Research Article - (2018) Volume 8, Issue 6
Long-Term Neurobehavioral Status and the Metabolism-Related Gene Expressions in Healthy Rat Hippocampus Following a Ketogenic Diet
- Corresponding Author:
- Hong Ni
Neurology Laboratory, Institute of Pediatric Research, Children’s Hospital of Soochow University, Suzhou 215003, China
Tel: 86512-67787306
Abstract
While as a well-established therapy for medically intractable epilepsy, clinical evidence of relevant adverse events of ketogenic diet (KD) has also been reported. We asked whether this kind of diet would have deleterious effects on normal brain function by evaluating KDinduced biochemical changes in hippocampus as well as neurobehavioral changes occurring in normal animals. Fifty-four Sprague-Dawley rats on postnatal day 28 (P28) were randomly assigned to three groups: normal rats fed with KD qd (daily for 4 weeks, NS+KD) or qod (every other day for 4 weeks, NS+KOD), and normal rats fed with standard normal laboratory diet (NS+ND). Neurobehavioral changes were observed on P35, P42 and P49. The hippocampal mossy fiber sprouting, the expression levels of zinc transporters (ZnTs) and lipid metabolism related genes were detected by Timm staining, real-time RT-PCR and Western blot analysis on P58, respectively. The results revealed that there were no significant differences in the three reflection tests (surface righting, cliff avoidance, forelimb suspension) among the three groups. KD-treated NS+KOD and NS+KD groups showed a significant delay of negative geotaxis reflex only on P35, but not on P42 and P49. In respect with open field test, daily KD treatment only leaded a reduction in exploratory activity and increased grooming times, but induced no significant changes in the score of vertical activity and delay time. KD qod treated rats (NS+KOD) displayed a slight delay in the place navigation test on P35 compared with that in NS+KD group. There were no significant differences in Timm staining among the three groups. In parallel with these changes, KD treatment (both NS+KD and NS+KOD) induced significantly down-regulated mRNA levels of Apoa1, Pdk4, and upregulated expression of ApoE, ANXN7, cPLA2 in hippocampus when compared with NS+ND group (except ApoE in NS+KOD group). Notably, both the mRNA and protein levels of cPla2 in NS+KOD rats were significantly down-regulated compared with that of NS+KD group, but still markedly higher than that in the NS+ND group. No significant difference was found in concern with ZnTs among the three groups. Our data suggest that early life daily KD treatment in normal rats KD may have no long-term adverse effects on most of the neurobehavioral parameters, but minor neurobehavioral damage may exist. The hippocampal lipid metabolism signaling pathway, especially cPLA2, may be the target of protective effect of KD on long-term brain injury after developmental seizures.
Keywords
Ketogenic diet, Zinc transporter, ApoE, Cpla2, ANXN7, Apoa1, Pdk4
Introduction
The ketogenic diet (KD) is a valuable therapy for medically intractable epilepsy since the 1920s. KD has recently been utilized in a variety of neurological and mental disorders such as Alzheimer, Parkinson’s disease, autism spectrum disorders and ACTH-resistant West syndrome [1,2]. Interestingly, KD could also effectively prevent exercise-induced oxidative stress of Taekwondo athletes [3]. On the other hand, however, it is reported that KD can result in a variety of complications including protein-losing enteropathy, arterial stiffness, nephrolithiasis, cholelithiasis, declined linear growth status, trace mineral deficiencies and increased tendency in mood problems [4-9], which limits its usefulness.
Animal data also indicates that KD is associated with long-term adverse effects of biotin deficiency, dyslipidemia, a proinflammatory state, signs of hepatic steatosis, glucose intolerance, and a reduction in β- and α-cell mass [10,11]. However, previous studies mainly observed the effect of KD on the function of peripheral organs, while the study of brain function was less frequent. One animal study using 8-weekold young-adult CD-1 mice exposed to the KD in utero showed that prenatal exposure to the KD influenced the offspring neuro-anatomy and behavior in adulthood [12]. Another study using CD-1 mouse neonates whose mothers were fed a KD prior to and during gestation demonstrated that gestational KD altered maternal metabolic status as well as offspring physiological growth and brain structure [13]. In human condition, Shiohama et al. reported that a ketogenic diet at 4 months of age induced a reversible white matter lesions during ketogenic diet therapy in glucose transporter type 1 deficiency syndrome [14]. Lambrechts et al. investigated possible adverse effects of KD on cognition, behavior, psychosocial adjustment, and quality of life in school-aged children and adolescents. They found a tendency toward an increase in mood problems [15].
Based on the upon evidence, and combined with the fact that KD has been wildly used in pediatric neurological diseases, it is necessary a more comprehensive study on the neurotoxicological repertoire, particularly on the KD effects on brain tissue. On the balance, the aim of the present study was to investigate the effect of a high-fat low-carbohydrate ketogenic diet on neurobehavioral parameters of normal young rats.
Energy metabolism signals in hippocampus have been advanced to explain the anticonvulsant effect of KDs [16]. In particular, we have recently shown modulatory effects of KD on the activity of zinc/lipid signal-related genes, such as ZnT-3, ApoE, ApoJ and ACAT-1 in Sprague-Dawley rats submitted to recurrent neonatal seizures [17]. To further clarify the causal relationship of zinc and lipid metabolism signals, in this study KD-induced biochemical changes in hippocampus, mainly the expression of zinc and lipid transporter related genes occurring in normal animals were compared. Since the weaning date of the rats was 21 days after birth, the experiment chose to start the ketogenic diet 28 days after weaning, that is, one week after the adaptive diet.
Materials and Methods
▪ Animal preparation
Sprague-Dawley rats (number = 54) at postnatal day 8 (P8) were obtained from the Chinese academy of sciences, shanghai experimental animal center, China. Rats were kept in an environment-controlled room which was away from bright light and noise under a 12 h/12h light/dark cycle. The animals were treated in accordance with the guidelines set by the National Institutes of Health for the humane treatment of animals. At weaning day P21, rats were randomly divided into three groups (n = 18/each group): normal rats fed with KD qd (daily, NS+KD) or qod (every other day, NS+KOD), and normal rats fed with standard normal laboratory diet (NS+ND). At P28, rats in NS+KD and NS+KOD groups received KD, while rats in NS+ND group received normal diet. The formula of KD was reported in detail previously [17]. KD (70% fat, 20% protein and no carbohydrate) and normal diet (50% carbohydrate, 20% protein and 4.5% fat) were obtained from Chinese academy of sciences, shanghai experimental animal center, China. All rats were given food and water ad libitum for 4 weeks.
▪ Neurobehavioral tests
Neurological behavioral parameters of brain damage (Negative geotaxis reflex, Plane righting reflex, Cliff avoidance reflex and forelimb suspension test) were observed on P35, P42 and P49 according to the procedure previously described [18,19].
▪ Open field test
The open-field test was performed on P42 and P49 as described previously [20,21]. A horizontal (crossing) score was recorded as the number of squares that the animal crossed in the time period. A vertical (rearing) score was recorded as the number of times that the animal reared in the same period. The delay time (time sitting in the central square) and grooming times was also recorded.
▪ Timm staining
Since we aimed to investigate the effect of KD on the expression of zinc transporters in normal hippocampus, and the expression of zinc transporters involved in the pathogenesis of hippocampal mossy fiber sprouting, thus in this study, we also examined the effect of KD on the mossy fiber sprouting of hippocampus in normal rats. On P58, a subset of rats were sacrificed by decapitation Timm staining (n=6/ each group). The pyramidal/infra-pyramidal CA3 region and the inner molecular layer of the dentate gyrus of hippocampus were assessed on each section. Mossy fiber sprouting was analyzed using a semi-quantitative scale for terminal sprouting in the CA3 and the dentate gyrus [22].
▪ Real-time RT-PCR study
Randomly selected six rats from each group were anesthetized with chloropent (3 ml/kg, i.p.) on P58. The method has been described in detail previously [21]. The primers and probes of the twelve genes were designed against GenBankpublished sequences with the software Primer Express 2.0 (Applied Biosystems), of which the sequences were listed in Table 1. The ΔCT method of relative quantification was used to determine the fold change in expression. Firstly, the real-time PCR threshold cycle (CT) of the target mRNAs and the internal control β-actin was determined. Secondly, the ratio target genes: β-actin were calculated as follows: target genes: β-actin =2CT (target)-CT (β-actin) (ΔCT=CT Target – CT β-actin). The fold change in expression was then obtained (2-ΔCT –method) [23].
| Gene | Genbank accession number | Primer sequence |
|---|---|---|
| ApoE | NM_001270681.1 | F: 5’-ACCTAATGGAGAAGATACA-3’ R: 5’-GAGAATCTTTATTAAGCAAGG-3’ probe:5’-FAM-AACTCCATTGCCTCCACCAC-TAMRA-3’ |
| Apoa1 | NM_012738 | F:5’-CGATCAGATGCGCGAGAAC-3’ R:5’-TACTCGATCAGGGTAGGGTGGTT-3’ Probe:5’-FAM-CCCAGCGCCTGACCGAGATCAA-TAMRA-3’ |
| PDK4 | NM_053551 | F:5’-GCTCACACAAGTCAATGGAAAATT-3’ R:5’-ATGTGGTGAAGGTGTGAAGGAA-3’ Probe:5’-FAM-CCAGGCCAACCAATCCACATCGTG-TAMRA-3’ |
| Annexin A7 | NM_130416 | F: 5’-TTGTGGATGTCGTGTCTA-3’ R: 5’-GGCATCATAGTATGTAGGAG-3’ probe:5’-FAM-CGTTCCAATGACCAGAGGCA-TAMRA-3’ |
| cPLA2 | NM_133551 | F: 5’-AGATCCTTATCAGCACAT-3’ R: 5’-CACAGGGTTTATATCATTATTG-3’ probe: 5’-FAM-TTGTTCGCTTCCTGCTGTCA-TAMRA-3’ |
| ZnT-1 | NM_022853 | F: 5'- CGTTGTTGTGAATGCCTTGGT-3' R:5'-GGGTTCACACAAAAGTCGTCTTC-3' probe::5'-FAM-TTCTACTTTTCCTGGAAGGGTTGTA-TAMRAM-3' |
| ZnT-3 | NM_001013243 | F:5'- TGGGCGCTGACGCTTACT-3' R: 5'- GTCAGCCGTGGAGTCAATAGC-3' probe::5'-FAM-ACCACGTTGCCTCCGCACACCT-TAMRAM-3' |
| ZnT-4 | NM_172066.1 | F:5’- GCTGAAGCAGAGGAAGGTGAA -3’ R: 5’- TCTCCGATCATGAAAAGCAAGTAG -3’ probe:5’-FAM-CAGGCTGACCATCGCTGCCGT-TAMRA -3’ |
| ZnT-5 | NM_001106404.1 | F: 5’- CCAGCACATGTCTGGCCTAA -3’ R: 5’- TTTGCAGTACTTCATGGATTCCA -3’ probe:5’-FAM-CACTGGCTTCCACGATGTCCTGGCTAT– TAMRA-3’ |
| ZnT-6 | NM_001106708.1 | F: 5’- CGGCATTATCCCAGGACTCA -3’ R: 5’-CCAGCAAGATCGATCAGAACAA -3’ probe:5’-FAM- TTCTTGCCCCGCATGAACCCG -TAMRA-3’ |
| ZnT-7 | XM_001073594.1 | F: 5’- TTGGGATCCGCGTCTGA -3’ R: 5’- CCCTCTAGAAGTGACTCGGTATGG -3’ probe:5’-FAM-TCGTCTCTGCTGTCACTGCCGCC–TAMRA- 3’ |
Table 1: Oligonucleotide primers for real-time RT-PCR analysis.
▪Western blot analysis
Protein levels were detected by western blot method [21] on P58 (n=6/each group). Polyvinylidene fluoride membranes blots after blocking solution TBS-T were incubated with one of the following antibodies: a rabbit anticPLA2 polyclonal antibody (1:800, Eno Gene) ,a goat anti-ApoE polyclonal antibody (1:800, Santa Cruz), a goat anti-Clusterin polyclonal antibody (1:500, Santa Cruz) in Tris buffered saline containing 0.2% Tween-20 (TBST) and 5% nonfat dry milk overnight at 4℃. The blot was then incubated with the secondary antibody for about 2 h at ambient temperature. Antibody reactions were exposed with Kodak X-ray film using the ECL detection system Amersham. The relative changes of the intensity of each immunoreactive band were evaluated with Sigma Scan Pro 5 and were normalized to a loading control β-actin.
▪ Statistical analysis
The Timm staining, the mRNA and the protein levels were analyzed with post hoc comparisons using a Bonferroni test after ANOVA. The behavioral parameters were analyzed by nonparametric Kruskal-Wallis test. by SAS 8.0 statistical software. Data were presented as the mean ± SD, and statistical significance was considered as a P<0.05.
Results
▪ Neurological behavior
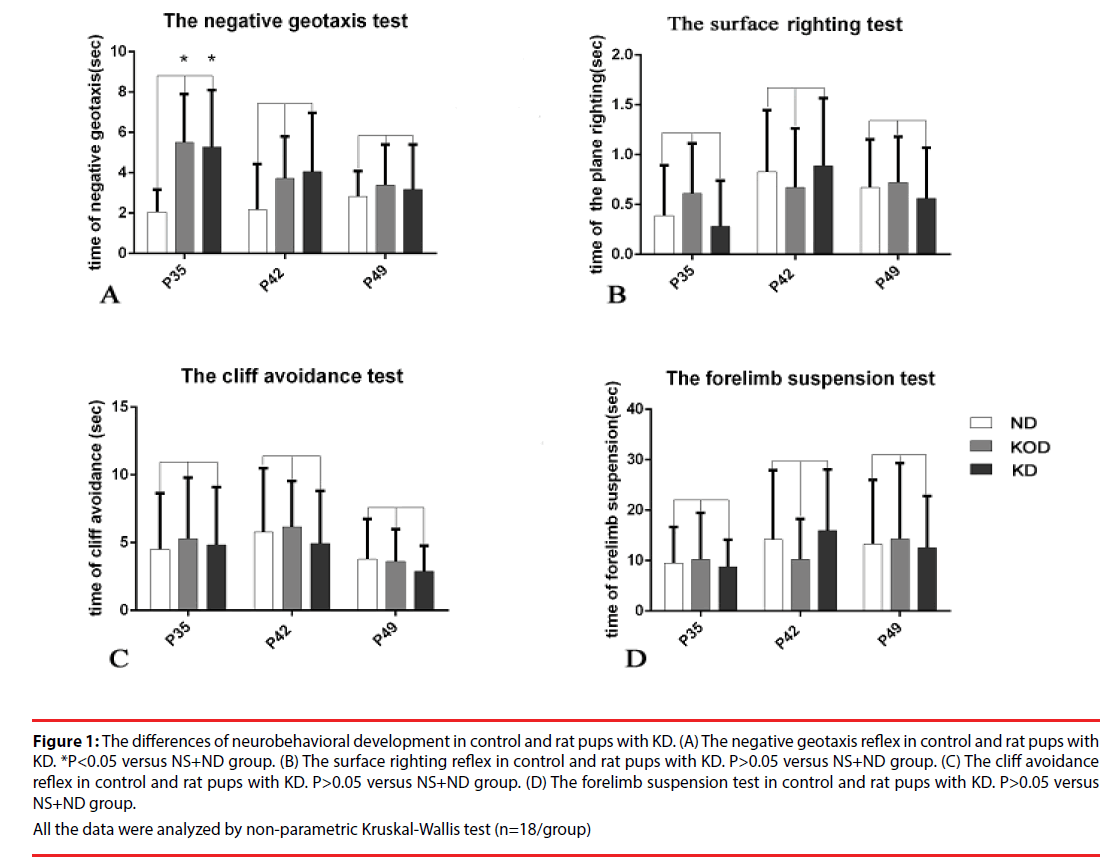
The effects of chronic treatment by KD on neurological development may be presented by different neurological reflexes. As shown in Figure 1, KD-treated NS+KOD and NS+KD groups showed a significant delay of negative geotaxis reflex only on P35 (P<0.05, Figure 1A) . No other differences were observed in the surface righting reflex, cliff avoidance reflex and forelimb suspension test (Figure 1 B-D).
Figure 1: The differences of neurobehavioral development in control and rat pups with KD. (A) The negative geotaxis reflex in control and rat pups with KD. *P<0.05 versus NS+ND group. (B) The surface righting reflex in control and rat pups with KD. P>0.05 versus NS+ND group. (C) The cliff avoidance reflex in control and rat pups with KD. P>0.05 versus NS+ND group. (D) The forelimb suspension test in control and rat pups with KD. P>0.05 versus NS+ND group.
All the data were analyzed by non-parametric Kruskal-Wallis test (n=18/group)
▪ Open field test
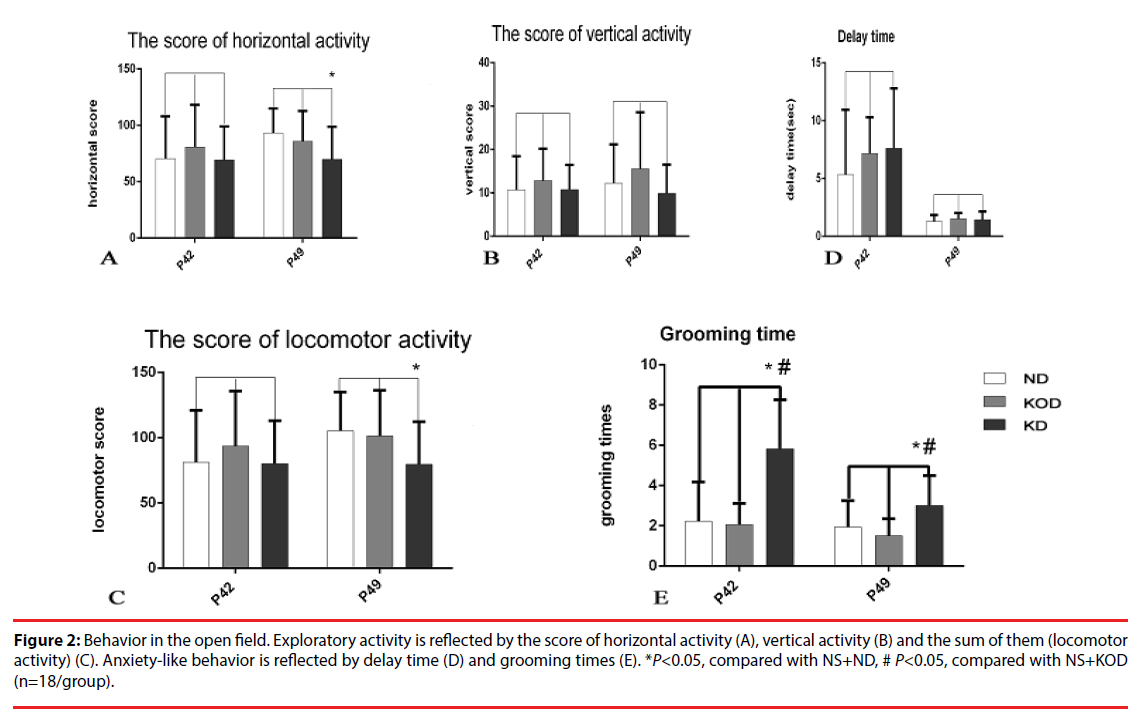
This test evaluates the activity in a novel environment, as well as anxiety and exploration. As shown in Figure 2, administration of KD induced no changes in vertical activity and delay time. However, a reduction in exploratory activity was observed as shown by the reduced score of horizontal activity and locomotor activity in daily KD-treated animals (NS+KD group) when compared with the control. In addition, animals of NS+KD group showed anxiety-like behavior as exhibited by increased grooming times compared with the other two groups.
Figure 2: Behavior in the open field. Exploratory activity is reflected by the score of horizontal activity (A), vertical activity (B) and the sum of them (locomotor activity) (C). Anxiety-like behavior is reflected by delay time (D) and grooming times (E). *P<0.05, compared with NS+ND, # P<0.05, compared with NS+KOD (n=18/group).
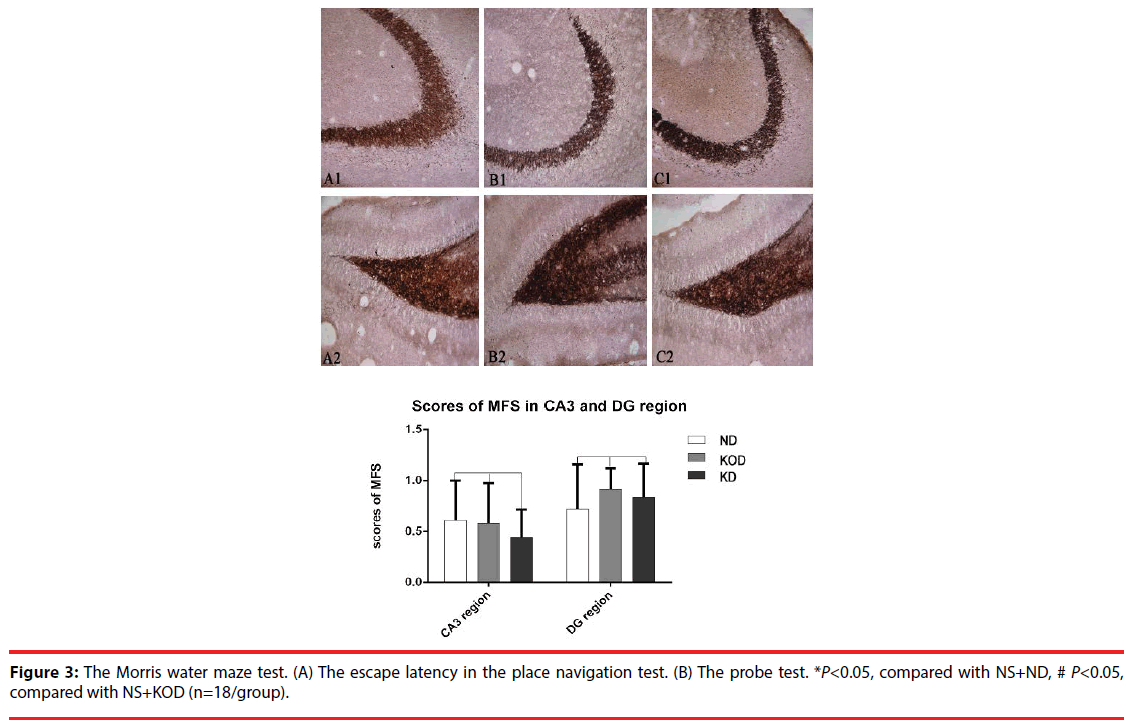
▪ Timm staining
As shown in Figure 3, the Timm staining patterns in the region of stratum pyramidale of CA3 subfield (Fig.3A-C) and supragranular of dentate gyrus (Figure 3D-F) are not obviously different among the NS+ND, NS+KOD and NS+KD groups.
▪ Real- time RT-PCR analysis
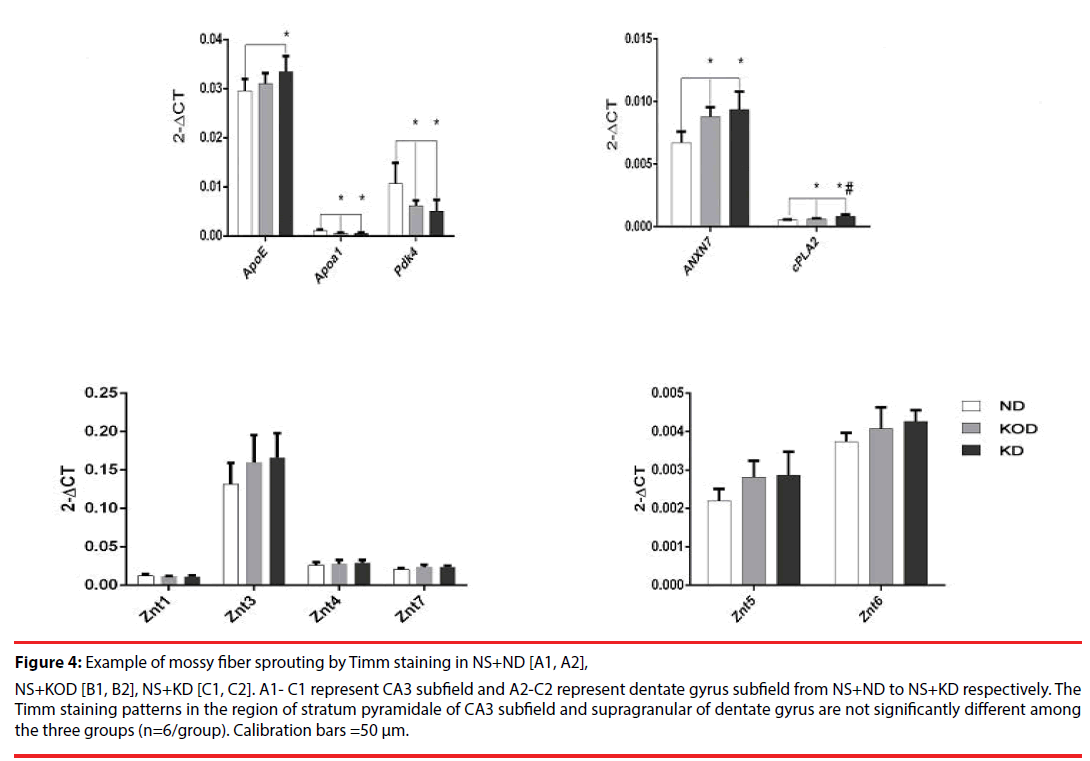
Real time RT-PCR was employed to evaluate the relative gene expression for apolipoprotein E (ApoE), Apoa1(apolipoprotein A-I), Pdk4 (dehydrogenase kinase, isozyme 4), clusterin (ApoJ, apolipoprotein J), annexin 7 (ANX7) and cPLA2 (Ca2+ -dependent phospholipase A2) that are involved in regulation of lipid metabolism; ZnT-1 (zinc transporter 1), ZnT-3~ZnT-7 that are involved in regulation of zinc metabolism, respectively. As shown in Figure 4, among the total twelve genes, four lipid metabolism-related genes showed down-regulated (Apoa1, Pdk4) or up-regulated (ANXN7, Cpla2) expressions in KD-treated NS+KD and NS+KOD groups compared with that in the NS+ND group. In addition, the mRNA levels of ApoE in NS+KD group was increased significantly compared with that in NS+ND group. Further, daily KD-treated rats (NS+KD) showed a significant upregulation of mRNA expression of cPLA2 in hippocampus when compared with NS+KOD rats. No significant differences were observed in ZnTs expressions among the three groups.
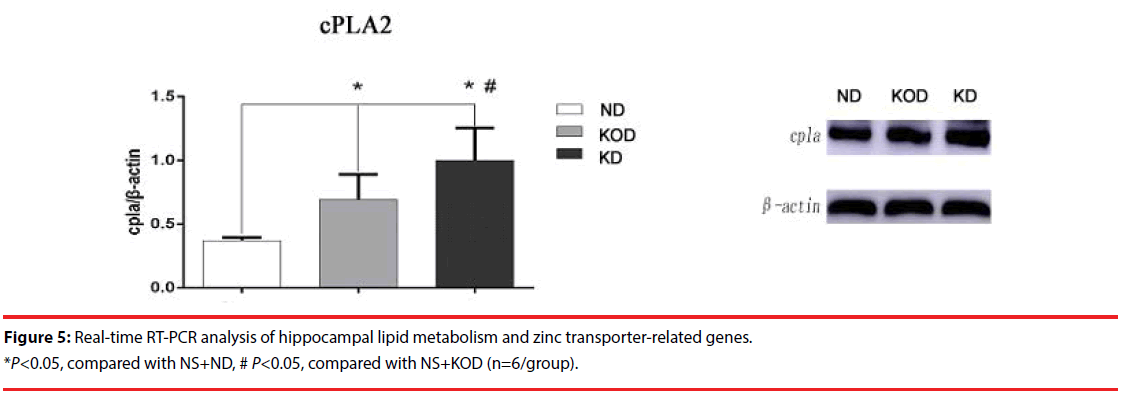
Figure 4: Example of mossy fiber sprouting by Timm staining in NS+ND [A1, A2], NS+KOD [B1, B2], NS+KD [C1, C2]. A1- C1 represent CA3 subfield and A2-C2 represent dentate gyrus subfield from NS+ND to NS+KD respectively. The Timm staining patterns in the region of stratum pyramidale of CA3 subfield and supragranular of dentate gyrus are not significantly different among the three groups (n=6/group). Calibration bars =50 μm.
▪Western blot analysis
To validate the RT-PCR results, western blot analysis was performed on selected genes to evaluate the relative protein levels of CLU and cPLA2 in hippocampus. In consistent with RT-PCR results, KD-treated rats (NS+KOD and NS+KD) had a higher amount of cPLA2 in hippocampus when compared with NS+ND rats; meanwhile, there was higher level of cPLA2 in NS+KD than that in NS+KOD (p<0.05). In addition, there was long-term increase of CLU of KD treated NS+KD rats compared with that in the other two groups (Figure 5).
Discussion
We have recently characterized the neurobehavioral effects of ketogenic diet following flurothyl seizure-induced brain damage, where we have shown the improved neurobehavioral parameters by KD in epileptic rats [17]. However, in regard to the two control groups (NS+ND vs NS+KD), there were no significant difference with relevance to place righting test, cliff avoidance test and open field test. This is partly in accordance with our present results. Here, we showed no obvious differences among three groups in surface righting test, cliff avoidance test, forelimb suspension test, negative geotaxis test (P42, P49), vertical activity and delay time in open field test. However, in present study, we found significant differences between NS+ND vs NS+KD groups in the locomotor/horizontal activity and grooming times in open field test, as well as negative geotaxis test on P35. These new findings are quite in accordance with some clinical and experimental studies. For example, Beilharz et al. showed that intake of a high fat-Western diet is associated with deficits in hippocampal-dependent place recognition memory [24]. Sobesky et al. found that rats fed a high-fat diet demonstrated impaired hippocampal memory function using contextual pre-exposure fear conditioning (CPE-FC) [25]. Chwiej et al. further demonstrated that KD could induce structural changes of proteins from the α-helix to the β-sheet secondary structure in the DG hippocampal area [26]. Using four-weekold Long-Evans males treated with the KD for 4 subsequent weeks, it was found that rats fed with the KD showed increased social exploration in three different experimental settings, meanwhile there was no any difference in mobility or anxiety-related behaviors or working memory between the animals fed with the KD or standard rodent chow [27]. Taken as a whole, the current data suggest that early life daily KD treatment in normal rats KD may have no long-term adverse effects on most of the neurobehavioral parameters, but minor neurobehavioral damage may exist, which merit further investigations.
Knowledge regarding the effects of KD on brain gene expressions is limited. It has been reported that 8 weeks KD treatment reduced the levels of BDNF in the striatum of young Wistar rats [28]. Adult mice fed a ketogenic diet for 3 weeks increased dopaminergic activity in the motor and somatosensory cortex regions [29]. Simeone TA demonstrated that 10- to 14-day in vivo KD treatment altered the pathologic sharp waves (SPWs)- high-frequency oscillations (HFO) complexes generated by the hippocampal CA3 region of epileptic Kv1.1α knockout (KO) mice in vitro using extracellular multielectrode array recordings. The KD also improved spike-timing reliability of KO CA3 principal cells, decreased mossy fiber excitability, increased mossy fiber- CA3 paired-pulse ratios [30]. However, the effects of chronic KD treatment on related gene expression in hippocampus have been poorly investigated previously. Recently, we have demonstrated increased expression of ZnT-3, MT-3 as well as lipid metabolism-related ApoE, ApoJ and ACAT-1 in hippocampus following neonatal seizures which was inhibited by chronic KD treatment [17]. The results highlight zinc/ lipid transporter signals being potential targets of KD for the treatment of zinc/lipid peroxidation following developmental seizures. However, which signal plays the key role remains elusive.
Here, we have expanded our previous findings by showing, for the first time, that rats treated with KD (both NS+KD and NS+KOD groups) had a significant down-regulated expression of Apoa1, Pdk4 and upregulaion of ApoE, ANXN7 and cPLA2 in hippocampus when compared with non-KD treated NS+ND group (except ApoE in NS+KOD group). Meanwhile, ZnTs expressions were not significantly different among the three groups. It has been reported that ZnT-3 is regulated by hormones and fatty acids [31]. Lee et al. showed that estrogen decreased ZnT-3 expression and synaptic vesicle zinc levels in mouse brain through changing AP-3 delta expression [32]. These findings combined with our present results reinforce the importance of the lipid metabolism signals as an inductor of alterations in the gene expressions in hippocampus, and such changes might contribute to the pathophysiology of KD in normal brain disorders.
It is interesting to point out that the expression of cPLA2 in present study is quite in parallel with the neurobehavioral and cognitive changes in KD treated rats. The unregulated activation of cPLA2 has been well documented to be pivotal to the pathogenesis of several neurodegenerative diseases [33,34], such as Parkinson’s disease and Alzheimer’s disease [35,36]. Bate C and Williams A recently showed that the αSNinduced activation of cPLA2 resident within synapses in cultured neurons was responsible for synapse damage which was reduced by pretreatment with the cPLA2 inhibitors [37]. In a mice model of spinal cord injury (SCI), Liu et al. found that SCI significantly increased cPLA2 expression and activation. Remarkably, blocking cPLA2 ameliorated motor deficits, and reduced cell loss and tissue damage after SCI [38]. There are few studies on brain cPLA2 expression after epilepsy attack. Sandhya TL once found that cPLA2 immunoreactivity was increased in hippocampal neurons at 1 and 3 days and at 1, 2, 4 and 11 weeks in astrocytes after kainate injection suggesting that cPLA2 may be involved in neurodegeneration [39]. Combined with our present study, it is reasonable to speculate that cPLA2 may be an attractive therapeutic target for KD to ameliorate long-term brain injury following developmental seizures and deserves further study.
Conclusion
In conclusion, this study demonstrates that early life daily KD treatment in normal rats KD may have no long-term adverse effects on most of the neurobehavioral parameters, but minor neurobehavioral damage may exist. The hippocampal lipid metabolism signaling pathway, especially cPLA2, may be the target of protective effect of KD on long-term brain injury after developmental seizures which merit further investigations.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81471337, 81271458).
References
- Verrotti A, Iapadre G, Pisano S, et al. Ketogenic diet and childhood neurological disorders other than epilepsy: an overview. Expert. Rev. Neurother 17(1), 461-473 (2017).
- Bostock EC, Kirkby KC, Taylor BV. The Current Status of the Ketogenic Diet in Psychiatry. Front. Psychiatry 8(1), 43 (2017).
- Rhyu HS, Cho SY, Roh HT. The effects of ketogenic diet on oxidative stress and antioxidative capacity markers of Taekwondo athletes. J. Exerc. Rehabil 10(1), 362-366 (2014).
- Moriyama K, Watanabe M, Yamada Y, et al. Protein-Losing Enteropathy as a Rare Complication of the Ketogenic Diet. Pediatr. Neurol 1(10) 009 (2015).
- Eric Kossoff. Danger in the Pipeline for the Ketogenic Diet? Epilepsy. Curr 14(1), 343–344 (2014).
- Wibisono C, Rowe N, Beavis E, et al. Ten-Year Single-Center Experience of the Ketogenic Diet: Factors Influencing Efficacy, Tolerability, and Compliance. J. Pediatr 10(1), 1016 (2015).
- Desai AA, Thompson LM, Abdelmoity AT, et al. Management of symptomatic cholelithiasis while on ketogenic diet: a case report. Pediatr. Neurol 51(1), 439-440 (2014).
- Groleau V, Schall JI, Stallings VA, et al. Long-term impact of the ketogenic diet on growth and resting energy expenditure in children with intractable epilepsy. Dev. Med. Child. Neurol 56(1), 898-904 (2014).
- Lambrechts DA, Bovens MJ, de la Parra NM, et al. Ketogenic diet effects on cognition, mood, and psychosocial adjustment in children. Acta. Neurol. Scand 127(1), 103-108 (2013).
- Yuasa M, Matsui T, Ando S, et al. Consumption of a low-carbohydrate and high-fat diet (the ketogenic diet) exaggerates biotin deficiency in mice. Nutrition 29(1), 1266-1270 (2013).
- Ellenbroek JH, van Dijck L, Töns HA, et al. (2014) Long-term ketogenic diet causes glucose intolerance and reduced β- and α-cell mass but no weight loss in mice. Am. J. Physiol. Endocrinol. Metab 306(1), E552-E558.
- Sussman D, Germann J, Henkelman M. Gestational ketogenic diet programs brain structure and susceptibility to depression & anxiety in the adult mouse offspring. Brain. Behav 29(1), e00300 (2014).
- Sussman D, Ellegood J, Henkelman MA. Gestational ketogenic diet alters maternal metabolic status as well as offspring physiological growth and brain structure in the neonatal mouse. BMC. Pregnancy. Childbirth 13(1), 198 (2013).
- Shiohama T, Fujii K, Takahashi S, et al. Reversible white matter lesions during ketogenic diet therapy in glucose transporter 1 deficiency syndrome. Pediatr. Neurol 49(1), 493-496 (2013).
- Lambrechts DA, Bovens MJ, de la Parra NM, et al. Ketogenic diet effects on cognition, mood, and psychosocial adjustment in children. Acta. Neurol. Scand 127(1), 103-108 (2013).
- Giordano C, Marchiò M, Timofeeva E, et al. Neuroactive peptides as putative mediators of antiepileptic ketogenic diets. Front. Neurol 5(1), 63 (2014).
- Tian T, Ni H, Sun BL. Neurobehavioral Deficits in a Rat Model of Recurrent Neonatal Seizures Are Prevented by a Ketogenic Diet and Correlate with Hippocampal Zinc/Lipid Transporter Signals. Biol. Trace. Elem. Res (2015).
- Rothstein S, Simkins T, Nuñez JL. Response to neonatal anesthesia: effect of sex on anatomical and behavioral outcome. Neuroscience 152(1), 959-969 (2008).
- Escudeiro SS, Lima NB, Patrocínio MC, et al. Behavioral and neurochemical effects of alpha-lipoic Acid in the model of Parkinson's disease induced by unilateral stereotaxic injection of 6-ohda in rat. Evid. Based. Complement. Alternat. Med 571378 (2013).
- Guo Y, Zhang H, Gao J, et al. Study of genes associated with the 'anger-in' and 'anger-out' emotions of humans using a rat model. Exp. Ther. Med 9(1), 1448-1454 (2015).
- Ni H, Yan JZ, Zhang LL, et al. Long-term effects of recurrent neonatal seizures on neurobehavioral function and related gene expression and its intervention by inhibitor of cathepsin B. Neurochem. Res 37(1), 31-39 (2012).
- Sliwa A, Plucinska G, Bednarczyk J, et al. Post-treatment with rapamycin does not prevent epileptogenesis in the amygdala stimulation model of temporal lobe epilepsy. Neurosci. Lett 509(1), 105-109 (2012).
- de Araújo DP, De Sousa CN, Araújo PV, et al. Behavioral and neurochemical effects of alpha-lipoic Acid in the model of Parkinson's disease induced by unilateral stereotaxic injection of 6-ohda in rat. Evid. Based. Complement. Alternat. Med 571378 (2013).
- Beilharz JE, Maniam J, Morris MJ. Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats. Brain. Behav. Immun 7(1), 134-41 (2014).
- Sobesky JL, Barrientos RM, De May HS, et al. High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1β, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain. Behav. Immun 42(1), 22-32 (2014).
- Chwiej J, Skoczen A, Janeczko K, et al. The biochemical changes in hippocampal formation occurring in normal and seizure experiencing rats as a result of a ketogenic diet. Analyst 140(1), 2190-2194 (2015) .
- Kasprowska-Liśkiewicz D, Liśkiewicz AD, Nowacka-Chmielewska MM, et al. The ketogenic diet affects the social behavior of young male rats. Physiol. Behav 179(1), 168-177 (2017).
- Vizuete AF, de Souza DF, Guerra MC, et al. Brain changes in BDNF and S100B induced by ketogenic diets in Wistar rats. Life. Sci 92(1), 923-928 (2013).
- Church WH, Adams RE, Wyss LS. Ketogenic diet alters dopaminergic activity in the mouse cortex. Neurosci. Lett 571(1), 1-4 (2014).
- Simeone TA, Samson KK, Matthews SA, et al. In vivo ketogenic diet treatment attenuates pathologic sharp waves and high frequency oscillations in in vitro hippocampal slices from epileptic Kv 1.1α knockout mice. Epilepsia 55(1), e44-49 (2014).
- Smidt K, Rungby J. ZnT3: a zinc transporter active in several organs. Biometals 25(1), 1-8 (2012).
- Lee JY, Kim JH, Hong SH Estrogen decreases zinc transporter 3 expression and synaptic vesicle zinc levels in mouse brain. J. Biol. Chem 279(1), 8602-8607 (2004).
- Farooqui AA, Horrocks LA. Phospholipase A2-generated lipid mediators in the brain: The good, the bad, and the ugly. Neuroscientist 12(1), 245–260 (2006).
- Farooqui AA, Ong WY, Horrocks LA. Inhibitors of brain phospholipase A2 activity: Their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Phamacol. Rev 58(1), 591–620 (2006).
- Klivenyi P, Beal MF, Ferrante RJ, et al. Mice deficient in group IV cytosolic phospholipase A2 are resistant to MPTP neurotoxicity. J. Neurochem 719(1), 2634–2637 (1998).
- Sanchez-Mejia RO, Newman JW, Toh S, et al. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer’s disease. Nat. Neurosci 11(1), 1311-1318 (2008).
- Bate C, Williams A. α-Synuclein-induced synapse damage in cultured neurons is mediated by cholesterol-sensitive activation of cytoplasmic phospholipase A2. Biomolecules 5(1), 178-93 (2015).
- Liu NK, Deng LX, Zhang YP, et al. Cytosolic phospholipase A2 protein as a novel therapeutic target for spinal cord injury. Ann. Neurol 75(1), 644-658 (2014).
- Sandhya TL, Ong WY, Horrocks LA, et al. A light and electron microscopic study of cytoplasmic phospholipase A2 and cyclooxygenase-2 in the hippocampus after kainate lesions. Brain. Res 788(1), 223-231 (1998).