothalamic pituitary adrenal axis; SNS, sympathetic nervous system; NTS, nucleus tractus solitaries; PB, parabrachial nucleus; VLM, ventrolateral medulla; PVN, paraventricular nuclei of the hypothalamus; CEA, central amygdala; BNST, bed nucleus of the stria terminalis; BLA, basolateral amygdala; vHIP, ventral hippocampus; mPFC, medial prefrontal cortex; IFN, interferon; Q-RTPCR, Quantitative real-time PCR; CRH, corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone
Introduction
Social defeat, characterized by social withdrawal, fear, and avoidance, is the key risk factor of mood disorders, such as autism, major depression disorder, post-traumatic stress disorder, bipolar disorder, and schizophrenia [1- 3]. Although the biological mechanisms have not been fully understood, the individual responses to prolonged or severe stressors contribute to the development and exacerbation of mental health disorders [4-7]. Social defeat induced by physical or psychological stress accompanied the response of immune system. Stress initiates the immune system through the discrete neural circuitry that includes the sympathetic nervous system (SNS) and the hypothalamic pituitary adrenal axis (HPA) [8]. HPA activation leads to the release of GC in circulation and activation of the SNS leads to the increased release of catecholamines in circulation (epinephrine, Epi) and in tissues (norepinephrine, NE). The immune system is regulated by GC and NE through multiple pathways [9-11]. Moreover, peripheral inflammation influences the behavior via neural and humoral pathways [12,13]. The neural pathway occurs via afferent nerves. As an example, the vagal nerve has a proven role in mediating infection-induced behavior. The afferent vagus nerve projects to distinct brain regions, including the nucleus tractus solitarius (NTS), parabrachial nucleus (PB), ventrolateral medulla (VLM), paraventricular nuclei of the hypothalamus (PVN), central amygdala (CEA), and bed nucleus of the stria terminalis (BNST). These regions regulate motivation and mood [2]. On the other hand, the humoral pathway involves the delivery of cytokines from the peripheral immune system to the brain. The most familiar pathway to discuss first is the effect of cytokines on serotonin (5-hydroxytryptamine, 5-HT). Cytokines, specifically IL-2 and IFN, have been shown to directly increase the enzymatic activity of indolamine-2,3-dioxygenase (IDO), which increases the conversion of tryptophan to kynurenine and consequently decreases the production of serotonin. The depletion of tryptophan and subsequent decrease of 5-HT is a well-established feature of mood disorder pathophysiology [14-16].
Peripheral inflammation frequently causes sickness and this is a pattern of behavior that resembles depressive symptomatology, including lethargy, anorexia, decreased interest in exploring, reduced sexual activity, and increased time spent sleeping [17-19]. Data show that the central or peripheral LPS induced a depressivelike phenotype when quantified with an increased time of immobility in the tail-suspension test and the forced swim test [20,21]. In humans, an inflammatory response has been stimulated using vaccination, endotoxins, LPS, interferon (IFN), and IL-2 accompanying the depressionlike behavior [22-24]. Similarly, with cancer patients receiving immune boosting IL-2 and/ or IFN therapy, early depressive symptoms have been repeatedly demonstrated [25,26]. These results strongly suggest that the inflammation is a causative factor of mood symptoms. A previous study indicated that monocyte trafficking to the brain due to stress and inflammation influences mood and behavior [27]. By contrast, Lehmann et al. [28] reported that social defeat induces depressive-like states and microglial activation without involvement of peripheral macrophages. Interestingly, Filiano et al. [29] reported that the adaptive immune system, particularly IFN-γ, is implicated in disorders characterized by social dysfunction and suggests a co-evolutionary link between social behavior and anti-pathogen immune response driven by IFN-γ signaling. CNS neurons directly respond to IFN-γ derived from meningeal T-cells to elevate tonic GABAergic inhibition and prevent aberrant hyper-excitability in the medial prefrontal cortex (mPFC). Regulation of social defeat involves multiple brain regions including mPFC, amygdala, and hippocampus [30-32]. Generally, basolateral amygdala (BLA) plays an important role in the establishment and consolidation of fear information [33]. The coding of the fear information is modified by ventral hippocampus (vHIP) to determine the output and response to the repeated aversive stimulation [34].
Ample evidence supports the communication between the social behavior regulation of the brain and immune system, but some issues need to be clarified, for example: GC influences not only the peripheral immune system, but also the brain, such as the hippocampus which is very sensitive to GC. However, little is known about the response of hippocampus to the fluctuation of peripheral GC during the establishment and the extinction of the acute social defeat; Whether immune response alleviate with the social fear extinction; Given establishment of aversive stimulation depends on the BLA activity, whether inhibiting BLA suppresses the inflammation in vHIP. This study attempts to present preliminary evidence regarding these issues. Resident-intruder paradigm was used to induce the acute social defeat. Social behavior, peripheral GC, and inflammation of vHIP were measured at the designed time.
Material and Methods
▪ Animals
An adult male C57BL/6 mouse weighing 20-22 g and an adult male Kunming (KM) mouse weighing 35-40g were purchased from the Center of Experiment Animal, Lanzhou University. CX3CR1GFP/+ mouse, which express green fluorescent protein (GFP) in monocytes, dendritic cells, natural killer cells, and microglia in the brain, were purchased from the Jackson Laboratory and bred in the Laboratory Centre for Medical Sciences, Lanzhou University. In this mouse strain, Cx3cr1 gene was replaced by a GFP reporter gene, and all Cx3cr1-expressing cells were expected to become GFP-positive. Animals aged 8-10 weeks and weighing 20–22 g were used in this study. All animals were bred inhouse and maintained in an aseptic environment supplied with clean water and rodent chow ad libitum. Experimental procedures and protocols in this study were approved by the Ethics Committee of Lanzhou University, China.
▪ Reagents
Muscimol (Mus) was obtained from Sigma (St. Louis, MO, USA). Mouse monoclonal GAPDH and polyclonal stat1 antibodies were obtained from Santa Cruz Stat1 (Santa Cruz, CA, USA). Mouse NLRP3, CX3CL1, CX3CR1, TNF-α, IL- 18, IL-1β, and GAPDH primers were designed based on sequences retrieved from a gene bank and produced by Takara Biotechnological Company (Takara Biotech, Co., Ltd., Dalian, China). ELISA kits of glucocorticoid and ACTH were purchased from R&D (R&D, IL, USA). Kits for total RNA extraction and real-time PCR were purchased from Takara Biotechnological Company (Takara Biotech, Co., Ltd., Dalian, China). Total protein extraction kits were from the Institute of Beyotime Biotechnology (Beyotime Biotechnology, Shanghai, China). PVDF membrane was from Millipore (Bellerica, MA, USA).
▪ Social defeat
A KM mouse that was raised in a cage for two weeks was designated as the resident. Then, a C57BL/6 or CX3CR1GFP/+ mouse was introduced as the intruder. For 10 minutes, the C57BL/6 mouse was exposed in the cage with the KM mouse. The aggressive attack was stopped using a perforated plastic barrier, which allowed continuous visual, auditory, and olfactory contact. After 6 hours, the barrier was removed and physical contact was allowed for another 10 minutes. After the second attack, the C57BL/6 mouse was placed back in its home cage. The C57BL/6 or CX3CR1GFP/+ mice were randomly assigned to the control without any social attack (n = 12) and the social defeat with the social attack. Mice in social defeat were divided into subgroups based on the designated time at which the animals were sacrificed after social aggression (1, 3, 7, and 15 days; n = 12 per subgroup).
▪ Stereotactic injections
C57BL/6 or CX3CR1GFP/+ mouse were anesthetized with isoflurane after the completion of social aggression. Mus (40 ng/0.25 μl) or an equal volume of saline solution was bilaterally injected into the BLA (bregma coordinates: AP -1.7 mm; ML ±3.3 mm; and DV -3.8 mm from the brain surface). Mouse which were make incision in the head skin and opened the window in skull without social aggression served as the sham. After three days, the mice were sacrificed following behavioral examination.
▪ Open field test
The behavior of the mice while exploring a 50 cm × 50 cm open-field arena was assessed during a 5 minute test. Locomotor activity and freezing time were measured by a video tracking system (TM-vision, Chengdu Techman Software Co., Ltd, Chengdu, China).
▪ Social interaction
Social avoidance was analyzed according to Pizarro’s modified method [35]. Briefly, two 9 cm × 9 cm mesh enclosures were placed at the opposite sides of the open field. One enclosure contained a novel KM mouse as the social target and another enclosure did not contain a mouse as the social untarget. The experimental mouse was allowed to freely explore a 50 cm × 50 cm arena containing the target and untarget enclosures. Time spent in the social interaction zone (14 cm × 26 cm) surrounding the target enclosure and reference zones (14 cm × 26 cm) surrounding the untarget enclosure were measured using a video tracking system (TM-vision, Chengdu Techman Software Co., Ltd, Chengdu, China).
▪ Sucrose preference test
Three days before the experiment, the test mice were habituated to 1% sucrose for 48 hours. After overnight fluid deprivation, the mice were given two bottles of 1% sucrose or tap water for 2 hours and the volumes consumed were measured the next morning. The amount of sucrose consumed was normalized to that of water for each animal (calculated from a 2-hour consumption test following overnight fluid deprivation).
▪ Analysis of microglial activation
After behavioral testing, CX3CR1GFP/+ and wild type mice were deeply anesthetized with isoflurane and transcardially perfused with 20 mL heparinized saline solution and then with 30 mL 4% paraformaldehyde in 0.1 M phosphate buffer (PB) at pH 7.4 and 4 °C. Brains were harvested and stored in the same fixative for 90 minutes at 4 °C followed by at least 24 hours of immersion in 20% sucrose solution in 0.1 M PB with 4% paraformaldehyde. Serial coronal sections (30 μm thickness) were obtained using a cryostat microtome. To analyze the microglial activation in the ventral hippocampus, three sections per mouse were selected at -2.8 mm, -3.3 mm, and -3.8 mm from the bregma according to the mouse brain atlas. Microglia were observed using a fluorescence microscope and photographed using an Olympus microscope BX3 and Olympus DP73 digital camera. Eight views of the ventral hippocampus per section were used to count the cell number, and 20 microglial cells per section were selected randomly to measure the area of cell body using ImageJ (NIH, Bethseda, MD). There were five mice in each group.
▪ GFP positive cells in choroid plexus
Choroid plexus of lateral ventricle were isolated from CX3CR1GFP/+ and wild type mice and mounted on the sections. Choroid plexus were observed and photographed using fluorescence microscope with the digital camera. Density of GFP-positive cells was computed per unit area.
▪ ELISA assay
Blood was collected through the tail vein. Plasma was isolated from blood, and GC, ATCH were measured according to the operation manual. Each sample was measured in triplicate.
▪ RNA extraction and quantitative realtime PCR
Fresh vHIP tissues were isolated from the corresponding coronal sections under the stereomicroscope. Total RNA was extracted using RNAiso Plus reagent (Takara Biotech, Co., Ltd., Dalian, China) in accordance with the manufacturer’s instructions. DNA contamination was removed with RNase-free DNase. cDNA was synthesized from 1 μg of RNA with M-MuLV reverse transcriptase and random hexamer following the manufacturer’s instructions (Fermentas, Burlington, Canada). Quantitative real-time PCR (Q-RT-PCR) was performed using PIKoREAl96 detector (Thermo Scientific, USA). The sequence of primers for mouse NLRP3, CX3CL1, CX3CR1, TNF-α, IL-18,IL-1β and GAPDH are listed in Table 1. The assays were initiated for 5 minutes at 95 °C, 40 cycles of 15 seconds at 94 °C, and 1 minute at 60°C. The threshold cycles of the target gene and GAPDH were calculated. The amplifications of NLRP3, CX3CL1, CX3CR1, TNF-α, IL-18 and IL-1β cDNA were normalized to the expression of GAPDH. The relative mRNA expression
| Gene | Forward(5’-3’) | Reverse(5’-3’) |
|---|---|---|
| GAPDH | GCGAGACCCCACTAACATCAA | GTGGTTCACACCCATCACAAA |
| NLRP3 | ACTGAAGCACCTGCTCTGCAAC | AACCAATGCGAGATCCTGACAAC |
| CX3CL1 | ACCTATGGCCCTGACATCATCAC | CTTGCCAGCCCTCAGAATCAC |
| CX3CR1 | TCTACAAAGCCATTCCCATGTCC | CAATGTAAGCCTGCAAATGAGACC |
| TNF-α | CCCTTTACTCTGACCCCTTTATTGT | TGTCCCAGCATCTTGTGTTTCT |
| IL-18 | TTCTGCAACCTCCAGCATCA | AGTGAAGTCGGCCAAAGTTGTCT |
| IL-1β | TACAAGGAGAACCAAGCAACGA | TGCCGTCTTTCATTACACAGGA |
Table 1: List of primer sequence.
levels of NLRP3, CX3CL1, CX3CR1, TNF-α, IL-18 and IL-1β were calculated using the 2ΔCT method.
▪ Protein extraction and Western blot analysis
Fresh tissues of vHIP were isolated from the corresponding coronal sections under the stereomicroscope. Proteins were extracted using RIPA buffer that contained protease inhibitors. Extracted proteins (50 μg) were fractionated to 10% sodium dodecyl sulfate polyacrylamide gel and then transferred onto polyvinylidene fluoride membranes. The membranes were blotted with anti-Stat1 (1:1000), anti-GAPDH (1:5000), and horseradish peroxidase-conjugated second antibodies (1:5000). Immunoreactive protein bands were visualized by enhanced chemiluminescence using the Bioanalytical Imaging System (Azure Biosystems, INC, USA).
▪ Statistical analysis
Data were expressed as mean ± SEM. Statistical analysis was performed using SPSS Statistics software 17.0. Differences between the two groups were analyzed by Student’s t-test, whereas those among three or more groups were analyzed by one-way or two-ways analysis of variance with least significant difference test. Statistical significance was considered at P < 0.05.
Results
▪ Effects of acute social aggression on social behavior
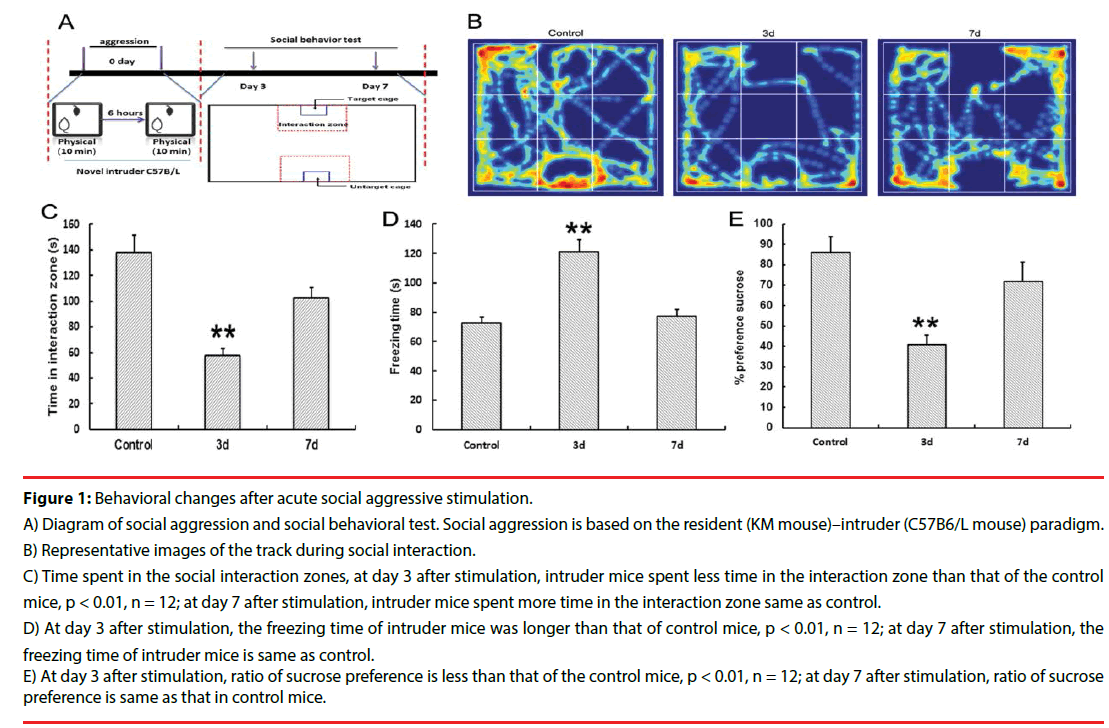
Spontaneous exploration behavior was examined using the open field test. As shown in Figure 1D, the freezing time of the intruder mice increased dramatically at day 3 after stimulation compared with that of the control mice (p < 0.01). On day 7 after stimulation, the freezing time of the intruder mice decreased. The time spent in the social interaction zone is shown in Figure 1B and b. On day 3 after social aggression, the intruder mice spent less time in the interaction zone compared with control mice (p < 0.01). Moreover, on day 7 after stimulation, time spent in the interaction zone of the intruder mice was the same as that of the control mice. The results of sucrose preference are shown in Figure 1E, where the ratio of sucrose preference decreased in intruder mice at day 3 after social attack compared with control mice (p < 0.01), whereas the ratio of sucrose reference increased on day 7 after stimulation. These data suggested that social avoidance triggered the depression-like behavior induced by social aggression. At day 7 after social aggression, time of social interaction increased (Figure 1).
Figure 1: Behavioral changes after acute social aggressive stimulation.
A) Diagram of social aggression and social behavioral test. Social aggression is based on the resident (KM mouse)–intruder (C57B6/L mouse) paradigm.
B) Representative images of the track during social interaction.
C) Time spent in the social interaction zones, at day 3 after stimulation, intruder mice spent less time in the interaction zone than that of the control mice, p < 0.01, n = 12; at day 7 after stimulation, intruder mice spent more time in the interaction zone same as control.
D) At day 3 after stimulation, the freezing time of intruder mice was longer than that of control mice, p < 0.01, n = 12; at day 7 after stimulation, the freezing time of intruder mice is same as control.
E) At day 3 after stimulation, ratio of sucrose preference is less than that of the control mice, p < 0.01, n = 12; at day 7 after stimulation, ratio of sucrose preference is same as that in control mice.
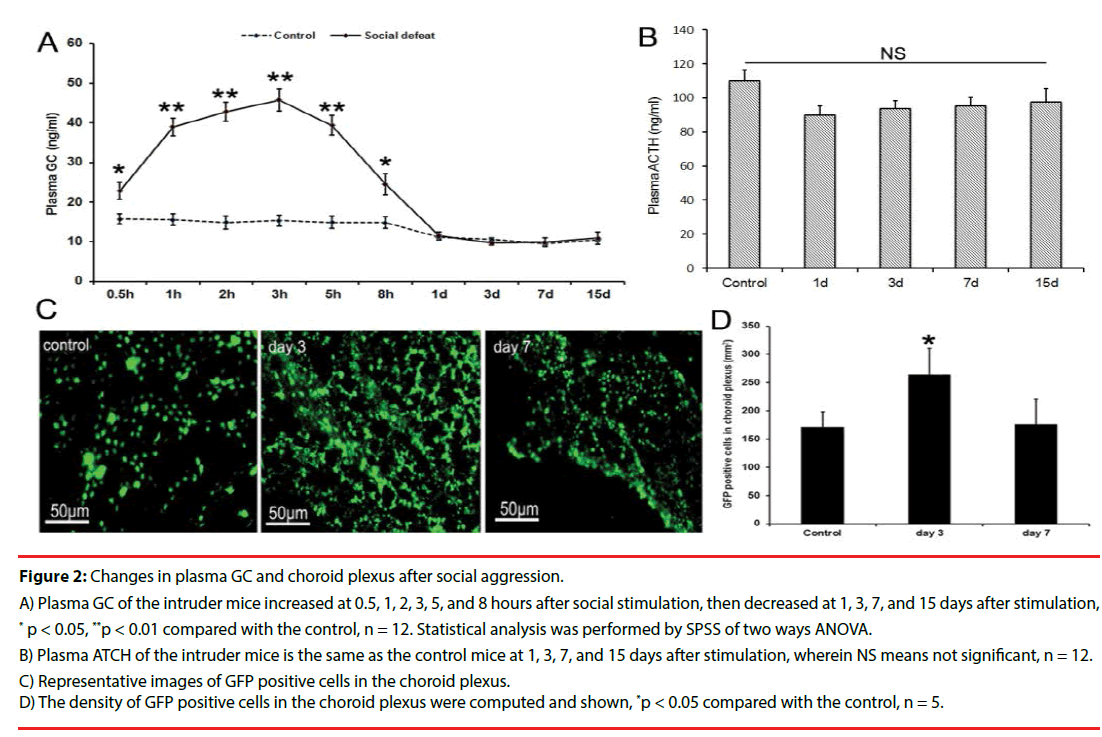
▪ Fluctuation of plasma GC and accumulation of monocyte in choroid plexus
The contents of plasma GC increased significantly at 0.5, 1, 2, 3, 5 hours after social attack in the intruder mice. At 8 hours after the social attack, the plasma GC began to decrease (Figure 2A). The contents of plasma GC and ATCH had no significant difference in the intruder mice compared with those of the control mice at days 1, 3, 7, and 15 after social attack (Figures 2A and 2B). High plasma GC was only maintained for 8 hours, but the number of monocytes labeled with GFP in choroid plexus increased in the intruder mice compared with that in the control mice at day 3 after social attack, whereas the number of monocytes reduced at day 7 after social attack (Figures 2C and 2D). These data suggested that the activation of the immune system was maintained longer than that of the content of plasma GC.
Figure 2: Changes in plasma GC and choroid plexus after social aggression.
A) Plasma GC of the intruder mice increased at 0.5, 1, 2, 3, 5, and 8 hours after social stimulation, then decreased at 1, 3, 7, and 15 days after stimulation, * p < 0.05, **p < 0.01 compared with the control, n = 12. Statistical analysis was performed by SPSS of two ways ANOVA.
B) Plasma ATCH of the intruder mice is the same as the control mice at 1, 3, 7, and 15 days after stimulation, wherein NS means not significant, n = 12.
C) Representative images of GFP positive cells in the choroid plexus.
D) The density of GFP positive cells in the choroid plexus were computed and shown, *p < 0.05 compared with the control, n = 5.
▪ Effects of the acute social aggression on inflammatory signals in vHIP
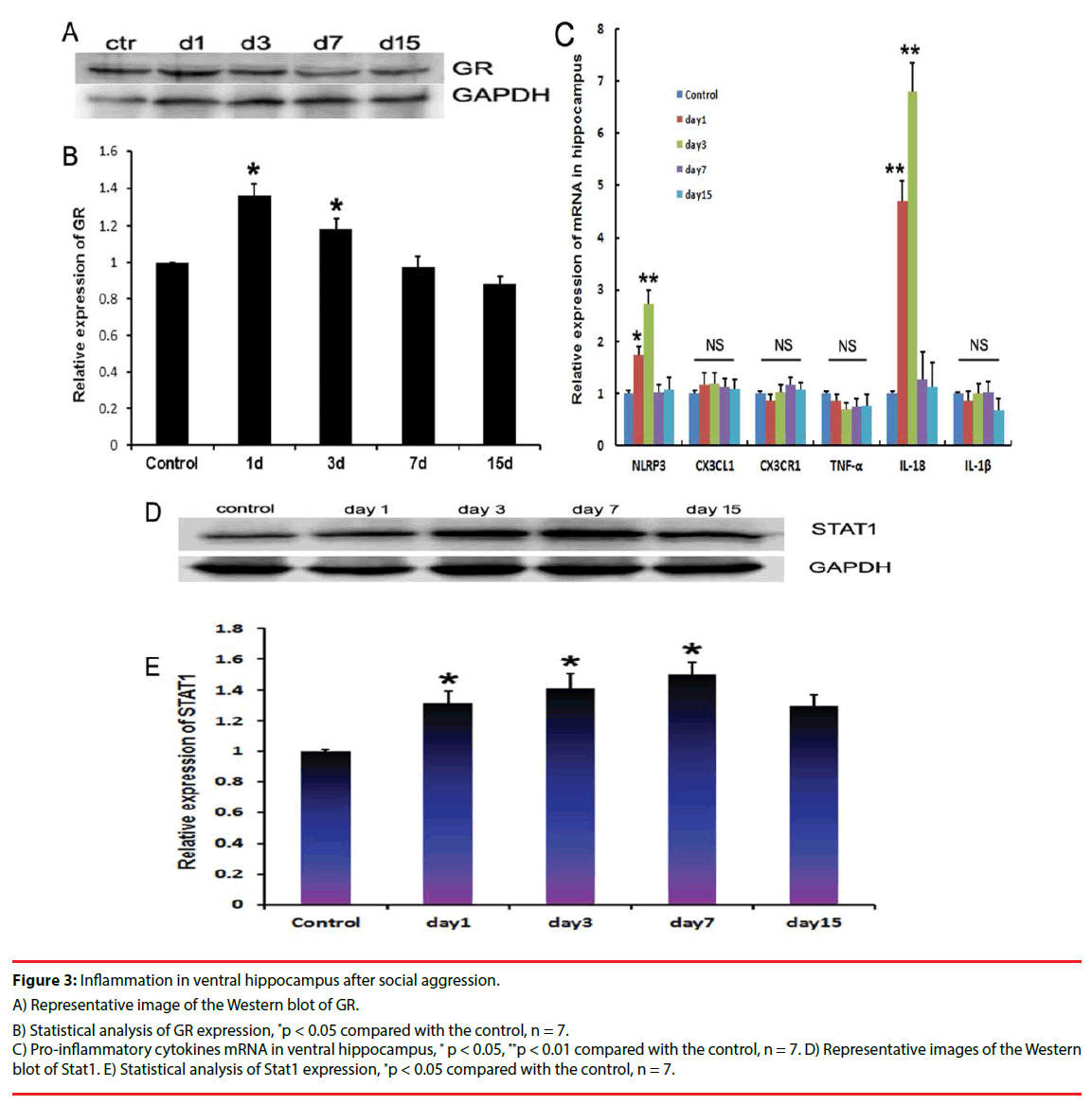
Social attack caused the GR expression in ventral hippocampus of the intruder mice to elevate from day 1 to day 3 after stimulation compared with the control mice (Figures 3A and 3B). To further evaluate the ventral hippocampal inflammatory characteristics, proinflammatory cytokines, including TNF-α, IL-1β, CX3CR1, CX3CL1, and IL-18, were measured using quantitative PCR. Results showed that the mRNA levels of NLRP3 and IL-18 increased significantly from day 1 to day 3 after stimulation in the intruder mice compared with that in the control mice. At day 15 after the social attack, IL-18 and NLRP3 expression levels were reduced (Figure 3C). NLRP3 and IL-18 belong to the effector molecules of inflammasome. Furthermore, Stat1, as the downstream molecule, was measured using immunoblotting. Results showed that the stat1 expression increased at day 1, day 3, and day 7 after stimulation in the ventral hippocampus of the intruder mice, and then decreased at day 15 compared with the levels in control mice (Figures 3D and 3E).
Figure 3: Inflammation in ventral hippocampus after social aggression.
A) Representative image of the Western blot of GR.
B) Statistical analysis of GR expression, *p < 0.05 compared with the control, n = 7.
C) Pro-inflammatory cytokines mRNA in ventral hippocampus, * p < 0.05, **p < 0.01 compared with the control, n = 7. D) Representative images of the Western blot of Stat1. E) Statistical analysis of Stat1 expression, *p < 0.05 compared with the control, n = 7.
▪ Effects of acute social aggression on microglia activation in ventral hippocampus
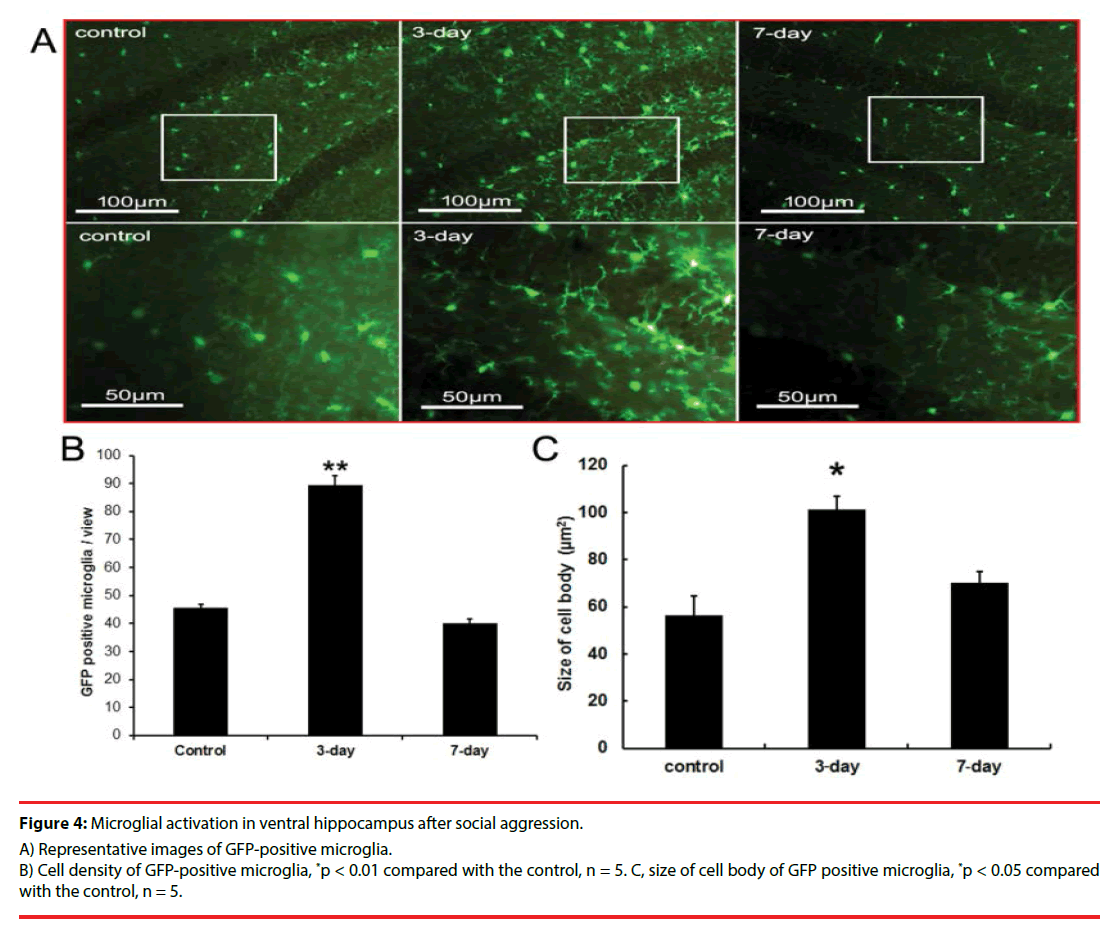
In the CX3CR1GFP/+ transgenic mice, microglia with amoeba-like morphology increased in the ventral hippocampus of intruder mice at day 3 after stimulation. On day 7, the microglial morphology and numbers became similar to those in the control mice (Figures 4A, 4B, and 4C). The time course of microglia activation in ventral hippocampus was consistent with the dynamic changes of behavior and resident inflammation.
Figure 4: Microglial activation in ventral hippocampus after social aggression.
A) Representative images of GFP-positive microglia.
B) Cell density of GFP-positive microglia, *p < 0.01 compared with the control, n = 5. C, size of cell body of GFP positive microglia, *p < 0.05 compared with the control, n = 5.
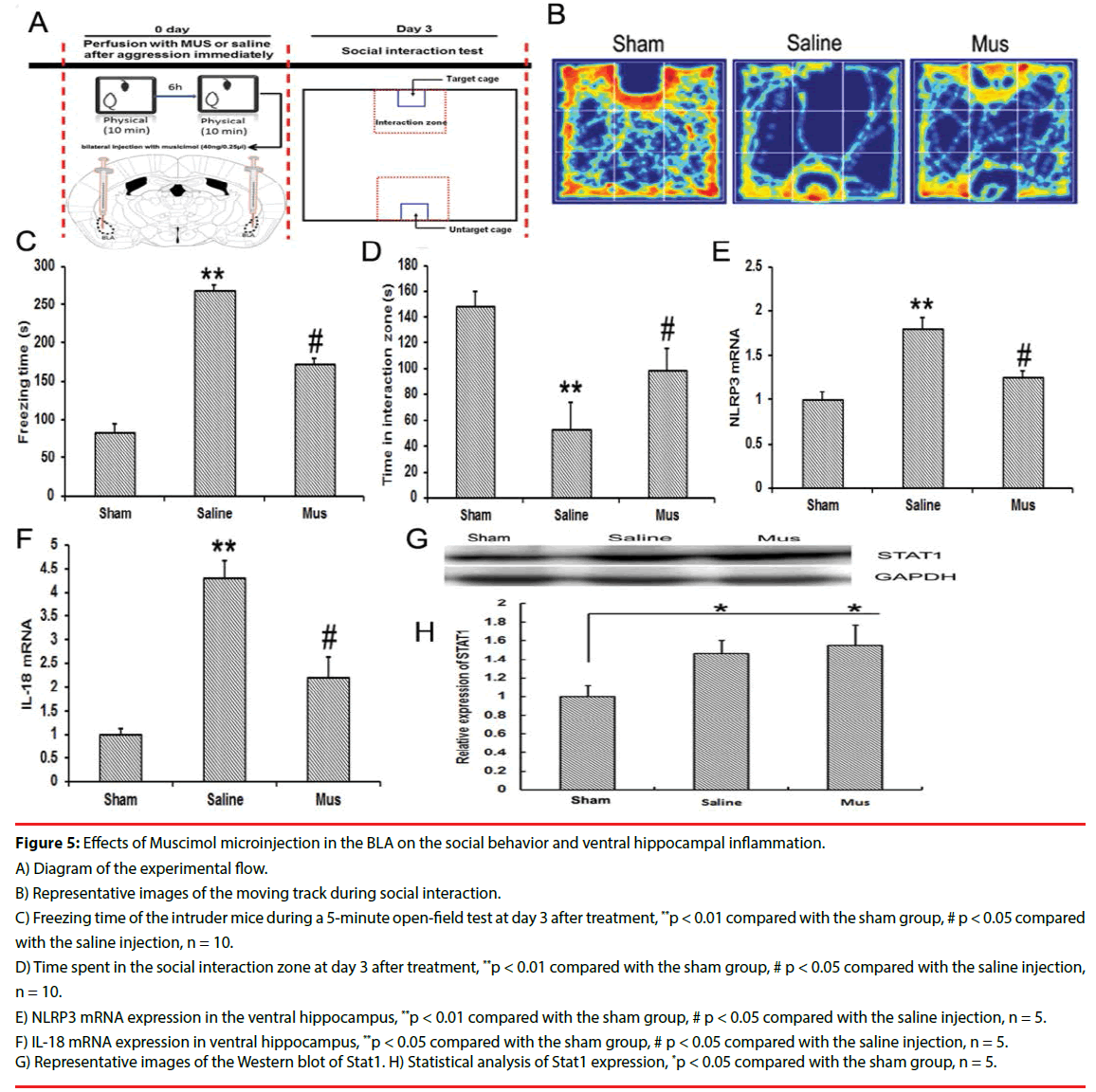
▪ Effects of BLA inhibition with Mus on social behavior and ventral hippocampal inflammation
As the GABA agonist, Mus was immediately microinjected into bilateral BLA after social attack. On day 3 after social aggression, Mus treatment significantly increased the time of social interaction and decreased the time of freezing compared with the saline injection (Figures 5B, 5C, and 5D). Simultaneously, Mus treatment reduced the levels of NLRP3 and IL 18 in ventral hippocampus compared with the saline treatment (Figures 5E and 5F). The Stat1 expression in ventral hippocampus was observed. Mus treatment did not significantly reduce the Stat1 levels compared with the saline treatment (Figures 5G and 5H).
Figure 5: Effects of Muscimol microinjection in the BLA on the social behavior and ventral hippocampal inflammation.
A) Diagram of the experimental flow.
B) Representative images of the moving track during social interaction.
C) Freezing time of the intruder mice during a 5-minute open-field test at day 3 after treatment, **p < 0.01 compared with the sham group, # p < 0.05 compared with the saline injection, n = 10.
D) Time spent in the social interaction zone at day 3 after treatment, **p < 0.01 compared with the sham group, # p < 0.05 compared with the saline injection, n = 10.
E) NLRP3 mRNA expression in the ventral hippocampus, **p < 0.01 compared with the sham group, # p < 0.05 compared with the saline injection, n = 5.
F) IL-18 mRNA expression in ventral hippocampus, **p < 0.05 compared with the sham group, # p < 0.05 compared with the saline injection, n = 5.
G) Representative images of the Western blot of Stat1. H) Statistical analysis of Stat1 expression, *p < 0.05 compared with the sham group, n = 5.
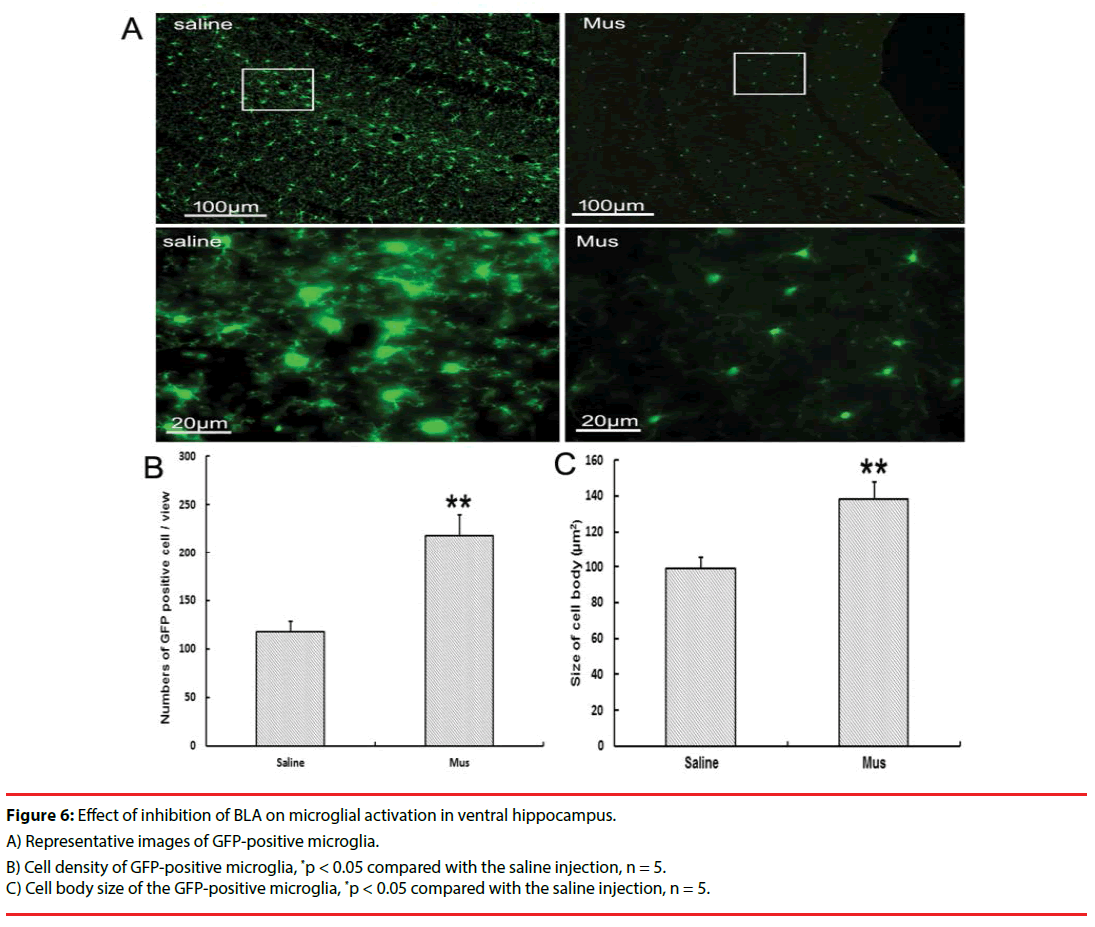
▪ Effects of inhibition of BLA on microglial activation in vHIP
In the CX3CR1GFP/+ transgenic mice, inhibiting BLA with Mus microinjection reduced the microglial activation at day 3 after social aggression (Figures 6A, 6B and 6C). Change in microglia was characterized by the decreased microglial proliferation and ameliorated the microglial swelling compared with the saline treatment.
Figure 6: Effect of inhibition of BLA on microglial activation in ventral hippocampus.
A) Representative images of GFP-positive microglia.
B) Cell density of GFP-positive microglia, *p < 0.05 compared with the saline injection, n = 5.
C) Cell body size of the GFP-positive microglia, *p < 0.05 compared with the saline injection, n = 5.
Discussion
The present study indicates that the time course of social behavior is consistent with the inflammation in vHIP, but not the fluctuation of plasma glucocorticoid in the development of acute social defeat. Plasma GC was maintained at high levels for 8 hours after stimulation and then recovered. Elevated GC induced by stress is beneficial to transiently adapt to environmental changes. GC secretion in animals under stress is mostly regulated by the activity of the HPA-axis and the negative feedback exerted by the levels of circulating GC acting upon glucocorticoid receptors (GR) [36-39]. This mechanism is triggered by a set of stress hormones, such as corticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH), and arginine vasopressin. Moreover, this mechanism is determined by stressor modality and its characteristic time course, as in the case of its effect on social status.
Hippocampus is sensitive to GC signaling, which is ascribed to high distribution of GR in hippocampus, especially in CA3 regions [40]. A previous study suggested that the depressive-like behavior induced by stress triggers the decrease in adult neurogenesis of the dentate gyrus. The status of neurogenesis is negatively regulated by GC signaling. Additionally, the expression of brain-derived neurotrophic factor (BDNF) in the hippocampus is negatively regulated by GC. Furthermore, BDNF is an important neurotrophic factor involved in modifying synaptic plasticity and neurogenesis. Ample evidence has indicated that neurogenesis and synaptic plasticity in hippocampus implicate the development of depression [41-43]. Clinical studies have found that hippocampal atrophy is significant in patients suffering from chronic major depression disorder [44]. In our work, the GR expression increased in the ventral hippocampus of intruder mice at day 1 and day 3 after social aggression.
Microglial cells were activated during brain injuries and immunological stimuli following dramatic alterations in morphology and cytokine release. For the microglia surrounding the neurons, subtypes of microglia can provide trophic support to neurons through the release of nerve growth factors. These microglial cells are capable of assisting synaptic plasticity. Microglia have been implicated as the “brain’s electricians,” in which the release of neurotrophic factors and anti-inflammatory cytokines from microglia has been shown to promote synaptic plasticity [45,46]. In the current study, microglial activation accompanied the production of inflammatory cytokines, especially inflammasome-related cytokines, including NLRP3 and IL-18, which significantly increased in the ventral hippocampus at day 1 and day 3 after social aggression. A previous study suggested that the microglial NLRP3 inflammasome activation mediates IL-1β-related inflammation in the prefrontal cortex of depressive rats [47]. Interestingly, dopamine controls systemic inflammation through inhibition of the NLRP3 inflammasome. Inhibition of dopamine reward circuit occurs in social defeat and depression [48]. Active microglia plays dual roles, on one hand, active microglia clear the accumulation of Aβ and α-synuclein through phagocytosis to ameliorate neural degeneration. On the other hand, chronic inflammation and microglial activation implicate the development of neural degenerative diseases [49-51]. Additionally, synapse pruning by active microglia modify the neural circuit and behavior [52]. In our study, social fear induced by social attack accompanied the microglial activation in vHIP. But the role of active microglia is still not clear in this process.
LPS injection through peritonea induced the activation of microglia accompanied with sickness behavior, which suggested that the peripheral inflammation connected the brain response [53]. In the present study, at early stages after aggression, plasma GC increased. Furthermore, the monocyte increased in the choroid plexus, which suggested that the interface between periphery and brain adapted to this stimulation. A recent study indicated that dynamic alteration of subtype of T-cells affect the regulation of the brain immunology. Although the plasma GC recovered transiently after stimulation, GR expression in vHIP remained higher in the accompanying microglial activation in the intruder mice than that in the control mice. These data suggested that the fluctuation of peripheral GC is not consistent with the central inflammation.
Recognition and consolidation to aversive information depends on the activity of the BLA. Inhibition of BLA increases the resistance to stress stimuli and reduces fear memory [54,55]. In the present study, Mus, the GABA agonist, was injected into bilateral BLA immediately after social aggression, which increased the social interaction, reduced the expression of NLRP3 and IL-18, and decreased the microglial activation in vHIP. These data suggested that the inflammation depends on the BLA activity induced by stress stimuli. The monocyte traffic into the brain is controversial, but evidence has suggested that the choroid plexus, which is the interface between peripheral and central immunology, simultaneously responds to brain stress and peripheral inflammation. Microglial cells consist of the brain immune system to fight against the inflammation and degeneration. Under normal conditions, microglia protects the nerve cells, maintain the neurobiological homeostasis and regulate the behavioral competency. Previous studies have shown microglial cells are responsible in the brain for inflammation and degeneration during ischemia and generation of harmful metabolite such as amyloid beta and tau protein [56,57]. Recently, some work suggested microglial cells play a critical role in disruption of neuroplasticity and aggravation of depression during psychological stress [58]. Our present study found social defeat caused the microglial activation, which serve as the second harmful factor involved in the damage of nerves. But the precise mechanism of active microglial underlying the development of social defeat remains to be further understood.
Conclusions
This study does not give the precise conclusion on the roles of immunoreactivity in the development of social defeat; however, plasma GC fluctuation is not the determining factor in the development of the social defeat and ventral hippocampal inflammation. It is still possible that the transient increase in glucocorticoid might be involved in the subsequent microglial and behavioral changes. In contrast, the activity of BLA is closely associated with the microglial activation and inflammation in vHIP during aversive stimulation. Inhibiting the BLA action reduced the social fear and decreased the microglial activation and inflammation in vHIP. These data suggested that the microglial activation and inflammation in vHIP may facilitate social defeat.
▪ Ethics Approval and Consent to Participate
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. Human subjects were not involved in this work.
Availability of Data and Materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors’ Contributions
Yu-Hong Jing and Chu-Chu Qi designed experiments; Xue-Zhu Ma, Qing-Jun Wang, and Hai-Chao Chen performed experiments; Li- Ping Gao and Jie Yin analyzed data; Yu-Hong Jing wrote the manuscript; Yu-Hong Jing secured financial support; all authors read the manuscript and approved the final version for submission.
Acknowledgments
We thank the Experimental Animal Center of Lanzhou University. This work was assisted by Xiao- Lan Ma, Yue Yi Zhuang and Yan-Qing Li, senior students of the medical school of Lanzhou University.
Funding
This work is partly supported by National Natural Science Foundation of China (No. 81370448 and 81570725) to Jing Yu Hong.
References
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends. Immunol 27(1), 24-31 (2006).
- Dantzer R, O'Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci 9(1), 46-56 (2008).
- Pace TW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain. Behav. Immun 25(1), 6-13 (2011).
- Maes M, Song C, Lin A, et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine 10(1), 313-318 (1998).
- Kalynchuk LE, Gregus A, Boudreau D, et al. Corticosterone increases depression-like behavior, with some effects on predator odor-induced defensive behavior, in male and female rats. Behav. Neurosci. 118(6), 1365-1377 (2004).
- Norman GJ, Karelina K, Zhang N, et al. Stress and IL-1beta contribute to the development of depressive-like behavior following peripheral nerve injury. Mol. Psychiatry 15(4), 404-414 (2010).
- Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol. Ther 130 (2), 226-238 (2011).
- Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat. Rev. Immunol 6 318-328 (2006).
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol 11(1), 625-632 (2011).
- Wohleb ES, Powell ND, Godbout JP, et al. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci 33(34), 13820-13833 (2013).
- Hanke ML, Powell ND, Stiner LM, et al. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain. Behav. Immun 26(7), 1150-1159 (2012).
- Hansen MK, O'Connor KA, Goehler LE, et al. The contribution of the vagus nerve in interleukin-1beta-induced fever is dependent on dose. Am. J. Physiol. Regul. Integr. Comp. Physiol 280(4), R929-34 (2001).
- Konsman JP, Veeneman J, Combe C, et al. Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur. J. Neurosci 28(12), 2499-2510 (2008).
- Capuron L, Neurauter G, Musselman DL, et al. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol. Psychiatry. 2003;54:906-14.
- Barton DA, Esler MD, Dawood T, et al. Elevated brain serotonin turnover in patients with depression: effect of genotype and therapy. Arch. Gen. Psychiatry 65(1), 38-46 (2008).
- Zhang J, Terreni L, De Simoni MG, Dunn AJ. Peripheral interleukin-6 administration increases extracellular concentrations of serotonin and the evoked release of serotonin in the rat striatum. Neurochem. Int 38(4), 303-308 (2001).
- Hart BL. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev 12(2), 123-137 (1988).
- Kent S, Bluthe RM, Kelley KW, et al. Sickness behavior as a new target for drug development. Trends. Pharmacol. Sci 13(1), 24-8 (1992).
- Raison CL, Miller AH. Malaise, melancholia and madness: the evolutionary legacy of an inflammatory bias. Brain. Behav. Immun 31(1), 1-8 (2013).
- Bluthe RM, Michaud B, Kelley KW, et al. Vagotomy blocks behavioural effects of interleukin-1 injected via the intraperitoneal route but not via other systemic routes. Neuroreport 7(1), 2823-2827 (1996).
- Bluthe RM, Laye S, Michaud B, et al. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur. J. Neurosci 12(1), 4447-4456.
- Reichenberg A, Yirmiya R, Schuld A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry 58(5), 445-452 (2001).
- Grigoleit JS, Kullmann JS, Wolf OT, et al. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PloS. one 6(1), e28330 (2011).
- Imran M, Manzoor S, Khattak NM, et al. Current and future therapies for hepatitis C virus infection: from viral proteins to host targets. Arch. Virol 159(5), 831-846 (2014).
- Capuron L, Ravaud A, Miller AH, et al. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain. Behav. Immun 18(1), 205-213 (2004).
- Eller T, Vasar V, Shlik J, et al. The role of IL-2 and soluble IL-2R in depression and antidepressant response. Curr. Opin. Investig. Drugs 10(7), 638-643 (2009).
- McKim DB, Patterson JM, Wohleb ES, et al. Sympathetic Release of Splenic Monocytes Promotes Recurring Anxiety Following Repeated Social Defeat. Biol. Psychiatry 79(1), 803-813 (2016).
- Lehmann ML, Cooper HA, Maric D, et al. Social defeat induces depressive-like states and microglial activation without involvement of peripheral macrophages. J. Neuroinflammation 13(1), 224 (2016).
- Filiano AJ, Xu Y, Tustison NJ, et al. Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature 535(1), 425-429 (2016).
- Uribe-Marino A, Gassen NC, Wiesbeck MF, et al. Prefrontal Cortex Corticotropin-Releasing Factor Receptor 1 Conveys Acute Stress-Induced Executive Dysfunction. Biol. Psychiatry 80(1), 743-753 (2016).
- Dulka BN, Ford EC, Lee MA, et al. Proteolytic cleavage of proBDNF into mature BDNF in the basolateral amygdala is necessary for defeat-induced social avoidance. Learn. Mem 23(4), 156-160 (2016).
- Pearson-Leary J, Eacret D, Chen R, et al. Inflammation and vascular remodeling in the ventral hippocampus contributes to vulnerability to stress. Transl. Psychiatry 7(1), e1160 (2017).
- Kitamura T, Ogawa SK. Engrams and circuits crucial for systems consolidation of a memory. Science 356(6333), 73-78 (2017).
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, et al. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76(2012), 804-812.
- Pizarro JM, Lumley LA, Medina W, et al. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain. Res 1025(1), 10-20 (2004).
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends. Neurosci 20(2), 78-84 (1997).
- Imaki T, Nahan JL, Rivier C, et al. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neuroscience 11(1), 585-599 (1991).
- Surjit M, Ganti KP, Mukherji A, et al. Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell 145(2011), 224-241.
- King BR, Nicholson RC. Advances in understanding corticotrophin-releasing hormone gene expression. Front. Biosci 12(1), 581-590 (2007).
- Azuma K, Zhou Q, Niwa M, et al. Association between Mastication, the Hippocampus, and the HPA Axis: A Comprehensive Review. Int. J. Mol. Sci 18(8), 1687 (2017).
- Antoniazzi CT, Metz VG, Roversi K, et al. Tactile stimulation during different developmental periods modifies hippocampal BDNF and GR, affecting memory and behavior in adult rats. Hippocampus 27(1), 210-220 (2017).
- van Hasselt FN, Cornelisse S, Zhang TY, et al. Adult hippocampal glucocorticoid receptor expression and dentate synaptic plasticity correlate with maternal care received by individuals early in life. Hippocampus 22(2012), 255-266.
- Mutlu O, Gumuslu E, Kokturk S, et al. Effects of chronic administration of adipokinetic and hypertrehalosemic hormone on animal behavior, BDNF, and CREB expression in the hippocampus and neurogenesis in mice. Fundam. Clin. Pharmacol 30(1), 4-13 (2016).
- Schoenfeld TJ, McCausland HC, Morris HD, et al. Stress and Loss of Adult Neurogenesis Differentially Reduce Hippocampal Volume. Biol. Psychiatry 82(12), 914-923 (2017).
- Han J, Harris RA, Zhang XM. An updated assessment of microglia depletion: current concepts and future directions. Mol. Brain 10(1), 25 (2017).
- Sipe GO, Lowery RL, Tremblay ME, et al. Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat. Commun 7(1), 10905 (2016).
- Pan Y, Chen XY, Zhang QY, et al. Microglial NLRP3 inflammasome activation mediates IL-1beta-related inflammation in prefrontal cortex of depressive rats. Brain. Behav. Immun 41(1), 90-100 (2014).
- Yan Y, Jiang W, Liu L, et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160(1), 62-73 (2015).
- Peelaerts W, Bousset L, Van der Perren A, et al. Alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522(1), 340-344 (2015).
- Baruch K, Deczkowska A, Rosenzweig N, et al. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer's disease. Nat. Medicine 22(1), 135-137 (2016).
- Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics 7(1), 354-365 (2010).
- Wong EL, Lutz NM, Hogan VA, et al. Developmental alcohol exposure impairs synaptic plasticity without overtly altering microglial function in mouse visual cortex. Brain. Behav. Immun 67(1), 257-278 (2017).
- Sylvia KE, Demas GE. Overcoming neonatal sickness: Sex-specific effects of sickness on physiology and social behavior. Physiol. Behav 179(2017), 324-332.
- Lee SC, Amir A, Haufler D, et al. Differential Recruitment of Competing Valence-Related Amygdala Networks during Anxiety. Neuron 96(2017), 81-8e5.
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature 517(1), 284-292 (2015).
- Shichita T, Sakaguchi R, Suzuki M, et al. Post-ischemic inflammation in the brain. Front. Immunol 3(1), 132 (2012).
- Nagele RG, Wegiel J, Venkataraman V, et al. Contribution of glial cells to the development of amyloid plaques in Alzheimer's disease. Neurobiol. Aging 25(1), 663-674 (2004).
- Kreisel T, Frank MG, Licht T, et al. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol. Psychiatry 19(6), 699-709 (2014).