Review Article - (2018) Volume 8, Issue 5
Is testosterone perspective available for neurodegenerative diseases?
- Corresponding Author:
- Orcun Avsar
Department of Biotechnology Yeditepe University, Istanbul, Turkey
Tel: +905325937388
Abstract
Neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease are increasingly spreading worldwide. Neuroinflammation, deposition of misfolded proteins, oxidative stress, mitochondrial dysfunction, and excitotoxicity are main biological processes in the development of neurodegeneration. Furthermore, noncoding RNAs, genetic mutations, and environmental factors are involved in the development of neurodegeneration. Recent advances in the knowledge of testosterone shows that the concentrations of testosterone might be effective in the pathogenesis of the neurodegenerative diseases and testosterone has been implicated in the modulation of dopaminergic system. It has been proposed that the degree of the oxidative stress identifies whether testosterone affects neuronal function negatively or positively. In other words, testosterone can be neurotoxic or neuroprotective according to the environment. In the light of several evidences, testosterone should be evaluated as a crucial factor for the prevention, diagnosis, and treatment of neurodegenerative diseases by clinicians.
Keywords
Testosterone, Neurodegeneration, Parkinson’s disease, Alzheimer’s disease, Multiple sclerosis
Introduction
The sex hormone, testosterone is a pivotal molecule. Its concentrations might be determinative between healthy and pathological brains. Testosterone may be involved in the development of neurodegenerative and neuropsychiatric disorders and in this regard, testosterone might be neuroprotective and therefore show therapeutical properties for neurodegenerative diseases [1]. In this review, the potential evidences of testosterone for neurodegeneration and neurodegenerative diseases are discussed. Furthermore, therapeutic potential of exogenous testosterone is discussed for neuropsychiatric and neurodegenerative diseases.
Testosterone as a pivotal molecule
▪ General information about testosterone
Testosterone (T), a male sex hormone that subserves reproductive and sexual functions, was discovered in 1930s [2]. F.C. Koch and L. McGee isolated 20 mg testosterone from bovine testicles in 1927. Then, E. Laqueur and A. Butenandt isolated testosterone from human testicle and characterized it chemically in 1935 [3]. Testosterone is generated by testes of men, overies of females and adrenal glands and transported by the sex hormone binding globulins (SHBG) and albumins. The level of SHBG is enhanced with age in males, for this reason, the level of free testosterone is decreased and at the age of 60 and over, it reaches the lowest levels in males [4].
Testosterone has several effects on different body tissues, involving brain. In addition to its reproductive function, testosterone is responsible for body hair, libido, sexual function, reduced risk of osteoporosis, increased muscle mass, and the development of central nervous system. The free form of testosterone can pass the blood-brain barrier and affect neurons [5]. Low testosterone levels are associated with aging and several diseases such as diabetes, obesity, sexual disfunction, stroke [2]. On the other hand, excessive testosterone levels might be detrimential to the cardiovascular system [6].
▪ Testosterone and dopaminergic system
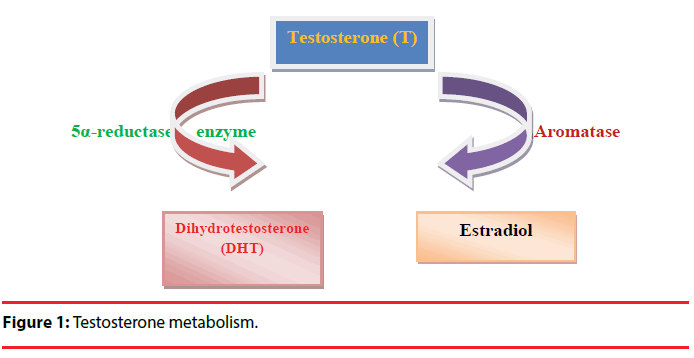
Testosterone is one of the most studied hormonal factors in human behavior. Testosterone is associated with human behaviors such as low risk aversion, social dominance, extraversion, mating effort, competitiveness, and affiliative behaviors [7]. Moreover, it was reported that there was a positive correlation between the quantity of spicy food (eating behavior) and endogenous salivary testosterone [8]. Testosterone may regulate the behaviors of organisms by affecting central dopaminergic system [9]. Testosterone affects brain functions including motor, cognitive, and motivational behaviors and dopamine release in midbrain circuits [10]. Testosterone modulates gene expression via activation of androgen receptors (ARs) in a direct way or may interact with estrogen receptors after aromatization. 5α-reductase enzymes (5αR) may convert testosterone to dihydrotestosterone (DHT). DHT has more affinity for AR than testosterone. Furthermore, testosterone might be converted into estradiol by the enzyme, aromatase (Figure 1) [10]. ARs are located in the dopaminergic neurons of the striatum and testosterone adjusts striatal dopaminergic function [11]. Moreover, it has been reported that catechol-o-methyltransferase (COMT), tyrosine hydroxylase (TH), monoamine oxidase A (MAOA), monoamine oxidase B (MAOB) gene expression levels in the substantia nigra of adolescent male rats are associated with testosterone [10].
Animal studies suggest that dopaminergic neurons are inhibited by circulating testosterone. Castration leads to enhanced dopamine release in striatum. On the other hand, some other studies propose that testosterone supplements may elevate striatal dopamine concentrations and dopamine turnover in animals [10].
Dopamine is the endogenous inhibitor of prolactin, therefore, dopamine agonists are used for the treatment of prolactinoma. Furthermore, prolactin reduces the level of testosterone in an indirect way. In the light of this knowledge, dopamine agonists may enhance testosterone concentrations by suppression of prolactin [12].
▪ Testosterone and oxidative stress
Oxidative stress is described as an unbalanced redox that includes excessive production of reactive oxygen species (ROS) and decreased antioxidant potency, involving enzyme-related activities such as catalase, superoxide dismutase, and the GSH oxidation-reduction system, and several non-enzyme-derived antioxidant substances [13]. Excessive production of ROS may lead to the injury and death of neurons. These ROS are significantly implicated in the pathogenesis of various neurodegenerative diseases such as Parkinson’s Disease (PD), glaucoma, amyotrophic lateral sclerosis (ALS), Alzheimer’s Disease (AD), Huntington’s Disease (HD), and HIV-associated neurocognitive disorder, via activation of glial cells (microglia and astrocytes) promoted by the stimulation of neurodegeneration and neuroinflammation [14].
It has been suggested that testosterone might be neuroprotective when oxidative levels are minimum, on the other hand, when the oxidative stress levels are incerased testosterone may elevate the damage of oxidative stress [14].
Oxidative stress is one of the most significant factors that causes dopaminergic system disturbances. The factor, aging, leads to dopamine auto-oxidation [9] and testosterone may enhance oxidative stress-related neurotoxicity in dopaminergic neurons and then lead to apoptosis [15]. Furthermore, it has been demonstrated that short-term and long-term stress may cause the reduction of blood testosterone levels and modified oxidative redox [13]. On the other hand, testosterone is able to protect against the damage of oxidative stress. It has been suggested that the degree of the oxidative stress adjust whether testosterone affects neuronal function negatively or positively [14].
▪ Testosterone and neurodegeneration
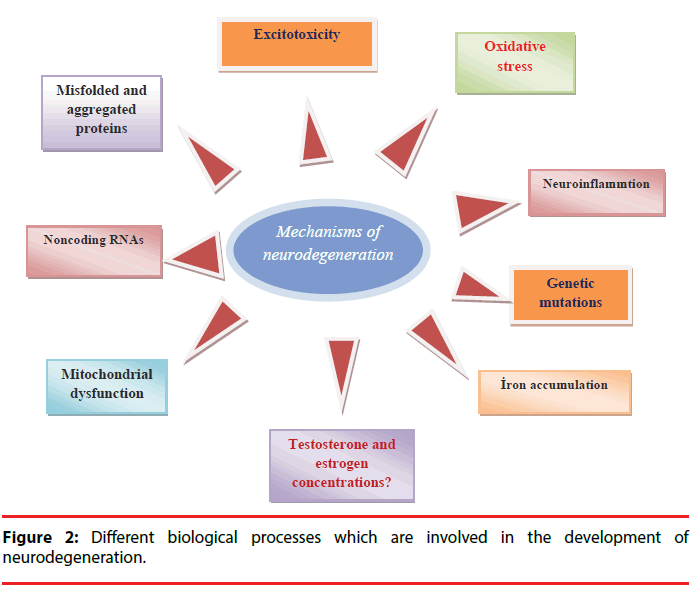
Brain is a very sensitive organ because of the unreplaceable nature of neurons in the body [16]. Neurodegeneration (irreversible tissue loss) defines the gradual and progressive deterioration in neuronal functions such as movement, motivation, and memory due to the structural alterations of neurons or neuron death. These changes give rise to accumulation of toxic proteins in the brain, and the loss of mitochondria functions [17]. Neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease are increasingly spreading worldwide. Neuroinflammation, deposition of misfolded proteins, oxidative stress, mitochondrial dysfunction, and excitotoxicity are main biological processes in the development of neurodegeneration and brain tissue is much more sensitive to oxidative stress than other tissues due to high levels of peroxidizable fatty acids, iron, and limited antioxidant activity [13]. Furthermore, noncoding RNAs, genetic mutations such as PARK1, PARK4, PARK8, PS1, PS2, FTD, APOE, and environmental factors such as pesticides, fungicides, substances from addictive drugs, heavy metals, viruses, might be involved in the development of neurodegeneration (Figure 2) [18,19].
Testosterone has bidirectional and converse effects -antioxidant and oxidative stressor- depend on the conditions in the brain [13]. It has been reported that testosterone can enhance oxidative stress-induced neurotoxicity in dopaminergic neurons in rats and then leads to loss of dopamine neurons and neurodegeneration [15]. Depletion of testosterone by orchieoctomy may increase the oxidative stress in brain. Castration in male mice gives rise to the loss of dopaminergic neurons in the striatum and nigra, and then, stimulation of PDassociated pathogenesis [20]. Moreover, it has been shown that testosterone is associated with cognitive decline in male subjects with increased oxidative stress levels [15]. These evidences might be one of the explanations of the mechanisms of testosterone in neurodegenerative diseases.
The secretion of sexual hormones, testosterone and estrogen, is reduced with aging. Particularly, estrogen exert neuroprotective activity in clinical and animal studies. Estrogen modulates beta-amyloid accumulation and acts as a neuroprotective agent [13]. Furthermore, estrogen has neuroprotective effects on AD and PD. In other words, estrogen is clinically effective for the treatment of PD and AD, and this action may occur by the conversion of testosterone into estradiol, the potent form of estrogen in the brain [13].
It has been demonstrated that testosterone is neuroprotective in healthy male subjects. On the other hand, the neuroprotective features of testosterone is reduced with increased age and then leads to enhanced ROS production and neurodegeneration [15].
Testosterone treatment for neurodegenerative diseases
Testosterone may have protective effects on neurodegenerative diseases such as Alzheimer’s disease (AD), mild cognitive impairment (MCI), amyotrophic lateral sclerosis (ALS), vascular cognitive impairment (VCI) or depression [5]. It has been suggested that testosterone sustains neurogenesis and promotes synaptic density in the hippocampus, preventing adult brain from neurodegeneration [21]. Testosterone treatment in human subjects might modulate the balance between protective and degenerative processes, therefore, it maintains synapses and neurons [22]. It has been demonstrated that testosterone might be neuroprotective in rat studies involving the most studied multiple sclerosis (MS) model, experimental autoimmune cephalomyelitis (EAE) [22]. It has been shown that testosterone has a vital role for the progression of spinal and bulbar muscular atrophy (SBMA). SBMA is a neurodegenerative disease and influences middle aged males due to the decreases testosterone levels and testosterone replacement therapy may improve the symptoms of the disease [23].
It has been reported that testosterone supplements might improve the symptoms (motor) in Parkinson’s Disease, and motor behavior deficiences in aged male rats and castrated male rats, and activate dopaminergic system [9]. The activated dopaminergic system by testosterone supplements may be associated with the decreased oxidative stress [9]. Meydan et al. has demonstrated that testosterone as a neuroprotective agent suppresses orchiectomyinduced oxidative damage [24]. Furthermore, the decreased levels of dopamine, tyrosine hydroxylase and dopamine transporters in old male rats are enhanced by testosterone supplements [9].
17β-trenbolone is an anabolic-androgenic steroid and used for muscle growth. Several bodybuilders inject this agent in large doses and for very long time in order to enhance muscle mass and strength. It has been shown that 17β-trenbolone is accumulated in the fetus and adult rat brain, particularly in the hippocampus. Aβ accumulation is modulated by 17β-trenbolone and 17β-trenbolone promotes the apoptosis of primary neurons of hippocampus in vitro. It has been suggested that 17β-trenbolone is implicated in neurodegeneration and the individuals who are exposed to this substance by several ways are affected [25].
Testosterone replacement therapy also effective in the treatment of verbal and spatial memory, and depression in men with increased age [26].
▪ Testosterone treatment for PD
Parkinson disease is a progressive neurodegenerative disesase characterized by motor disturbances such as rigidity, portural instability, bradykinesia, resting tremor, and Lewy body formations. Aging and gender are two main clinically risk factors for PD [27]. Moreover, male subjects have a 2-fold enhanced incidence for PD than female subjects [15]. The common age of onset is between 50 and 70. Loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) leads to dopamine deficiency, then the symptoms of PD [20]. In a study conducted with rats, Parkinsonismstimulating neurotoxin 1-methyl, 4-phenyl, 1,2,3,6-tetrahydropyridine caused to severe nigrostriatal dopamine depletion, reduced serum testosterone levels, and loss of Leydig cells [28]. It has been reported that testosterone deficiency is seen in individuals with PD. Testosterone deficiency is more prevalent in subjects with PD when compared with control groups [29]. Therefore, testosterone deficiency should be evaluated during the diagnosis of PD. Moreover, it has been demonstrated that testosterone replacement therapy may supply a significant improvement of the motor symptoms (resting tremor and fine motor control) in parkinsonian patients with testosterone deficiency [30].
Several patients with PD suffer from nonmotor symptoms involving anxiety, reduced energy level, depression and sexual dysfunction. Moreover, some patients also have sleep disorders and hypothyroidism [31]. Low free testosterone concentrations in males with PD might be related with nonmotor symptoms of PD such as fatigue, apathy, erectile dysfunction, reduced libido, mood alterations, and reduced enjoyment in life [32]. In different studies, it has been reported that testosterone replacement therapy contributes to improvements in libido, energy, mood, and strength [32]. Furthermore, the symptoms, which might be recoverable to anxiolytics, antidepressants, and antiparkinsonian drugs, could be responsive to the testosterone treatment [31]. Two major hypotheses are proposed to clarify the low testosterone concentrations: dopaminergic medications may lead to reduction of testosterone, or the testosterone level might be a biomarker of the pathology of PD-related brain regions such as hypothalamus [12]. Okun et al. [12] has reported that none of the dopaminergic medications, levodopa and pramipexole, reduced the testosterone concentrations in early PD. They have proposed that the low concentrations of testosterone might be the indicatory of the intrinsic PD pathogenesis.
Kenangil et al. has reported that free testosterone concentrations in male subjects with PD are not related with apathy and fatigue [33]. On the other hand, testosterone deficiency may give rise to the development of apathy and fatigue in men with PD. And it has been suggested that testosterone replacement therapy must be evaluated as a potential medication for apathy in males with PD [32].
It has been proposed that sudden loss of testosterone in young population might enhance the risk of PD in later life [20].
▪ Testosterone treatment for AD
Alzheimer’s Disease is a neurodegenerative disease and characterized by disturbances in language and speech, progressive cognitive decline, and sensorimotor dysfunctions [34]. Age-related loss of sex steroid hormones (estrogen and testosterone) has been correlated with the enhanced risk for AD in both females and males [35]. The decrease of testosterone levels is correlated with enhanced levels of Aβ peptides, neuronal cell death, and hyperphosphorylation of tau proteins [36]. For these reasons, the loss of testosterone and the metabolites, DHT and estradiol might be risk factors for the pathogenesis of AD and dementia. It has been shown that androgen modulates Aβ levels via AR and estrogen receptor (ER) and also testosterone may enhance the amounts of neprilysin in order to clear Aβ accumulation. Furthermore, the metabolite of testosterone, DHT, can elevate the levels of neprilysin and then decrease the amounts of Aβ [37]. AD is the most common form of dementia. Decreased concentrations of serum testosterone are significant AD risk factors for dementia in males and take roles in the alteration of the pathogenesis of AD [38].
It has been reported that higher free testosterone levels are associated with lower cerebral betaamyloid in females and free testosterone is positively associated with cognition in male subjects. It has been proposed that free testosterone may inhibit the accumulation of beta-amyloid in females and disrupt neurodegeneration in males [39]. In addition to those, the reduction of estrogen levels might be a significant risk factor for AD in females. It has been suggested that estrogen can reduce the concentrations of beta-amyloid peptides in neurons and also show antioxidant properties [26].
Testosterone has several neuroprotective effects in the brain such as reduction of Aβ accumulation, stimulation of neuron viability, and attenuation of tau hyperphosphorylation [35,38]. On the other hand, in studies conducted with men in old ages, clinically and statistically meaningful effects have not been observed and in the treatment group, adverse cardiovascular effects have been experienced [40].
It has been shown that testosterone treatment leads to reduction of the protein expression of Aβ 1-42 and in this way, testosterone may improve cognitive performance via AR to remove beta amyloid and increase synaptic plasticity [41]. Synaptic vesicle proteins are very important for dopamine neurotransmission and synaptic vesicle proteins recirculation is reduced by Aβ oligomers. It has been demonstrated that exogenous testosterone turns loss of synaptic vesicle proteins, Aβ-stimulated neurite damage, and exocytosis dysfunction [36]. Moreover, it has been shown that testosterone can enhance p75-nerve growth factor receptor and NGF and reduce the concentrations of beta-amyloid peptides in rat neurons [26]. All these evidences suggest that testoterone can be protective against AD-associated neurodegeneration [37].
▪ Testosterone treatment for multiple sclerosis
Multiple sclerosis (MS) is an assumed T cellmediated autoimmune and demyelinating disease with neuroinflammation, dysregulated immune response, and neurodegeneration which lead to demyelination and axonal damage and primarily affecting young females. MS is significantly associated with brain atrophy and loss of brain volume [42]. Vascular alterations such as the destabilization of blood brain barrier are the prevalent properties in MS lesions [43]. Gray matter (GM) atrophy has been proposed as a marker for neurodegeneration and disease progression in multiple sclerosis and gray matter lessions in MS are associated with synaptic loss and neuronal cell death [22]. Matrix metalloproteinases (MMPs) which are zincinvolving endopeptidases are involved in the pathogenesis of MS due to neuroinflammation and blood-brain barrier distruptors [44]. Environmental pollution is a risk factor due to the stimulation of glial activation, neuroinflammation, cerebrovascular damage, and oxidative stress [45] and oxidative stress is the main process in the pathogenesis of MS [46].
In male subjects, MS is associated with enhanced disease progression, cognitive decline, and brain atrophy and it has been suggested that testosterone can modulate MS pathogenesis by affecting immune system [47]. Testosterone has immunomodulatory effects and can reduce generation of IFNγ, IL-1β, TNFα and other proinflammatory molecules by macrophages and monocytes [48]; enhance secretion of antiinflammatory IL-10 by T cells; decrease the proliferation of T cell [49]. Testosterone and estradiol replacement therapies can improve the symptoms of MS [50] and are well-tolerated, safe, and neuroprotective [51] and testosterone treatment significantly slows down brain atrophy, increases natural killer cells [52,53].
There is no available effective therapies for cognitive impairment and neurodegeneration in MS [54]. It has been demonstrated that testosterone can increase cognitive performance, and synaptogenesis, on the other hand, it decreases dendritic and neuronal atrophy, microglial activation, and astrogliosis [54].
Conclusion
Neurodegenerative diseases are significant problems at both individual and population levels. The pathologies of neurodegenerative diseases are very complicated, therefore, their prevention and treatment are extremely challenging and currently few treatment options are available [55]. Specific drugs such as L-dopa for PD, donepezil, memantine for AD, riluzole for ALS, are available in order to minimize the symptoms [56]. On the other hand, ketogenic diet might be beneficial for neurodegenerative diseases by reducing the oxidative damage, supplying energy for focal brain hypometabolism, and enhancing mitochondrial biogenesis. Nevertheless, the long-term effects of ketogenic diet is not known [57,58].
Testosterone is involved in the development of central nervous system and maintains the normal function during maturation [59]. The free form of testosterone can pass the bloodbrain barrier and stimulate the differentiation of neurons and neurite outgrowth increase [1]. Age-related deficiency of testosterone might increase the neurodegeneration and lead to diseases such as AD and PD. The levels of oxidative stress designates whether testosterone replacement therapy has negative or positive effects. Testosterone replacement therapy might be useful under low oxidative stress conditions, on the other hand, it may lead to unintented consequences under increased oxidative stress conditions [60]. It has been reported that the levels of testosterone is low in PD and other neurodegenerative diseases. It is an increasing agreement that non-medical use of testosterone has a neurodegenerative potential and it has been demonstrated that only very high testosterone levels lead to neuronal excitotoxicity; on the other hand, lower concentrations might be protective [14].
All the studies remark the potential role of testosterone in neurodegeneration [14] and the translation of neuroprotective effects of testosterone into safe and effective treatments for neurodegenerative diseases are encouraging [10,21].
It requires more research to determine the mechanisms and pathways of testosterone therapies in neurodegenerative diseases. Testosterone therapy has possible side effects and it can exacerbate existent prostate cancer in some male subjects. For this reason, it has been recommended that prostate specific antigen levels should be monitored before and during testosterone treatment [1].
In conclusion, I deeply believe that testosterone should be studied in more depth for its promising property as a treatment in various neurodegenerative diseases. Furthermore, testosterone should be evaluated as a crucial factor for the prevention, diagnosis, and treatment of neurodegenerative diseases by clinicians.
Conflicts of Interest
Authors declared no conflict of interest.
References
- Gold SM, VoskuhL RR. Testosterone replacement therapy for the treatment of neurological and neuropsychiatric disorders. Curr. Opin. Investig. Drugs 7(7), 625-630 (2006).
- Shores MM. Testosterone treatment and cardiovascular events in prescription database studies. Asian. J. Androl 19(2), 1-7 (2017).
- Birgner C. Anabolic androgenic steroids and central monoaminergic systems. PhD thesis, Department of Pharmaceutical Biosciences, Uppsala University, USA (2008).
- Iqbal MJ, Dalton M, Sawers RS. Binding of testosterone and oestradiol to sex hormone binding globulin, human serum albumin and other plasma proteins: evidence for non-specific binding of oestradiol to sex hormone binding globulin. Clin. Sci (Lond) 64(3), 307-314 (1983).
- Białek M, Zaremba P, Borowicz KK, Czuczwar SJ. Neuroprotective role of testosterone in the central nervous system. Pol. J. Pharmacol 56(5), 509-518.
- Xie W, Ren M, Li L, et al. Perinatal testosterone exposure potentiates vasculardysfunction byERβ suppression in endothelial progenitor cells. PLoS One 12(1), e0182945 (2017).
- Määttänen I1, Jokela M, Hintsa T, et al. Testosterone and temperament traits in men: Longitudinal analysis. Psychoneuroendocrinology, 38(10), 2243-2248 (2013).
- Begue L, Bricout V, Boudesseul J, et al. Some like it hot: testosterone predicts laboratory eating behavior of spicy food. Physio. Behavior 139: 375-377 (2015).
- Cui R, Kang Y, Wang L, et al. Testosterone propionate exacerbates the deficits of nigrostriatal dopaminergic system and downregulates nrf2 expression in reserpine-treated aged male rats. Front. Aging. Neurosci 31(9), 172 (2017).
- Purves-Tyson TD, Handelsman DJ, Double KL, et al. Testosterone regulation of sex steroid-related mRNAs and dopamine-related mRNAs in adolescent male rat substantia nigra. BMC Neuroscience 13(1), 95 (2012).
- Siegel JA, Young LA, Neiss MB, et al. Estrogen, testosterone, and sequential movement in men. Behav. Neurosci 122(5), 955-962 (2008).
- Okun MS, Wu SS, Jennings D, et al. Testosterone level and the effect of levodopa and agonists in early Parkinson disease: results from the INSPECT cohort. J. Clin. Mov Disord 26(1), 1-8 (2014).
- Son SW, Lee JS, Kim HG, et al. Testosterone depletion increases the susceptibility of brain tissue to oxidative damage in a restraint stress mouse model. J. Neurochem 136(1), 106-117 (2016).
- Pomara C, Neri M, Bello S, et al. Neurotoxicity by Synthetic Androgen Steroids: Oxidative Stress, Apoptosis, and Neuropathology: A Review. Curr Neuropharmacology 13(1), 132-145 (2015).
- Holmes S, Singh M, Su C, et al. Effects of oxidative stress and testosterone on pro-inflammatory signaling in a female rat dopaminergic neuronal cell line. Endocrinology 157(7), 2824-2835 (2016).
- Angelova DM, Brown DR. Iron, aging, and neurodegeneration. Metals 5(1), 2070-2092 (2015).
- Kovacs GG. Molecular pathological classification of neurodegenerative diseases: Turning towards precision medicine. Int. J. Mol. Sci 17(2), 189 (2016).
- Salta E1, De Strooper B. Noncoding RNAs in neurodegeneration. Nat. Rev. Neurosci 18(10), 627-640 (2017).
- Nieoullon A. Neurodegenerative diseases and neuroprotection: current views and prospects. J. App. Med 9(1), 173-183 (2011).
- Khasnavis S, Ghosh A, Roy A, et al. Castration induces Parkinson disease pathologies in young male mice via inducible nitric-oxide synthase. J. Biol. Chem 288(29), 20843-20855 (2017).
- Romero-Martínez Á, Ruiz-Robledillo N, Moya-Albiol L. Depressive mood and testosterone related to declarative verbal memory decline in middle-aged caregivers of children with eating disorders. Int. J. Environ. Res. Public. Health 13(3), 286 (2016).
- Kurth F, Luders E, Sicotte NL, et al. Neuroprotective effects of testosterone treatment in men with multiple sclerosis. Neuroimage. Clin 4(1), 454-460 (2014).
- Chevalier-Larsen ES, Merry DE. Testosterone treatment fails to accelerate disease in a transgenic mouse model of spinal and bulbar muscular atrophy. Dis. Model. Mech 5(1), 141-145 (2012).
- Meydan S, Kus I, Tas U, et al. Effects of testosterone on orchiectomy-induced oxidative damage in the rat hippocampus. J. Chem. Neuroanat 40(4), 281-285 (2010).
- Ma F, Liu D. 17β-trenbolone, an anabolic-androgenic steroid as well as an environmental hormone, contributes to neurodegeneration. Toxicol. Appl. Pharmacol 282(1), 68-76 (2015).
- Hammond J, Le Q, Goodyer C. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J. Neurochem 77(5), 1319-1326 (2011).
- Holmes S, Abbassi B, Su C, et al. Oxidative stress defines the neuroprotective or neurotoxic properties of androgens in immortalized female rat dopaminergic neuronal cells. Endocrinology 154(11), 4281-4292 (2013).
- Ruffoli R, Giambelluca MA, Scavuzzo MC, et al. MPTP-induced Parkinsonism is associated with damage to Leydig cells and testosterone loss. Brain. Res 1229(10), 218-223 (2008).
- Okun MS, Fernandez HH, Rodriguez RL, et al. Testosterone therapy in men with Parkinson disease. Arch. Neurol 63(5), 729-735 (2006).
- Mitchell E, Thomas D, Burnet R. Testosterone improves motor function in Parkinson’s disease. J. Clin. Neurosci 13(1), 133-136 (2006).
- Okun MS, McDonald WM, DeLong MR. Refractory nonmotor symptoms in male patients with Parkinson disease due to testosterone deficiency: a common unrecognized comorbidity. Arch. Neurol 59(5), 807-811 (2002).
- Ready RE, Friedman J, Grace J, et al. Testosterone deficiency and apathy in Parkinson’s disease: a pilot study. J. Neurol. Neurosurg. Psychiatry 75(1), 1323-1326 (2004).
- Kenangil G, Orken DN, Ur E, et al. The relation of testosterone levels with fatigue and apathy in Parkinson’s disease. Clin. Neurol. Neurosurg 111(5), 412-414 (2009).
- Barron AM, Fuller SJ, Verdile G, et al. Reproductive hormones modulate oxidative stress in Alzheimer's disease. Antioxid. Redox. Signal 8(11-12), 2047-59 (2006).
- Carroll JC, Rosario ER The potential use of hormone-based therapeutics for the treatment of Alzheimer’s disease. Curr. Alzheimer. Res 9(1), 18-34 (2012).
- Lau CF, Ho YS, Hung CHL, et al. Protective effects of testosterone on presynaptic terminals against oligomeric β-amyloid peptide in primary culture of hippocampal neurons. Biomed. Res.Int 2014(1), 103906 (2014).
- Pike CJ, Carroll JC, Rosario ER, et al. Protective actions of sex steroid hormones in Alzheimer’s disease. Front. Neuroendocrinol 30(2), 239-258 (2009).
- Verdile G, Asih PR, Barron AM, et al. The impact of luteinizing hormone and testosterone on beta amyloid (Aβ) accumulation: Animal and human clinical studies. Horm. Behav 76(1), 81-90 (2015).
- Lee JH, Byun MS, Yi D, et al. Sex-specific association of sex hormones and gonadotropins, with brain amyloid and hippocampal neurodegeneration. Neurobiol. Aging 58(1), 34-40 (2017).
- Winslow BT, Onysko MK, Stob CM, et al. Treatment of Alzheimer disease. Am. Fam. Physician 83(12), 1403-1412 (2011).
- Huo DS, Sun JF, Zhang B, et al. Protective effects of testosterone on cognitive dysfunction in Alzheimer’s disease model rats induced by oligomeric beta amyloid peptide 1-42. J. Toxicol. Envir. Health 79(19), 856-863 (2016).
- Yokote H, Kamata T, Toru S, et al. Serum retinol levels are associated with brain volume loss in patients with multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin 3(3), 2055217317729688 (2017).
- Iacobaeus E, Sugars RV, Törngvist Andren A, et al. Dynamic changes in brain mesenchymal perivascular cells associate with multiple sclerosis disease duration, active inflammation, and demyelination. Stem. Cells. Transl. Med 6(10), 1840-1851 (2017).
- Boziki M, Grigoriadis N. An update on the role of matrix metalloproteinases in the pathogenesis of multiple sclerosis. J. Med. Chem 14(2), 155-169 (2018).
- Palacios N, Munger KL, Fitzgerald KC, et al. Exposure to particulate matter air pollution and risk of multiple sclerosis in two large cohorts of US nurses. Environ. Int 109(1), 64-72 (2017).
- Guo X, Namekata K, Kimura A, et al. ASK1 in neurodegeneration. Adv. Biol. Regul 66(1), 63-71(2017).
- Airas L Hormonal and gender-related immune changes in multiple sclerosis. Acta. Neurol. Scand 132(199), 62-70 (2015).
- Gold SM, VoskuhL RR Estrogen and testosterone therapies in multiple sclerosis. Prog. Brain. Res 175(1), 239-251 (2009).
- Bove R. Autoimmune diseases and reproductive aging. Clin. Immunol 149(2), 251-264 (2013).
- Antonio M, Patrizia F, Ilaria I, et al. A rational approach on the use of sex steroids in multiple sclerosis. Recent. Pat. CNS. Drug. Discov 3(1), 34-9 (2006).
- Sicotte NL, Giesser BS, Tandon V, et al. Testosterone treatment in multiple sclerosis: a pilot study. Arch. Neurol 64(5), 683-688 (2007).
- Spence RD, Voskhul RR. Neuroprotective effects of strogens and androgens in CNS inflammation and neurodegeneration. Front. Neuroendocrinol 33(1), 105-115 (2012).
- Gold SM, Chalifoux S, Giesser BS, et al. Immune modulation and increased neurotrophic factor production in multiple sclerosis patients treated with testosterone. J. Neuroinflammation 5(1), 32 (2008).
- Ziehn MO, Avedisian AA, Dervin SM, et al. Therapeutic testosterone administration preserves excitatory synaptic transmission in the hippocampus during autoimmune demyelinating disease. J. Neurosc 32(36), 12312-12324 (2012).
- Newberg AB, Serruya M, Wintering N, et al. Meditation and neurodegenerative diseases. Ann. N. Y. Acad. Sci 1307(1), 112-123 (2014).
- Dunkel P, Chai CL, Sperlagh B, et al. Clinical utillity of neuroprotective agents in neurodegenerative diseases: current status of drug development for Alzheimer’s, Parkinson’s and Huntington’s diseases, and amyotrophic lateral sclerosis. Expert. Opin. Investig. Drugs 21(9), 1267-1308 (2012).
- Paoli A, Bianco A, Damiani E, et al. Ketogenic diet in neuromuscular and neurodegenerative diseases. Biomed. Res. Int 2014(1), 474296 (2014).
- Horowitz AM, Villeda SA. Therapeutic potential of systemic brain rejuvenation strategies for neurodegenerative disease. F1000Res 6(1), 1291 (2017).
- Pan W, Han S, Kang L, et al. Effects of dihydrotestosterone on synaptic plasticity of the hippocampus in mild cognitive impairment male SAMP8 mice. Exp. Ther. Med 12(3), 1455-1463 (2016).
- Cunningham RL, Singh M, O’Bryant SE, et al. Oxidative stress, testosterone, and cognition among Caucasian and Mexican American men with and without Alzheimer’s disease. J. Alzheimers. Dis 40(3), 563-573 (2014).