Research Article - Neuropsychiatry (2017) Volume 7, Issue 5
Interictal Ionized Magnesium/ Total Serum Magnesium Ratio in Serbian Population with Drug Resistant Epilepsy- Whether is Severe Epilepsy in Fact Brain Injury?
- Corresponding Author:
- Dr. Gorica DjokiÃâÃâ¡, MD
clinical neurologist, neuropsychopharmacologist, PhD student in Neuroscience, Assistant Director, Clinic for Psychiatric Disorders “Dr Laza Lazarevic”, Visegradska 26, Belgrade, Serbia
Tel: 00381113636456
Fax: 00381113636461
Abstract
Introduction:
We hypothesized that our patients with drug resistant epilepsy have low interictal ionized magnesium/ total serum magnesium ratio to indicate on the protracted brain injury.
Objective:
The aim of this study was to examine Interictal ionized magnesium/total serum magnesium ratio in patients with drug resistant epilepsy and to consider illness and AEDs related predictors of the possible brain injury.
Methods:
Patients with drug resistant epilepsy of unknown cause were tested for interictal total serum magnesium concentrations and serum ionized magnesium concentrations at the endpoint visit 14 years later. Ionized magnesium/total serum magnesium ratio cut off point was 0. 60. Groups were monitored in relation to the: seizures types, seizures frequency, duration of epilepsy, appearance of status epilepticus (SE), and longest used first line antiepileptic drugs (AEDs).
Results:
According to our results, 60, 6% (N= 63) of the patients with drug resistant epilepsy of unknown cause had lower interictal ionized magnesium/total serum magnesium ratio (mean ratio 0.53 ± 0.05). Odds Ratio was 29.19 (95% CI 10.94 to 77.90%. In addition we have found significant differences among groups in: age (p=0.001), the length of suffering from epilepsy (p=0.017), the seizure frequency (p=0.000), the experiencing of the SE (p=0.000), and the longest used first line AEDs (p=0.012) with 54% of the phenobarbital in the study group.
Conclusion:
Patients with drug resistant epilepsy of unknown cause have low interictal ionized magnesium/ total serum magnesium ratio which can indicate on the protracted brain injury. More evidence is necessary to proclaim ionized magnesium as marker of the protracted brain injury induced by drug resistant epilepsy. Predictors for the low interictal ionized magnesium/total serum magnesium ratio in this group of patients are: status epilepticus, older age in relation with longer suffering from epilepsy, more frequent seizures, and longest total intake of the phenobarbital.
Keywords
Drug resistant epilepsy, Ionized magnesium, Brain injury, Status epilepticus, Seizure frequency, Phenobarbital, Interictal
Introduction
Proportion of the intractable seizures among people with epilepsy (PWE) is about 15.6%, and cumulative risk for developing inadequate seizure control is about 30%. Epilepsy with intractable seizures or drug resistant epilepsy, according to International League against Epilepsy (ILAE) is defined as “failure of adequate trials of two tolerated, appropriately chosen and used antiepileptic drugs schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom” [1].
Although the seizures are more frequently observed in patients with electrolyte disorders (hyponatremia, hypocalcemia, and hypomagnesemia), those kinds of seizures are classified as acute symptomatic seizures and they are unrelated to epilepsy and intractable seizures [2].
Magnesium can potentially modulate seizure activity because of its role as antagonist of the excitatory calcium influx through N-methyl-Daspartate (NMDA) receptors [3]. Accordingly, lower serum magnesium concentrations were found in PWE than in controls [4,5]. Reducing in serum magnesium concentration was more pronounced in PWE who had status epilepticus and severe epilepsy than in those with mild epilepsy [6]. But, hypomagnesemia can be induced by a variety of reasons, from starvation, alcohol intake, diarrhea and vomiting, renal tubular defects, to intake of antimicrobials or even antiepileptic drugs (AEDs) [7-9]. Therefore, numerous studies has proved that serum ionized magnesium monitoring is more precise clinical marker for brain condition [10]. Decrease of serum or plasma ionized magnesium levels associated with brain injury were found in experimental and clinical studies on child traumatic brain injury (TBI), severe close TBI in adults, open TBI, stroke, blast trauma, combined neurotrauma models, and epilepsy [11-17]. Serum ionized magnesium was significantly lower in PWE than in healthy controls, and levels were lower postictal (within 24hour after seizure) than interictal [18,19].
We hypothesized that our patients with drug resistant epilepsy (DRE) have low interictal ionized magnesium/ total serum magnesium ratio to indicate on the epilepsy induced protracted brain injury.
Objective
The aim of this study was to examine Interictal ionized magnesium/ total serum magnesium ratio in patients with drug resistant epilepsy and to consider illness and AEDs related factors that may contributed to the possible epilepsy induced brain injury.
Methods
The study was designed as a clinical cohort study which included patients who met criteria for DRE of unknown cause (epilepsies of unknown cause are epilepsies that in the past were termed “cryptogenic” including those that were considered “undetermined“, and even partially those that were considered idiopathic, but without precise genetic defect in which seizures are the core symptom of the disorder) [20]. The cohorts were defined in regards to presence or absence of decreased serum Interictal ionized magnesium/total serum magnesium ratio on the endpoint visit, 14 years after baseline visit.
This study is a part of a cohort study on the new onset interictal psychiatric disorders in patients with drug resistant epilepsy of unknown cause, and influence of the seizure frequency, illness duration, low interictal ionized magnesium/ total serum magnesium ratio, and experiencing status epilepticus on their occurrence.
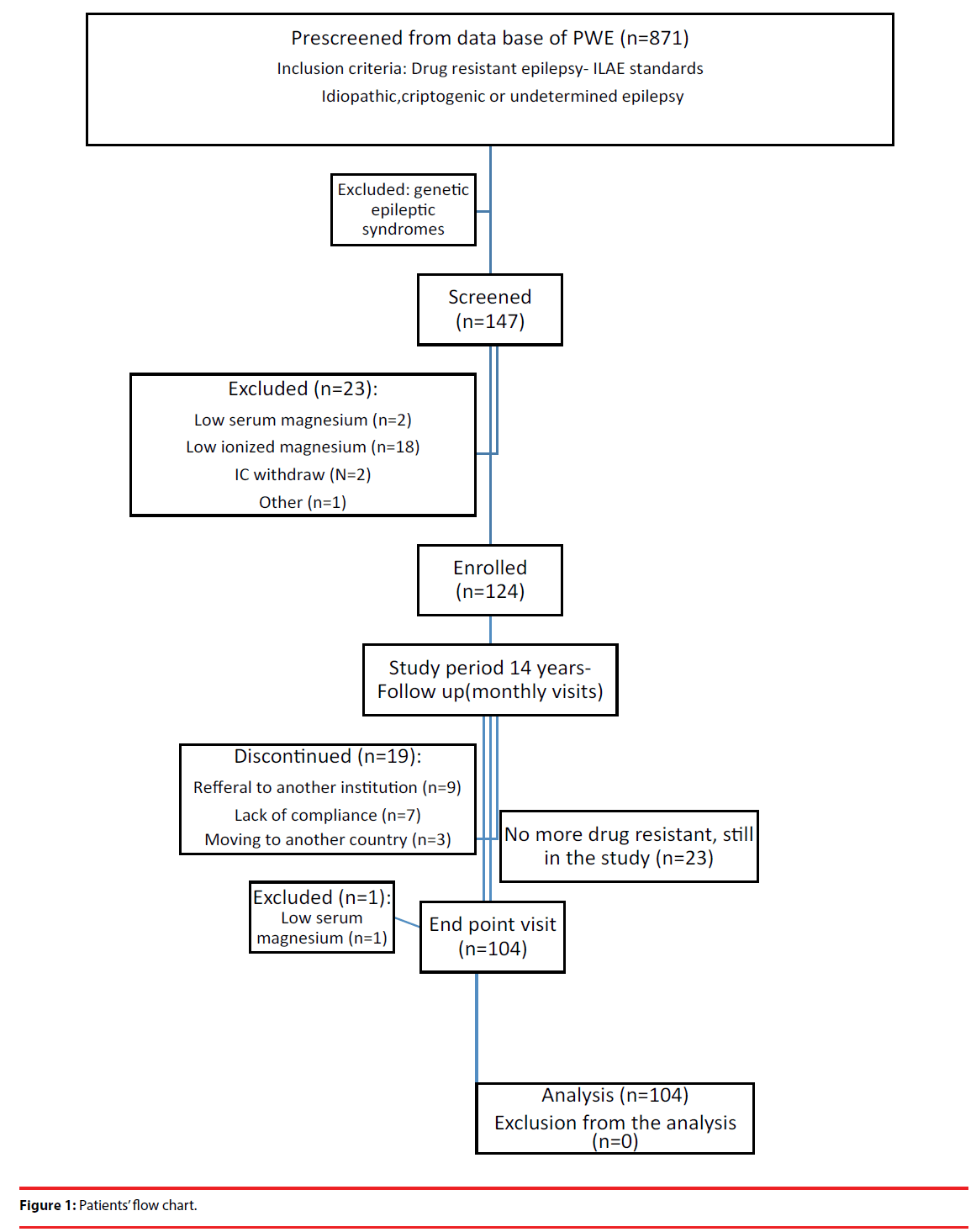
We have prescreened for the study patients from authors’ clinical database of PWE, and enroll them in the study during the year 2001. Study was completed in December 2015.
Patients were prescreened for the study according to inclusion criteria of the ILAE standards for drug resistant epilepsy of unknown cause (current terminology) i.e. idiopathic, cryptogenic and undetermined DRE, with exclusion of genetic epileptic syndromes (terminology used at the study start time) [21]. Exclusion criteria at screening visit and at endpoint visit was hypomagnesemia of any etiology, and additional exclusion criteria at screening visit was low Interictal ionized magnesium/ total serum magnesium ratio.
We have enrolled 124 female and male outpatients between the age of 18 and 50 years (less than 65 years at the time of endpoint visit), treated in Neurology Department, Institute for Neuropsychiatric Disorders “Dr Laza Lazarevic”(current name Clinic for Psychiatric Disorders „Dr Laza Lazarevic“). The study was completed after 14 years by 104 patients (Figure 1).
All patients gave their informed consent prior to screening. The study was approved by the ethical committee of the Clinic for Psychiatric Disorders “Dr Laza Lazarevic”3, and has been performed in accordance with the ethical standards.
Patients were tested for interictal [22] total serum magnesium concentrations and serum ionized magnesium concentrations at the endpoint visit 14 years later. Ionized magnesium/total serum magnesium ratio cut off point was 0. 60 [23].
Serum samples were stored in a freezer at -20°C until analyze was performed, but not longer than 7 days because of the samples stability. Total serum magnesium concentration was measured by a colorimetric method, and serum ionized magnesium concentration was measured by a NOVA 8 device with ion selective electrodes in both sampling on the same devices.
Study group (Ionized magnesium/total serum magnesium ratio lower than 0. 60) and control group (Ionized magnesium/total serum magnesium ratio higher than 0.60) were monitored in relation to the: seizures types, seizures frequency, duration of epilepsy, appearance of status epilepticus (SE) for at least once during illness, and longest used first line antiepileptic drugs (AEDs).
Seizure frequency was determined on the basis of the average seizure frequency during the study noted in a patient’s seizure calendar and average seizures frequency was assort in a two groups (one or more seizures per week, or one or more seizures per three months).
The experience of status epilepticus is determined on the basis of the documented appearance of the convulsive or non- convulsive SE for at least once during illness.
Longest used first line AEDs (carbamazepine, valproate, phenobarbital, or lamotrigine) was determined by longest total intake of the drug during the study, whether as a monotherapy or polytherapy.
Among the patients with partial seizures (N=49) most of them have partial complex seizures (N=37) diagnosed as temporal lobe epilepsy (TLE).
Between 104 patients who completed the 14 years long study, 23 of them are no more drug resistant due to new add-on AEDs therapy. Among 20 patients who didn’t complete the study are those who left the study for various reasons (lack of compliance, referral to another institution, moving to another country), or those with hypomagnesemia at endpoint visit (exclusion criteria at endpoint visit) (Figure 1).
Statistical Analysis
All collected data were organized and evaluated using dedicated software (IBM SPSS 24.0, USA) and were analyzed by descriptive statistical parameters and regression models. Descriptive statistical methods were represented by measures of central tendency (mean and median), variability (standard deviation and variation interval) and were expressed in number of patients and percentages. We used Medcalc for calculating odds ratio and diagnostic test evaluation calculator. For testing data of different categories (gender, age, illness related factors and therapy), Pearson’s χ2 and ANOVA tests were applied. We used linear regression models to examine illness and AEDs related predictors of the potential brain injury in patients with DRE of unknown cause. Level of statistical significance was set at p<0.05.
Results
According to our results, 60.6% (N=63) of the patients with drug resistant epilepsy of unknown cause had lower interictal ionized magnesium/ total serum magnesium ratio, while 39.4% (N= 41) had normal ratio. Odds ratio was 29.19 (95% CI 10.94 to 77.90), and Relative risk for having low interictal ionized magnesium/ total serum magnesium ratio in DRE was 3.07 (95% CI 2.36 to 4.00). Mean interictal Ionized magnesium/total serum magnesium ratio in the study group was 0.53 ± 0.05, while in the control group was 0.70 ± 0.06 (Table 1).
| Study group Lower IMg/Mg ¹ |
Control group Normal IMg/Mg² |
|
|---|---|---|
| N (%) | 63 (60.6) | 41 (39.4) |
| IMg/Mg ratio³ X ± SD; Med (min-max) |
0,53 ± 0,05; 0,55 (0,42-0,60) | 0,70 ± 0,07; 0,69 (0,61-0,90) |
| Odds ratio | 29.19 (95%CI 10.94 to 77.90); z statistic 6.737; P<0.0001 | |
| Relative risk for low IMg/Mg | 3.07 (95%CI 2.36 to 4.00); z stat 8.32; P<0.0001 | |
²Normal interictal ionizes magnesium/total serum magnesium ratio >0.60
³Interictal ionizes magnesium/total serum magnesium ratio
Table 1: Interictal ionized magnesium/ total serum magnesium ratio.
Distribution of demographic characteristics in the observed groups shows that there were no gender differences between the groups (p=0.452), while we found statistical significant age differences among groups (p=0.001), with odds ratio for having low ionized magnesium ratio after the age of 50 years of 2.93(95%CI 1.29 to 6.65) (Table 2).
| Demographic characteristics | Study group | Control group | Significance (p) |
|---|---|---|---|
| Gender N (%) Male Female |
41 (65.1) 22 (34.9) |
28 (68.3) 13 (31.7) |
a0.452 |
| Age X ± SD (min-max) |
53.08 ± 9.78 (32-64) | 46.05 ± 10.77 (32-64) | b0.001* |
| Odds ratio for older age (≥50) 2.93 (95%CI 1.29 to 6.65); z stat 2.57; P=0.01 | |||
Table 2: Distribution of the demographic characteristics in the groups.
The significant differences between groups were present compared to length of suffering from epilepsy (p=0.017) with odds ratio for longer illness duration (more than 35years) of 7.06 (95%CI 2.73 to 18.2), and seizure frequency (p=0.000), with odds ratio for more frequent seizures of 20.46 (95% CI 7.033 to 59.53%). There was no significant difference between groups relative to seizure types (p=0.530), with odds ratio for generalized seizures of 0.95 (95% CI 0.43 to 2.09) (Table 3).
| Study group | Control group | Significance (p) | |
|---|---|---|---|
| Duration of epilepsy (years) X ± SD (min-max) | 38.44 ± 10.11 (16-57) | 33.15 ± 11.21 (16-58) | ͨ0.017* |
| Odds ratio for longer illness duration (≥35 year) 7.06(95%CI 2.73 to 18.21); z stat 4.041; P=0.0001 | |||
| Type of seizures n (%) Generalised Partial |
33 (52,4) 30 (47,6) |
22 (53,7) 19 (46,3) |
ª0,530 |
| Odds ratio (generalized seizures) 0.95 (95% CI 0.43 to 2.09); z stat 0.128; P=0.90 | |||
| Seizure frequency n (%) At least one per week At least one in three months |
57 (90.5) 6 (9.5) |
13 (31.7) 28 (68.3) |
a0.000* |
| Odds ratio for more frequent seizures 20.46 (95% CI 7.033 to 59.53%); z stat 5.540; P<0.0001 | |||
Table 3: Illness related groups differences.
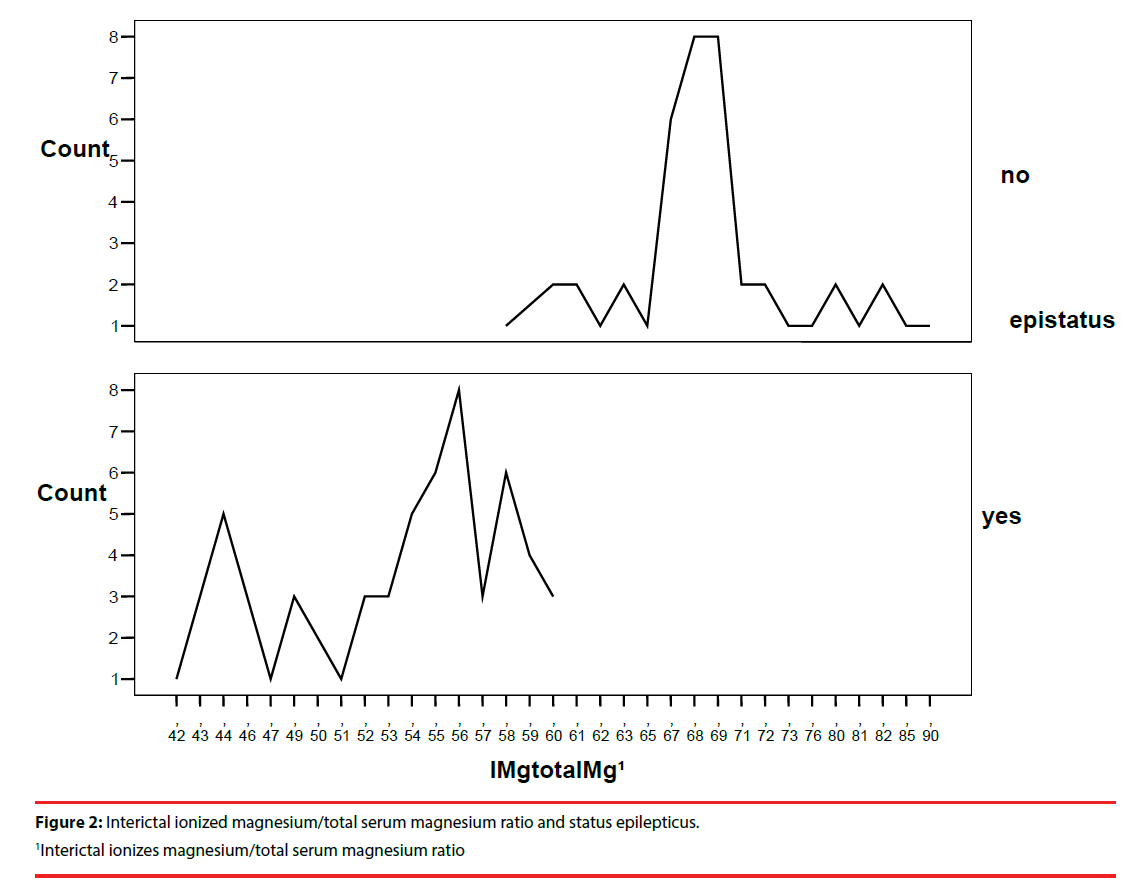
We found high statistical significance for the difference between two groups in the occurrence of the status epilepticus (p=0.000) with 95.2% of the patients who had the SE in study group, and relative risk for having low interictal ionized magnesium/total serum magnesium ratio was 14.67 (Table 4). Linear diagram of the interictal ionized magnesium/total serum magnesium ratio in regards to status epilepticus is in Figure 2.
| Study group | Control group | Significance (p) | |
|---|---|---|---|
| Status epilepticus n (%) | 60 (95,2) | 0 (0) | ª0,000* |
| Without status epilepticus | 3 (4,8) | 41 (100) | |
| Relative Risk for low IMg/Mg¹ 14.67(95%CI 4.92 to 42.72); z stat 4.82; P<0.0001 | |||
¹Interictal ionizes magnesium/total serum magnesium ratio
Table 4: Interictal ionized magnesium/ total serum magnesium ratio and status epilepticus.
Significant differences between the groups were also found in relation to the longest used first line AEDs (p=0.012), and study group AEDs profile was: 54% phenobarbital, 22.3% carbamazepine, 14.3% valproate, and 9.5% lamotrigine use. There was no AEDs pattern in control group where AEDs were presented in similar percentage (29.3% phenobarbital, 26.8% carbamazepine, 24.4% valproate, 19.5% lamotrigine).
Linear regression analysis marked older age (p=0.01), longer suffering from epilepsy (p=0.011), more frequent seizures (p=0.000), experiencing of the status epilepticus (p=0.000), and longest use of phenobarbital (p=0.037), as predictors of the lower ionized magnesium/ total serum magnesium ratio and potential brain injury.
Discussion
Numerous experimental and clinical studies have showed that lower ionized magnesium levels can be considered as a significant predictor of the brain injury and even of long- term neurobehavioral outcome [24]. As magnesium is a potential modulator of seizure activity because of its ability to antagonize the excitatory calcium influx through the NMDA receptor, it was expected to find lower ionized magnesium and higher ionized calcium levels during the seizures [18], and even to link low ionized magnesium levels to sudden unexpected death in epilepsy [25], or to consider magnesium supplementation in the overall management of the people with refractory epilepsy [26]. This is the first paper considering interictal ionized magnesium/total serum magnesium ratio in DRE.
Based on a present knowledge, we hypothesized that patients with drug resistant epilepsy have low interictal ionized magnesium/ total serum magnesium ratio to indicate on the epilepsy induced protracted brain injury. We found significantly lower interictal ionized magnesium/ total serum magnesium ratio in 60, 6 % of patients with DRE of unknown cause, thereby supporting the evidence on protracted brain injury in refractory seizures [18,19,26]. Additional evidence for this claim was found in this long term study, in which we have excluded patients with low Interictal ionized magnesium/ total serum magnesium ratio at screening visit, or patients with hypomagnesemia of any etiology at screening and endpoint visit. In this way, we have excluded even a least likely possibility that our patients had chronic magnesium deprivation such as in TRPM6 gene mutation, which lowers seizure threshold [27].
By examining the illness and AEDs related factors which can lead to a decrease in Interictal ionized magnesium/ total serum magnesium ratio, thus contributing to the possible brain injury, we have not found significant gender differences among study and control group. Previous studies in healthy adults have shown that ionized magnesium and total magnesium concentrations in men were not different from those in young women or in menopausal women [28], and similar pattern is present in our patients with DRE, but cannot be used to compare with our results.
We have not found enough results from literature to compare low ionized magnesium and types of seizures. We already know that decreased ionized magnesium levels provoke generalized seizures, so it is expected that we have more generalized than partial seizures. But in this “chicken or egg” situation where longer suffering from epilepsymore seizures-more frequent seizures induced lower Interictal ionized magnesium/total serum magnesium ratio, and low magnesium induced more seizures (mostly generalized), situation is not so clear. We have more generalized seizures but odds ratio was 0.95 for 95% CI 0.43 to 2.09, and differences were not significant. We assume that DRE of unknown cause is by itself a risk factor, and that the status epilepticus, more frequent seizures and longer suffering from epilepsy are more dominant for developing protracted brain injury than type of seizures.
Age, duration of epilepsy, and seizure frequency were significantly different among study and control group. There are not enough literature data in this field to compare with our results, but there are some studies on total magnesium, or ionized magnesium in pediatric population where age, seizure frequency and duration of treatment did not influence the plasma magnesium levels [29,30]. Our results indicate that lower interictal ionized magnesium/ total serum magnesium ratio is associated with older age (53.08 ± 9.78), longer suffering from epilepsy (38.44 ± 10.11), and more frequent seizures, which places our study on the side of other studies proving that intractable epilepsy damage the brain [31-33]. For more precise results, we have excluded 20 patients at the screening due to lower ionized magnesium or low total serum magnesium (Figure 1). Suppose that we didn’t exclude them from the study at the screening visit, our results on the “age dependent- longer suffering from epilepsy “model, and “longer suffering from epilepsy- more seizures” model would not have such importance.
Maybe the most intriguing, severe, and the previously least explored illness related factor which we monitored in our study, is occurrence of status epilepticus. There is a lot of evidence that magnesium is efficient in treatment of the SE [34], less evidence on lower serum magnesium levels in SE [35], but there is almost no evidence on the state of ionized magnesium during the SE or after it. More than 15% of the PWE had SE at least once during the illness, and patients with drug resistant epilepsy is even more at risk [36]. Status epilepticus in humans and animal models results in significant cerebral damage and in increased risk of subsequent seizures, associated with a characteristic pattern of neuronal loss particularly affecting the hippocampus [37-41]. Exactly as we knew that SE could cause brain damage, and that the low ionized magnesium levels are could be a clinical marker of the brain injury, we have anticipated that low ionized magnesium/total serum magnesium ratio would be in significant correlation with SE. This proved to be true because 95, 2% of the patients in the study group had SE, and that there is significant difference between the groups in the occurrence of the SE, with relative risk to have low interictal ionized magnesium/total serum magnesium ratio of 14.67 if you experienced status epilepticus. This proves that the appearance of status epilepticus is the most accurate factor for lower interictal ionized magnesium/total serum magnesium ratio and therefore for the protracted brain injury.
Some data in the literature show that some AEDs, in the first place phenobarbital, could cause brain damage, and another one claiming that other AEDs doesn’t cause additional brain damage [42,43]. Our results indicate that there was a significant difference among groups in relation to the longest used first line AEDs, and the study group AEDs profile was with the domination of the phenobarbital and the minimum of the valproate and lamotrigine, while at the same time the control group had no specific AEDs pattern. Our results support the evidence on the phenobarbital’s potential for brain damage.
Conclusion
Patients with drug resistant epilepsy of unknown cause have low interictal ionized magnesium/ total serum magnesium ratio which can indicate on the protracted brain injury. We assume that DRE of unknown cause is by itself a risk factor for the low interictal ionized magnesium/total serum magnesium ratio, but more evidence are necessary to proclaim ionized magnesium as marker of the protracted brain injury induced by drug resistant epilepsy. Predictors for the low interictal ionized magnesium/total serum magnesium ratio in this group of patients are: status epilepticus, older age in relation with longer suffering from epilepsy, more frequent seizures, and longest total intake of the phenobarbital.
References
- Kwan P, Arzimangolou A, Berg AT, et al. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission onTherapeutic Strategies. Epilepsia 51(6), 1069-1077 (2010).
- Nardone R, Brigo F, Trinka E. Acute Symptomatic seizures caused by electrolyte dsturbances. J. Clin. Neurol 12(1), 21-33 (2016).
- Sinert R, Zehtabchi S, Desai S, et al. Serum ionized magnesium and calcium levels in adult patients with seizures. Scand. J. Clin. Lab. Invest 67(3), 317-326 (2007).
- Yuena AWC, Sandera JW. Can magnesium supplementation reduce seizures in people with epilepsy? A hypothesis. Epilepsy. Res 100(1-2), 152-156 (2012).
- Oladipo OO, Ajala MO, Okubadejo N, et al. Plasma magnesium in adult Nigerian patients with epilepsy. Niger. Postgrad. Med. J 10(4), 234-237 (2003).
- Gupta SK, Manhas AS, Gupta VK, et al. Serum magnesium levels in idiopathic epilepsy. J. Assoc. Physicians. India 42(6), 456-457 (1994).
- Drueke TB, Lacour B. Magnesium homeostasis and disorders of magnesium metabolism. In: Feehally J, Floege J, Johnson RJ, eds. Comprehensive Clinical Nephrology(3rd edn), Philadelphia, PA, Mosby 136-138 (2007).
- Weber S, Schneider L, Peters M, et al. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J. Am. Soc. Nephrol 12(9), 1872-1881 (2001).
- Shah GM, Kirschenbaum MA. Renal magnesium wasting associated with therapeutic agents. Miner. Electrolyte. Metab 17(1), 58-64 (1991).
- Kahramana S, Ozgurtasb T, Kayali H, et al. Monitoring of serum ionized magnesium in neurosurgical intensive care unit: preliminary results. Clinica. Chimica. Acta 334(1-2), 211-215 (2003).
- Mendez DR, Corbett R, Macias C, et al. Total and Ionized Plasma Magnesium Concentrations in Children after Traumatic Brain Injury. Pediatric. Res 57(3), 347- 352 (2005).
- Dhandapani SS, Gupta A, Vivekanandhan S, et al. Serum ionic magnesium in traumatic brain injury. IJNT 2(2), 103-106 (2005).
- Cernak I, Savic VJ, Kotur J, et al. Characterization of plasmamagnesium concentration and oxidative stress following graded traumatic brain injury inhumans. J. Neurotrauma 17(1), 53-68 (2000).
- Bayir A, Ak A, Kara H, et al. Serum and cerebrospinal fluid magnesium levels, Glasgow Coma Scores, and in-hospital mortality in patients with acute stroke. Biol. Trace. Elem. Res 130(1), 7-12 (2009).
- Cojocaru IM, Cojocaru M, Burcin C, et al. Serum magnesium in patients with acuteischemic stroke. Rom. J. Intern. Med 45(3), 269-273 (2007).
- Cernak I, Radosevic P, Malicevic Z, et al. Experimental magnesium depletion in adultrabbits caused by blast overpressure. Magnes. Res 8(3), 249-259 (1995).
- Cernak I. Animal models of Head Trauma. NeuroRx 2(3), 410-422 (2005).
- Sinert R, Zehtabchi S, Desai S, et al. Serum ionized magnesium and calcium levels in adult patients with seizures. Scand. J. Clin. Lab. Invest 67(3), 317-326 (2007).
- Sood AK, Handa R, Malhotr RC, et al. Serum,CSF, RBC & urinary levels of magnesium & calcium in idiopathic generalised tonic clonic seizures. Indian. J. Med. Res 98(1), 152-154 (1993).
- Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of the epilepsies: Report of the Commission on Classification and Terminology 2005-2009. Epilepsia 51(4), 676-685 (2010).
- Engel J, International League against Epilepsy. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia 42(6), 796-803 (2001).
- Fisher RS, Scharfman HE, DeCurtis M. He can we identify ictal and interictal abnormal activity? Adv. Exp. Med. Biol 813(1), 3-23 (2014).
- Greenway DC, Hindmarsh JT, Wang J, et al. Reference interval for whole blood ionized magnesium in a healthy population and the stability of ionized magnesium under varied laboratory conditions. Clin. Biochem 29(6), 515-520 (1996).
- Bareyre FM, Saatman KE, Helfaer MA, et al. Alterations in Ionized and Total Blood Magnesium After Experimental Traumatic Brain Injury: Relationship to Neurobehavioral Outcome and Neuroprotective Efficacy of Magnesium Chloride. J. Neurochem 73(1), 271-280 ().
- Scorza FA, Cavalheiro EA, Cysneiros RM, et al. Serum magnesium and sudden unexpected death in epilepsy: A curious clinical sign or a necessity of life. Epilepsy. Res 101(3), 293-294 (2012).
- Yuen AW, Sander JW. Can magnesium supplementation reduce seizures in people with epilepsy? A hypothesis. Epilepsy. Res 100(1-2), 152-156 (2012).
- Osborn KE, Shytle RD, Frontera AT, et al. Addressing Potential Role of Magnesium Dyshomeostasis to Improve Treatment Efficacy for Epilepsy: A Reexamination of the Literature. J. Clin. Pharmacol 56(3), 260-265 (2016).
- Muneyyirci-Delale O, Dalloul M, Nacharaju VL, et al. Serum ionized magnesium and calcium and sex hormones in healthy young men: importance of serum progesterone level. Fertil. Steril 72(5), 817-822 (1999).
- Oladipo OO, Ajala MO, Okubadejo N, et al. Plasma magnesium in adult Nigerian patients with epilepsy. Niger. Postgrad. Med. J 10(4), 234-237 (2003).
- Al-Janabi JM, Marbut MM, Ahmed BS, et al. Determination of calcium & magnesium in the serum of epileptic patients. Tikrit. Med. J 11(2), 41-43 (2005).
- Bergen DC. Do seizures harm the brain? Epilepsy. Curr 6(4), 117-118 (2006).
- Kälviäinen R, Salmenpera T.Do recurrent seizures cause neuronal damage? A series of studies with MRI volumetry in adults with partial epilepsy. Progr. Brain. Res 135(1), 279-295 (2002).
- Wang B, Meng L. Functional brain network alterations in epilepsy: A magnetoencephalography study. Epilepsy. Res 126(1), 62-69 (2016).
- Zeiler FA, Matuszczak M, Teitelbaum J, et al. Magnesium sulfate for non-eclamptic status epilepticus. Seizure 32(1), 100-108 (2015).
- Jamil U, Badshah M, Nomani AZ, et al. Serum calcium and magnesium abnormalities in patients with status epilepticus: a single centre tertiary care experience. PJNS 10(3), 21-26 (2015).
- Fountain NB. Status epilepticus: risk factors and complications. Epilepsia 41(2), 23-30 (2000).
- Bronen RA. The status of status:seizures are bad for your brain health. Am. J. Neuroradiol 21(10), 1782-1783 (2000).
- Vingerhoets G. Cognitive effects of seizures. Seizure 15(4), 221-226 (2006).
- Arman F, Kaya D, Dincer A, et al. Serial EEG and MRI changes in status epilepticus-induced excitotoxic neuronal necrosis. Epileptic. Disord 13(4), 446-451 (2011).
- Cock HR, Hargreaves IP, Heales SJR, et al. Mitochondrial dysfunction associated with neuronal death following status epilepticus in rat. Epilepsy. Res 48(3), 157-168 (2002).
- Shorvon S, Ferlisi M. The Treatment of Super-refractory Status Epilepticus A Critical Review of Available Therapies and a Clinical Treatment Protocol. Brain 134(10), 2802-2818 (2011).
- Zhu HX, Cai FC, Zhang XP. Experimental stud on possibility of brain damage induced by chronic treatment with phenobarbital, clonazepam, valproic acid and topiramate in immature rats. Zhonghua. Er. Ke. Za. Zhi 45(2), 121-125 (2007).
- Jellet AP, Jenks K, Lucas M, et al. Standard dose valproic acid does not cause additional cognitive impact in rodent model of intractable epilepsy. Epilepsy. Res 110(1), 88-94 (2015).