Research Article - Neuropsychiatry (2016) Volume 6, Issue 5
Impact of working memory training on hot executive functions (decision-making and theory of mind) in children with ADHD: a randomized controlled trial
- Corresponding Author:
- Aitana Bigorra
Child and Adolescent Mental Health Unit, Hospital Universitari Mutua Terrassa, Barcelona, Spain
Tel: +34 93 736 59 03
Fax: +34 937887641
Abstract
Background
Children with attention deficit/hyperactivity disorder (ADHD) have deficits in working memory (WM) and in hot executive functions (EFs) that may be related. The main aim of this study was to analyze the efficacy of computerized Cogmed Working Memory Training™ (CWMT) on hot EF decision-making and theory of mind (ToM). Correlational analyses between WM and hot EFs at baseline were also performed to better clarify the nature of this interrelationship.
Methods
66 children with combined-type ADHD, aged 7 to 12 years, were included. Participants were randomized (1:1) to an experimental group (CWMT) (n=36) or a control group (non-adaptive training). At baseline, 1-2 weeks, and 6 months after the intervention, participants were assessed using performance-based measures of WM (backward digit span, letter-number sequencing of WISC-IV, and backward spatial span of WMS-III), decision-making (Iowa Gambling Task), and ToM (Happé’s Strange Stories and Folk Psychology Test).
Results
Statistically significant correlations were found between WM and ToM measures at baseline, but not between WM and decision-making. On adjusted multiple linear regression analysis, there were no significant improvements in any of the outcome measures at either time point.
Conclusions
There was no relationship between WM and decision-making in ADHD. A relationship was found between WM and ToM, but CWMT did not show far-transfer effects on ToM deficits in ADHD. Other implications of these results are discussed.
Keywords
Attention-deficit/hyperactivity disorder (ADHD), Working memory, Computerized cognitive training, Hot executive functions, Decision making, Theory of mind
Introduction
Attention deficit/hyperactivity disorder (ADHD) is the most common neurodevelopmental disorder of childhood [1]. Children with ADHD may have considerable difficulty in academic, psychosocial, and community functioning [1].
Deficits in executive functions (EFs), although not universal, are very common in ADHD individuals [2-5]. These functions are the mental capacities needed to formulate, plan, and perform the actions required to reach an objective [6]. Among the EFs, working memory (WM) have been shown to be repeatedly deficient in ADHD, as described in several meta-analyses [7-10]. WM facilitates active maintenance and manipulation of information without external stimuli for enough time to enable use of this information for some purpose [11]. WM has assumed a prominent role as a primary neurocognitive deficit or endophenotype in extant models of ADHD [2,8]. An intervention aimed at improving this cognitive ability would, therefore, be of considerable value in the treatment of ADHD.
Developmental theorists have proposed a neuropsychological subdivision of EFs. Zelazo et al. [12] differentiate between “cool” more abstract-cognitive EFs, such as WM, response inhibition, and cognitive flexibility, and “hot” affective EFs that involve incentives and motivation. Hot EFs include [13,14]: 1) delayed gratification and affective decision-making [15]; and 2) identification of the desires, thoughts, feelings, and intentions of others, and one’s own, also known as theory of mind (ToM) [16,17]. Although there is also evidence to the contrary [18-22], recent and increasingly robust evidence shows that ADHD individuals have deficits in ToM [19,23-32] and in decision-making [33- 35]. Deficits in hot EFs in ADHD could be due to deficits in general regulatory processes. For example, Barkley’s ADHD model [36] is particularly relevant in the context of a potential contribution of cool EFs to social cognition deficits in ADHD, and some authors have specifically noted that WM contributes to hot EF processes [37].
Several studies have described a relationship between cool EFs and ToM in children and adolescents with normal development [38-43] and in neurodevelopmental disorders such as in ADHD [29,44-47]. One specific cool EF domain more strongly associated with ToM is WM [40,41,48-51], probably because social cognition tasks require an individual to keep relevant social information in mind and to flexibly evaluate and process this information [52]. There are also other reasons to suspect that cool EFs and ToM might be related:1) evidence from brain-imaging studies has identified the frontal lobes as the seat of ToM abilities and cool EFs [53-55]. 2) ToM acquisition emerges with improvements in cool executive tasks in preschool age [56]. 3) individual differences in cool EFs and ToM correlate in individuals with normal development, even after adjusting for the effects of age and intellectual ability [38,39]. Furthermore, there may be directionality in this relationship, such that cool EFs predict ToM performance over time [57,58]. In view of the scarcity of studies that have examined this possibility, additional research including intervention research and longitudinal data is certainly needed.
There is some controversy regarding the relationship between decision-making and cool EFs. Some authors argue that cool EFs and decision-making are related and specifically cite WM, as WM provides the mechanism to hold on-line representations of various options and scenarios over a period of time [59-61]. Several studies have reported a role for WM in performing decision-making tasks [62,63]. This relationship may be asymmetrically dependent because decision-making seems to be influenced by the intactness or impairment of WM, but WM is not dependent on the intactness of decision-making [64]. On the other hand, some studies have found no relationship between WM and decision-making [65,66].
Klingberg et al. developed Robomemo® Cogmed Working Memory Training™ (CWMT), a computerized WM training program with several auditory and visuospatial WM tasks that are presented as attractive games designed for children [67]. This training has been used in various populations and has been effective for improving certain cognitive functions and psychiatric symptoms [67-71]. In healthy adults and in ADHD, CWMT is reported to produce changes in brain activity in areas involved in WM [72-75] and to facilitate dopaminergic transmission [76], which plays an important role in this cognitive function.
.The effect of training on non-trained task performance can be differentiated into neartransfer effects (post-training improvement of performance in tasks similar to the training tasks) and far-transfer effects (post-training improvement in tasks that are different in nature or appearance from the training tasks) [77]. Fartransfer effects occur when two different tasks share an underlying processing component and neuroanatomical areas or neural circuits [78].
In summary, despite growing evidence of the presence of ToM and decision-making deficits in ADHD, it remains unclear whether there really is a relationship between these cognitive skills and cool EFs such as WM. This also raises questions about whether improving cool EFs in ADHD could improve hot EFs in this population. To our knowledge, there are no studies evaluating the effectiveness of cognitive training in hot EFs in ADHD.
The main aim of this study was to analyze the far-transfer effect of an intervention using the Robomemo® CWMT on decision-making and ToM in a sample of children with ADHD with or without comorbid disruptive behavior disorders, by conducting a randomized, doubleblind, placebo-controlled, parallel-group clinical trial with an active control group and a 6-month post-intervention follow-up. An additional aim in this study was to analyze the relationship (correlation) between WM and decisionmaking and ToM in baseline in this sample of patients. Our hypotheses were that WM and ToM and decision-making would be related and that CWMT would produce far-transfer improvements in these cognitive skills.
Materials and Methods
▪ Study design
For the main objective, we conducted a randomized, double-blind, placebo-controlled, parallel-group clinical trial in which participants were randomized (1:1) to an experimental group (CWMT) or a control group (non-adaptive training). For the secondary objective, we performed a correlational study.
▪ Participants
A power analysis was calculated assuming the criterion of 1 SD group difference in visuospatial and verbal WM performance-based tasks because, in the absence of increased WM capacity, it is theoretically unclear why WM training should lead to improvements in far-transfer tasks [79]. We assumed 1 SD group difference, a risk of α=5% and a statistical power (1-β) of 95%, and a dropout rate of 20%. The sample size included 66 subjects.
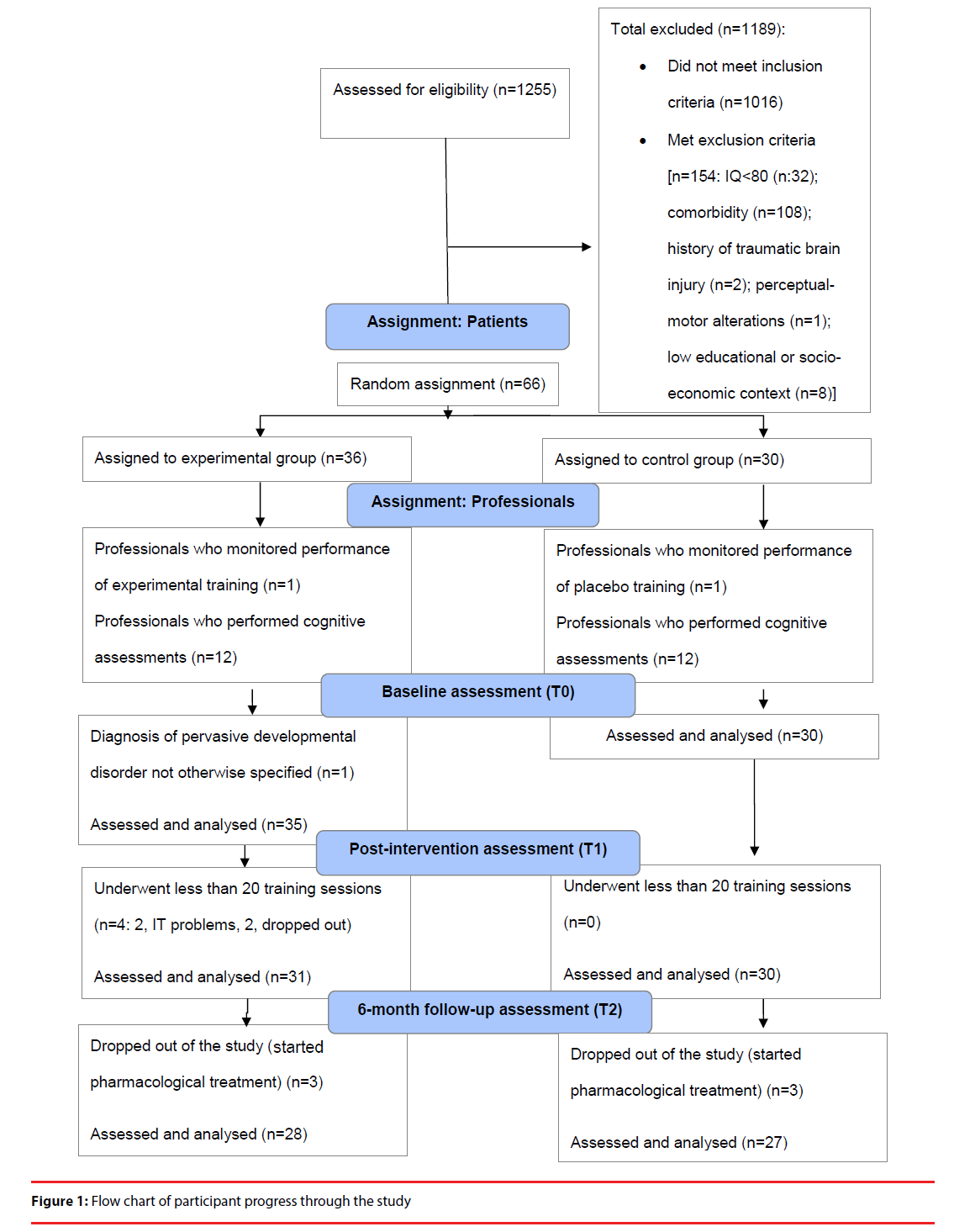
In total, 66 outpatients from the Child and Adolescent Psychiatric Unit of the Mutua de Terrassa University Hospital participated in the study. All had been diagnosed with combinedtype ADHD according to the DSM-IV-TR criteria. Comorbidity with other DSM-IV-TR disruptive behavior disorders (oppositional defiant disorder or conduct disorder) or elimination disorders was accepted. All diagnoses were confirmed using the Kiddie-Schedule for Affective Disorders and Schizophrenia, Present and Lifetime version (K-SADS-PL) [80] semistructured interview, which was administered to the participants’ parents. Other inclusion criteria included: 1) age between 7 and 12 years; 2) T score on the Conners ADHD index for parents and teachers > 70 at diagnosis; 3) no previous psychological or pharmacological treatment for ADHD; 4) access to a personal computer with an Internet connection. Exclusion criteria included: 1) IQ < 80; 2) comorbidity with autism spectrum disorder, psychosis, affective or anxiety disorder, consumption of toxic substances, learning disorder; 3) history of traumatic brain injury in the last two years; 4) perceptual-motor abnormalities that would preclude the use of a computer. Participants whose educational or socioeconomic context would make it unlikely for families to comply with the study requirements or follow the treatment procedure (families who did not speak Spanish or were monitored by social services due to suspected abuse/neglect) were also excluded from the study. Participants who participated in fewer than 20 training sessions or who initiated other pharmacological or psychological treatment during study participation were excluded from the subsequent data analysis.
A professional from the research team enrolled the participants and assigned them to either study group by random allocation using a computergenerated sequence. Study group allocation was blinded to the children, their families, their teachers, and the professionals who performed the cognitive assessments. Participants, their families, and their teachers were not aware of the differences between the experimental and control training (i.e., automatic adjustment of difficulty). The double-blind condition was maintained in all evaluations conducted throughout the study.
Following a thorough description of the study, verbal assent was obtained from the children and written informed consent from the parents. Upon completion of the study, participants in the control group were offered CWMT.
This study adhered to the principles outlined in the current legislation regarding clinical investigation (Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects, World Medical Association, 2004, Spanish Organic Law 15/1999 on Personal Data Protection, and Spanish Law 41/2002 on Patient Autonomy) and was approved by the Clinical Research Ethics Committee of the Mutua de Terrassa University Hospital. This study is registered as ISRCTN00767728 (www. controlled-trials.com).
This study forms part of a broader line of research on the effects of CWMT in ADHD children. A previous publication [81] analyzed the effects of CWMT on cool EFs, clinical symptoms, functional impairment, and academic achievement. Results from the same participant population are being published separately because each article describes results related to different objectives and theoretical aspects, which allows further analysis of the results without overextending a single publication.
▪ Intervention
The experimental group underwent the CWMT RoboMemo® (2005, Cogmed Cognitive Medical Systems AB, Stockholm, Sweden), involving the following WM tasks: visuospatial, auditory, and location memory, plus tracking of moving visual objects. The level of difficulty was automatically adjusted to the performance of each participant, thus generating a prolonged cognitive demand that exceeded existing capacity limits to keep the task challenging throughout the training phase and thereby maximize WM performance gains [82]. Each training session included 90 trials and lasted 30 to 45 minutes. Participants attended 5 sessions per week over a 5-week period for a total of 25 sessions. The control group (nonadaptive training) engaged in the MegaMemo (2005, Cogmed Cognitive Medical Systems AB, Stockholm, Sweden), which consists of the same WM tasks as CWMT but without adjustment for difficulty. The remaining characteristics were the same for both groups, and both training programs were the translated Spanish versions.
After randomization, participants were given the respective training programme (CWMT or nonadaptive training) on a CD that contained 25 training sessions. Training was conducted in the patient’s home, under the supervision of a family member. The training included performance feedback on each task and a reinforcement game at the end of each session. The family was advised to add an additional reward at the end of each session. The response to each session, training time and number of sessions completed were recorded in an Internet database. A member of the research team (coach) examined this information on a weekly basis and contacted each family via telephone to ensure adherence to the rules and to answer questions. Participants in the analysis received no other pharmacological or psychological treatment until the end of their participation in the study, as was verified by asking the families and checking the records of participants’ visits to the Unit.
▪ Measures
An improvement index score was calculated for participants in the experimental group by subtracting the start index (results for training days 2 and 3) from the max index (results from the two best training days).
The Wechsler Intelligence Scale for Children (WISC-IV) [83] was administered to the entire sample to check that all participants met the inclusion criteria (IQ > 80).
Outcome measures: Assessments of the outcome measures were conducted at baseline (T0), at 1 to 2 weeks post-training (T1), and at 6 months post-training (T2). Participants, their parents and teachers, and the professionals who performed the cognitive assessments were blinded to each child’s group assignment. Cognitive assessments were administered by appropriately trained psychology graduates in two sessions no more than one week apart and always in the same order.
For the evaluation of WM, we used: 1) backward digit span of the Wechsler Intelligence Scale for Children-IV (WISC-IV) [83] to measure verbal WM, 2) letter-number sequencing of the WISCIV [83] to measure verbal WM, and 3) backward spatial span of the Wechsler Memory Scale-III (WMS-III) [84] to measure visuospatial WM.
To evaluate decision-making, we used the Iowa Gambling Task (IGT) [85], an experimental paradigm designed to mimic real-life decisionmaking situations in the way it factors uncertainty, reward and punishment.
To evaluate ToM, we used: 1) Happé’s Strange Stories [86], a measure of advanced cognitive ToM [52], which is the ability to understand “cold” mental states, ie, infer others’ thoughts and beliefs [87]; and 2) The Folk Psychology Test [88], the children’s version of the Reading the Mind in the Eyes Test, adapted from the adult version [89] that aims to assess “mind-reading” ability by understanding emotional states through the expression of the eye region. It is a measure of advanced affective ToM [52], namely the ability to understand “hot” mental states, i.e., infer others’ emotions [87] (Supplementary Information includes a more detailed description of outcome measures).
▪ Statistical analysis
A descriptive statistical analysis was performed using the variables of age, sex, years of schooling, and comorbid disorders. The chi-square test or Fisher exact test was used, when appropriate, to compare baseline categorical variables between the groups and the Student t test or Mann-Whitney U test was used for quantitative variables.
We computed composite scores for WM and ToM because the measure of a cognitive ability is more robust when obtained by combining several tasks that measure the same processes. This reflects their shared performance or ability [79]. The arithmetic mean of the corresponding standardized scores was calculated as the final composite score. The WM composite score included backward digit span and letter-number sequencing of the WISC-IV and backward spatial span of WMS-III. The ToM composite score included Happé’s Strange Stories and Folk Psychology Test.
To evaluate the association between WM and ToM and decision-making, the Pearson correlation coefficient or Spearman’s rho was calculated at baseline, when appropriate.
To study the far-transfer effect of CWMT on ToM and decision-making, score changes between evaluations at study time points T0, T1, and T2 (T1-T0, T2-T1, T2-T0) were used as variables and analyzed using a general linear model, adjusted for age, sex and presence of a disruptive behavior disorder. In the evaluation of ToM (Happé’s Strange Stories and Folk Psychology Test), the WISC-IV (Wechsler Intelligence Scale for Children) Vocabulary subtest [83] was added as a predictor variable in the adjusted analysis because some studies have suggested a relationship between verbal ability and ToM [90,91]. The analyses were conducted as complete case analyses, i.e., did not include missing values. The effect size (d’), ie, the difference between the scores obtained (T1-T0, T2-T1, T2-T0) for each group divided by the pooled standard deviations of both groups at T0 [92], and the 95% CI were calculated and classified as small (0.2), moderate (0.5), or large (0.8). Statistical tests were conducted assuming two-tailed contrasts with an alpha significance level of 5%. The Statistical Package for the Social Sciences (SPSS®, version 17.0) was used for the statistical analyses.
The flow chart showing the participants’ progress through the study is presented in Figure 1. Of the 65 participants analyzed at T0, a total of 6.15% (n=4) completed fewer than 20 training sessions (2 due to technical problems, 2 who dropped out) and were not included in the subsequent data analysis. All other participants (93.85%) completed the 25 training sessions within a mean of 35.15 calendar days (SD: 3.15), with no statistically significant differences between the groups in this respect (Z=-0.54, df=59, p=0.59). Furthermore, 9.2% (n=6) started pharmacological treatment between T1 and T2 and were excluded from the study. There were no significant differences in the percentage of dropouts between the experimental and control groups in any study period (Fisher exact test: from T0 to T1: X2=3.65, df=1, p=0.08; from T1 to T2: X2=0.18, df=1, p=0.51; from T0 to T2: X2=2.41, df=1, p=0.12). Another participant was excluded from the final data analysis due to a diagnosis of pervasive developmental disorder not otherwise specified. Missing values refer to measures not administered for organizational or technical reasons (T0: 1 IGT; T1: 1 IGT). The study was conducted between June 2010 and December 2012.
Results
▪ Sociodemographic results
The demographic and clinical characteristics of the participants at T0 (baseline) are shown in Table 1. No significant differences between the groups were found for any of these variables or for the performance-based measures.
| Experimental group | Control | Statistical value | p-value | |
|---|---|---|---|---|
| Girls, % | 60 (n=21) | 50 (n=15) | 0.65 (χ2) | 0.46 |
| Age, years, mean (SD) | 8.79 (1.75) | 9.04 (1.68) | 0.44 | |
| Years of schooling, mean (SD) | 2.40 (1.80) | 2.57 (1.59) | -0.767 (Z) | 0.47 |
| Elimination disorder, % | 2.86 (n=1) | 6.67 (n=2) | 0.533 (χ2) | 0.59 |
| Oppositional defiant disorder, % | 31.43 (n=11) | 23.33 (n=7) | 0.529 (χ2) | 0.58 |
| Conduct disorder, % | 0 | 0 | --- | |
| IQ, mean (SD) | 100.63 (12.66) | 96.57 (11.26) | 1.91 (Z) | 0.18 |
| Ethnicity, % | 3.03 (χ2) | |||
| Spanish | 47.69 (n=31) | 43.08 (n=28) | ||
| Latin American | 3.08 (n=2) | 1.54 (n=1) | ||
| Other | 3.08 (n=2) | 1.54 (n=1) | 1.00 | |
| Race, % | 0.43 (χ2) | |||
| White | 45 (n=29) | 48 (n=27) | ||
| Arabic | 3.08 (n=2) | 0 (n=0) | ||
| African | 1.54 (n=1) | 3.08 (n=2) | ||
| American Indian | 4.61 (n=3) | 1.54 (n=1) | 0.51 | |
| Marital status of parents, % | 3.91 (χ2) | |||
| Married | 36.92 (n=24) | 35.38 (n=23) | ||
| Separated/divorced | 16.92 (n=11) | 7.69 (n=5) | ||
| Never married/single | 0 (n=0) | 3.08 (n=2) | 0.14 | |
| Years of schooling of parents*, mean (SD) | 11.63 (3.20) | 10.87 (2.94) | 0.437 (χ2) | 0.36 |
* Parent’s years of schooling was take as the highest value between the mother and father.
Table 1: Baseline sociodemographic and clinical characteristics of participants, and p-value of the difference between the groups.
▪ Relationship between WM and ToM/ decision-making
Statistically significant Pearson correlations were found between WM composite score and ToM composite score at baseline (r=0.47, p<0.001). Correlations were also significant between WM and each separate ToM measure: Happé’s Strange Stories (r=0.36, p=0.003) and Folk Psychology test total score (r=0.43, p<0.001). To calculate the correlations between WM and IGT, we used Spearman’s rho because the IGT total net score last 40 cards variable did not follow a normal distribution at baseline (p<0.05 in Kolmogorov-Smirnov test of normality). No significant correlations were found at baseline between IGT total net score last 40 cards and WM composite score (rho=-0.01, p=0.96).
▪ Efficacy of CWMT on hot EF
The mean improvement index in the experimental group was 30 (SD: 13.04). The mean and SD cognitive measurements at T0, T1, and T2 for the two groups are shown in Table 2.
| T0 | T1 | T2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Mean | SD | n | Mean | SD | n | d’* (95% CI) | Mean | SD | n | d’** (95%CI) | d’*** (95% CI) | |
| Cognitive measurements | |||||||||||||
| Working memory composite score | E | -0.00 | 0.79 | 35 | 0.29 | 0.66 | 31 | 0.81 (0.30 to 1.32) |
0.04 | 0.83 | 28 | -0.69 (-1.19 to -0.19) |
0.12 (-0.67 to 0.61) |
| C | 0.01 | 0.69 | 30 | -0.30 | 0.83 | 30 | -0.04 | 0.80 | 27 | ||||
| Iowa Gambling Task: total net score last 40 cards | E | 0.47 | 7.60 | 34 | 0.19 | 10.04 | 31 | 0.14 (-0.35 to 0.63) |
-0.64 | 13.80 | 28 | 0.17 (-0.32 to 0.66) |
0.30 (-0.19 to 0.79) |
| C | 0.13 | 9.41 | 30 | -1.31 | 6.74 | 29 | -3.56 | 7.26 | 27 | ||||
| Theory of Mind composite score | E | 0.06 | 0.83 | 35 | -0.01 | 0.80 | 31 | -0.18 (-0.67 to 0.31) |
-0.11 | 0.84 | 28 | -0.23 (-0.72 to 0.26) |
-0.41 (-0.90 to 0.08) |
| C | -0.07 | 0.89 | 30 | 0.01 | 0.83 | 30 | 0.11 | 0.78 | 27 | ||||
E, Experimental; C, Control; d’, effect size (small effect size, 0.2; moderate effect size, 0.5; large effect size, 0.8); * Comparison between T1 and T0 scores; **Comparison between T1 and T2 scores; ***Comparison between T2 and T0 scores.
Table 2: Mean values for cognitive measurements at baseline (T0), post-intervention (T1), and 6-month follow-up (T2) in the experimental and control groups.
The results of the general linear model analysis are shown in Table 3. There were no statistically significant differences between the groups for the last two IGT blocks of 20 choices (second half of the task) at any point in time (T1-T0: t=-1.44, df=4, p=0.89, T2-T1: t=1.20, df=4, p=0.24, T2- T0: t=0.78, df=4, p=0.44), and effect sizes were small (T1-T0: d’=0.14, 95% CI: -0.35 to 0.63; T2-T1: d’=0.17, 95% CI:-0.32 to 0.66; T2-T0: d’=0.30, 95% CI: -0.19 to 0.79). The single significant predictive variable was age, seen at T1 to T2 (t=2.06, df=4, p=0.04), with a positive beta (0.29), indicating that older children showed better performance.
| T1-T0 | T2-T1 | T2-T0 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor variable* | R2 ** | p of Beta*** | 95% CI for B *** | Predictor variable* | R2 ** | p of Beta*** | 95% CI for B *** | Predictor variable* | R2 ** | p of Beta*** | 95% CI for B *** | |
| Iowa Gambling Task (IGT): total net score, last 40 cards | - | -0.03 | 0.89 | -6.07 to 5.26 | age | 0.02 | 0.24 | -2.48 to 9.81 | - | -0.02 | 0.44 | -4.89 to 11.04 |
| Theory of Mind composite score | - | -0.08 | 0.45 | -0.60 to 0.27 | - | -0.08 | 0.93 | -0.43 to 0.40 | - | -0.10 | 0.57 | -0.64 to 0.35 |
Table 3: Regression analysis for differences in hot EFs at T1-T0, T2-T1 and T2-T0.
No statistically significant differences between the groups for ToM composite score were recorded at any point in time (T1-T0: t=-0.76, df=4, p=0.45; T2-T1: t=-0.09, df=4, p=0.93; T2-T0: t=-0.58, df=4, p=0.57), and effect sizes were small (T1-T0: d’=-0.18 95% CI:-0.67 to 0.31; T2-T1: d’=-0.23, 95% CI:-0.72 to 0.26) or small to moderate (T2-T0: d’=-0.41, 95% CI:-0.90 to 0.08) (Table 3). The same analysis produced similar results when ToM variables were considered separately (Folk Psychology Test: T1-T0: p=0.77; T2-T1: p=0.98; T2- T0: p=0.77; Happé’s Strange Stories: T1-T0: p=0.30; T2-T1: p=0.66; T2-T0: p=0.37). The complete results of this analysis are not included, but are available upon request.
Discussion
The results obtained in this study indicate, firstly, that WM relates differently with the two hot EFs evaluated, as WM and ToM show a correlation, but WM and decision-making do not. Secondly, an intervention using the Robomemo® CWMT in a sample of children with ADHD yielded no far-transfer effects post-training or at 6-months’ follow-up on hot EFs, decision-making, or advanced affective and cognitive ToM. To our knowledge, this is the first study analyzing the effectiveness of cognitive training on hot EF decision-making and ToM deficits in ADHD.
The correlational analyses performed between WM and ToM and decision-making at baseline makes it possible to elucidate different reasons why no such far-transfer effects were found. On the one hand, the results indicate that the lack of post-training improvement in decisionmaking was due to the absence of a relationship between WM and decision-making in ADHD. Additionally, our results indicate that ToM and WM are related in the sample of children with ADHD analyzed and, therefore, that CWMT is not effective in improving these cognitive skills and does not show far-transfer effects on ToM in ADHD.
The relationship between WM and ToM found is consistent with other results reported in the literature in subjects with normal development [40,41,48-51], and in subjects with attention and conduct problems [93], but to our knowledge, this is the first study to demonstrate a relationship between WM and ToM in ADHD. Although other studies have found a relationship between other EFs and ToM in ADHD [29,44-47], the relationship between WM and ToM seen in this study highlights the primary role of WM deficits in this neurodevelopmental disorder.
Despite the relationship between WM and ToM, CWMT does not improve ToM post-training or at 6-months’ follow-up in a sample of children with ADHD, which suggests that CWMT does not produce far-transfer effects in this cognitive skill. This absence of far-transfer effects was not explained by a lack of near-transfer WM improvements. In a previous publication with the same sample [81] in a randomized, doubleblind, placebo-controlled, parallel-group clinical trial with an active control group and a 6-month post-intervention follow-up, CWMT was seen to improve post-training WM with a large effect size, and the improvement remained significant over the long term [81]. Further, other fartransfer effects after CWMT were found, such as short and long term improvements in cool EFs, ADHD symptoms, and functional impairment related to school learning [81]. In contrast, other studies with ADHD samples have not found fartransfer effects with CWMT [94-97]. This has seriously questioned the effectiveness of such training, because finding evidence of far-transfer effects in cognitive training is by far the aspect considered most relevant to demonstrate its effectiveness [70]. Furthermore, some authors have noted methodological limitations in research on Cogmed [79,98,99], thus generating much controversy in the literature. To our knowledge, this is the first study analyzing the effectiveness of CWMT on ToM deficits in ADHD.
The absence of far-transfer effects in ToM in this study may be related to the specificity of the stimuli used in the CWMT. In one study [100], WM training with neutral material was compared to training with emotional material; only WM training including emotional material produced transfer to an affective executive control task (emotional Stroop task). The authors concluded that studies relying solely on neutral material may fail to target processes specific to the manipulation and processing of affective information; hence, affective effects would be selective to affective executive training [100].
More speculatively, the results of our study could indicate deficits in WM do not have a causal relationship with deficits in ToM in ADHD. The correlation between ToM and WM found in this study, and in previous ones, including longitudinal correlations, are an insufficient demonstration of causality, since any observed relationship may be mediated by some unknown third factor. The design used here (randomized controlled trial) allowed us to explore the possible existence of a causal relationship between WM and ToM and decision-making [101]. It is possible that the reason of the lack of improvement in ToM after cognitive WM training was that WM and ToM do not have a causal relationship. ToM deficits in ADHD may be causally related to other cognitive deficits [38,52,57,66,102] or may reflect primary difficulties, but not secondary consequences of more general cognitive dysfunctions [27]. Additional randomized trials with cognitive WM training that evaluate the effects on ToM are needed to confirm this possibility.
The absence of a relationship between WM and decision-making is consistent with results from other correlational studies [65,66,103] and also with results from other studies using an experimental design. For example, in a study with methadone maintenance patients, CWMT did not improve decision-making [104]. All these results are consistent with the absence of a relationship between WM and decision-making and with separable pathway models in ADHD [105-107], which include the dissociable contributions of (cool) executive dysfunction and motivational dysfunction. For example, in the dual-pathway model proposed by Sonuga-Barke [108], two dissociable neurodevelopmental pathways can lead to ADHD: The first is the executive dysfunction pathway, a top-down dysregulation characterized by poor inhibitory control, set-shifting, and reorienting of attentional resources. This pathway is subserved by cortical and subcortical networks (dorsolateral prefrontal cortex, dorsal anterior cingulate cortex, supramarginal gyrus, dorsal caudate nucleus, frontal eye fields, and supplementary motor cortex) [109]. The second is the motivational dysfunction pathway, a bottom-up dysregulation characterized by delay aversion associated with fundamental alterations in reward mechanisms [108]. It is subserved by frontolimbic circuits (subgenual and orbitofrontal cortices, amygdala, hippocampus, and ventral striatum) [110]. Additionally, the results obtained in this study,together with those from a previous report [81], indicate that CWMT can improve cool EFs but not decision-making in ADHD and, therefore, these two pathways show different responses to treatment.
Another explanation for the present results is that WM resources are necessary but not sufficient for the development of decision-making; that is, these cognitive functions develop independently from WM, but WM is relevant to the expression or application of these skills [64]. This hypothesis is based on a series of studies showing that an overload in WM worsens performance in tasks such as decision-making [62,63]. The design used in this study does not rule out this possibility.
Another possible explanation for the absence of effects of training on hot EFs could be related to the characteristics of the training used. In the experimental group, the level of difficulty was automatically adjusted to each participant’s performance, leading to prolonged cognitive demand that can be frustrating [111]. This could have minimized the effects of training on hot EFs, since children with ADHD have higher cognitive difficulties in situations that generate anger, frustration or negative emotions [112,113].
This study has some limitations: We cannot ensure that there are ToM and decision-making deficits in the sample used in this study because we did not have a sample of healthy comparison subjects. Although there is abundant evidence of the existence of hot EF deficits in ADHD, consensus on this issue is incomplete. Some studies have found no deficits in decisionmaking [20,22] or ToM [18,19,21] in ADHD. Limitations related to the sensitivity and ecological validity of some measures may explain the inconsistencies described in the literature, especially the ToM measures, which probably fail to capture the complexities of social interaction in the real world [114].
Several investigators in a research line focusing on differences between ADHD and conduct disorder based on underlying brain substrates have reported that hot EF deficits are specific to conduct disorder, but not to ADHD [115], although Groen [33] report evidence to support the contrary, for example. The absence of deficits in these areas could explain why improvements were not observed with training.
Linking to this argument, it may be that inclusion of ADHD children with comorbid disruptive behavior disorders in the sample hampered detection of changes in the outcome measures, since the skills related to hot EFs may differ in those disorders [25,115]. Nonetheless, the statistical analyses controlled for comorbidity with oppositional defiant disorder.
It may be difficult to draw conclusions about the relationship between WM and ToM and decision-making because these skills may continue to develop until late adolescence [116,117]. This notion is partially supported by the results of our study, because older children showed less difficulty in a decision-making task from post-training to 6 months’ follow-up. Further, some authors have argued that the IGT is too difficult for children [20], although it has been used in other studies in this population. Perhaps the inclusion of older subjects would have provided clearer results.
Due to the comprehensive evaluation used, we had to deal with the risk of committing a Type I error if we analyzed the measures separately. On the other hand, we risked committing a Type II error if we corrected for multiple comparisons using strict criteria. Instead, we chose to compute robust composite measures when possible. The analyses were not conducted on an intent-totreat basis, but rather as complete case analyses.
The results cannot be generalized to ADHD children with IQ<80, to children with comorbidities other than disruptive behavior disorders or elimination disorders, to children whose educational or socioeconomic context would make it unlikely for families to comply with the treatment procedure, to children under < 7 or > 12 years of age, or to children who have already received psychological or pharmacological treatment for ADHD.
Conclusions
Conclusions Robomemo® CWMT did not improve hot EFs decision-making and ToM in a sample of ADHD children at post-training or at 6 months of follow-up. This is explained primarily by the absence of relationship between decision-making and WM in this sample of children with ADHD, which in turn supports the view that different pathways exist in ADHD, with dissociable contributions of decision-making and cool EF deficits that respond differently to WM training. Secondly, because the results indicate the presence of a relationship between WM and ToM, the lack of improvement in this cognitive ability posttraining and at the 6-month follow-up seems to indicate that CWMT does not produce far-transfer effects on ToM. It should be noted, however, that there are other possible explanations (such as lack of a causal relationship between WM and ToM) and, consequently, the results require replication.
Acknowledgements
Maribel Ahuir, Llanos Artigao, Clara Barba, Andrea Bracho, Bernat Carreras, Noemi Carrillo, Marta Doñate, Cristina Enero, Alejandra Escura, Adrian Gaitan, Javi Sanchez, Pablo Vidal-Ribas, Maria Teresa Ordeig, Sylva-Astrik Torossian, Celine Cavallo, Helen Casas.
This study received financial support through the award 22è PREMI FERRAN SALSAS I ROIG – Salut Mental i Comunitat granted by the City Council of Rubi (Spain) in 2010.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5. 5 edition. Washington, D.C: American Psychiatric Publishing; 991 (2013).
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat. Rev. Neurosci 3(8), 617-628 (2002).
- Khoury JE, Milligan K. Comparing Executive Functioning in Children and Adolescents With Fetal Alcohol Spectrum Disorders and ADHD A Meta-Analysis. J. Atten. Disord(2016)
- Lambek R, Tannock R, Dalsgaard S, et al. Executive dysfunction in school-age children with ADHD.J. Atten. Disord15(8), 646-655 (2011).
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. J. Child. Psychol. Psychiatry37(1), 51-87 (1999).
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. Oxford University Press; 2004.
- Hervey AS, Epstein JN, Curry JF. Neuropsychology of adults with attention-deficit/hyperactivity disorder: a meta-analytic review. Neuropsychology 18(3), 485-503 (2004).
- Kasper LJ, Alderson RM, Hudec KL. Moderators of working memory deficits in children with attention-deficit/hyperactivity disorder (ADHD): a meta-analytic review. Clin. Psychol. Rev 32(7), 605-617 (2012).
- Martinussen R, Hayden J, Hogg-Johnson S, et al.A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child. Adolesc. Psychiatry 44(4), 377-384 (2005).
- Willcutt EG, Doyle AE, Nigg JT, et al.Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol. Psychiatry 57(11), 1336-1346 (2005).
- Baddeley A. Working memory: looking back and looking forward. Nat. Rev. Neurosci 4(10), 829-839 (2003).
- Zelazo, PD, Muller U. Executive function in typical and atypical development. In: U. Goswami, editor. Handbook of childhood cognitive development. Oxford: Blackwell Publishing. 445-469 (2002).
- Kerr A, Zelazo PD. Development of ‘hot’ executive function: the children’s gambling task. Brain. Cogn 55(1), 148-157 (2004).
- Séguin J, Zelazo P. Executive function in early physical aggression. In: Tremblay RE, Hartup WW, Archer J, editor. Developmental origins of aggression. New York: Guilford Press. 307-329 (2005).
- Damasio AR. Descartes’ error: emotion, reason, and the human brain. New York: Grosset/Putnam,(1994).
- Premack D, Woodruff G. Does the Chimpanzee Have a Theory of Mind? Behav. Brain. Sci4(4), 515-629 (1978).
- Perner J. Understanding the representational mind. Cambridge, MA: MIT Press/ Bradford Books; 1991.
- Charman T, Carroll F, Sturge C. Theory of mind, executive function and social competence in boys with ADHD. Emot. Behav. Difficulties6(1), 31-49 (2001).
- Dyck MJ, Ferguson K, Shochet IM. Do autism spectrum disorders differ from each other and from non-spectrum disorders on emotion recognition tests? Eur. Child. Adolesc. Psychiatry10(2), 105-116 (2001)
- Geurts HM, van der Oord S, Crone EA. Hot and cool aspects of cognitive control in children with ADHD: decision-making and inhibition. J. Abnorm. Child. Psychol 34(6), 813-824 (2006).
- Gonzalez-Gadea ML, Baez S, Torralva T, et al. Cognitive variability in adults with ADHD and AS: disentangling the roles of executive functions and social cognition. Res. Dev. Disabil 34(2), 817-830 (2013).
- Skogli EW, Egeland J, Andersen PN, et al.Few differences in hot and cold executive functions in children and adolescents with combined and inattentive subtypes of ADHD. Child. Neuropsychol. J. Norm. Abnorm. Dev. Child. Adolesc 20(2), 162-181 (2014).
- Bora E, Pantelis C. Meta-analysis of social cognition in attention-deficit/hyperactivity disorder (ADHD): comparison with healthy controls and autistic spectrum disorder. Psychol. Med 46(4), 699-716 (2016).
- Braaten EB, Rosén LA. Self-regulation of affect in attention deficit-hyperactivity disorder (ADHD) and non-ADHD boys: Differences in empathic responding. J. Consult. Clin. Psychol 68(2), 313-321 (2008).
- Buitelaar JK, van der Wees M, Swaab-Barneveld H, et al.Theory of mind and emotion-recognition functioning in autistic spectrum disorders and in psychiatric control and normal children. Dev. Psychopathol11(1), 39-58 (1999).
- Corbett B, Glidden H. Processing affective stimuli in children with attention-deficit hyperactivity disorder. Child. Neuropsychol 6(2), 144-155 (2000).
- Da Fonseca D, Seguier V, Santos A, et al.Emotion understanding in children with ADHD. Child. Psychiatry. Hum. Dev40(1), 111-121 (2009).
- Demurie E, De Corel M, Roeyers H. Empathic accuracy in adolescents with autism spectrum disorders and adolescents with attention-deficit/hyperactivity disorder. Res. Autism. Spectr. Disord5(1), 126-134 (2011).
- Shin DW, Lee SJ, Kim BJ, et al.Visual attention deficits contribute to impaired facial emotion recognition in boys with attention-deficit/hyperactivity disorder. Neuropediatrics 39(6), 323-327 (2011).
- Sinzig J, Morsch D, Lehmkuhl G. Do hyperactivity, impulsivity and inattention have an impact on the ability of facial affect recognition in children with autism and ADHD? Eur. Child. Adolesc. Psychiatry 17(2), 63-72 (2008).
- Sodian B, Hülsken C, Thoermer C. The self and action in theory of mind research. Conscious. Cogn12(4), 777-782 (2003).
- Uekermann J, Kraemer M, Abdel-Hamid M, et al.Social cognition in attention-deficit hyperactivity disorder (ADHD). Neurosci. Biobehav. Rev 34(5), 734-743 (2010).
- Groen Y, Gaastra GF, Lewis-Evans B, et al.Risky Behavior in Gambling Tasks in Individuals with ADHD – A Systematic Literature Review. PloS. ONE8(9), e74909 (2013).
- Mowinckel AM, Pedersen ML, Eilertsen E, et al.A meta-analysis of decision-making and attention in adults with ADHD. J. Atten. Disord 19(5), 355-367 (2015).
- Patros CHG, Alderson RM, Kasper LJ, et al.Choice-impulsivity in children and adolescents with attention-deficit/hyperactivity disorder (ADHD): A meta-analytic review. Clin. Psychol. Rev 43(1), 162-174 (2016).
- Barkley RA, Murphy K, Kwasnik D. Psychological adjustment and adaptive impairments in young adults with ADHD. J. Atten. Disord 1(1), 41-54 (1996).
- Ilkowska M, Engle RW. Working Memory Capacity and Self-Regulation. In: Member RHHF, editor. Handbook of Personality and Self-Regulation, Wiley-Blackwell 263-290 (2010).
- Carlson SM, Moses LJ. Individual differences in inhibitory control and children’s theory of mind. Child. Dev 72(4), 1032-1053 (2001).
- Carlson SM, Moses LJ, Breton C. How specific is the relation between executive function and theory of mind? Contributions of inhibitory control and working memory. Infant. Child. Dev 11(2), 73-92 (2002).
- Keenan T. Memory span as a predictor of false belief understanding. New. Zealand. J. Psychology27(1),36-43 (1998).
- Keenan T, Olson DR, Marini Z. Working Memory and Children’s Developing Understanding of Mind. Aust. J. Psychol50(2), 76-82 (1992).
- Vetter NC, Altgassen M, Phillips L, et al.Development of Affective Theory of Mind Across Adolescence: Disentangling the Role of Executive Functions. Dev. Neuropsychol 38(2), 114-215 (2013).
- Vetter NC, Leipold K, Kliegel M, et al.Ongoing development of social cognition in adolescence. Child. Neuropsychol 19(6), 615-629 (2013).
- Caillies S, Bertot V, Motte J, et al. Social cognition in ADHD: irony understanding and recursive theory of mind. Res. Dev. Disabil 35(11), 3191–3198 (2014).
- Mary A, Slama H, Mousty P, et al.Executive and attentional contributions to Theory of Mind deficit in attention deficit/hyperactivity disorder (ADHD). Child. Neuropsychol 22(3), 345-365 (2016).
- Moshirian Farahi SM, Asgari Ebrahimabad MJ, Moshirian Farahi SMM. Investigation of Theory of Mind in ADHD and Normal Children and its Relationship with Response Inhibition. Iran. J. Cogn. Educ(2014).
- Papadopoulos TC, Panayiotou G, Spanoudis G, et al.Evidence of poor planning in children with attention deficits. J. Abnorm. Child. Psychol 33(5), 611-623 (2005).
- Davis HL, Pratt C. The development of children‘s theory of mind: The working memory explanation’. (Special issue). Cognitive development. Australian. J. Psychology47(1), 25-31 (1995).
- Drayton S, Turley-Ames KJ, Guajardo NR. Counterfactual thinking and false belief: the role of executive function. J. Exp. Child. Psychol 108(3), 532-548 (2011).
- Gordon AC, Olson DR. The relation between acquisition of a theory of mind and the capacity to hold in mind. J. Exp. Child. Psychol 68(1), 70-83 (1998).
- Maehara Y, Saito S. I see into your mind too well: working memory adjusts the probability judgment of others’ mental states. Acta. Psychol (Amst) 138(3), 367-376 (2011).
- Vetter NC. Theory of Mind Development in Adolescence and its (Neuro)cognitive Mechanisms. [Dresden]: Technische Universität Dresden(2013).
- Sabbagh MA, Taylor M. Neural correlates of theory-of-mind reasoning: An event-related potential study. Psychol. Sci 11(1), 46-50 (2000).
- Abu-Akel A, Shamay-Tsoory S. Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia49(11), 2971-2984 (2011).
- Soliva JC, Carmona S, Fauquet J, et al.Neurobiological substrates of social cognition impairment in attention-deficit hyperactivity disorder:gathering insights from seven structural and functional magnetic resonance imaging studies. Ann. N. Y. Acad. Sci1167(1), 212-220 (2009).
- Wellman HM, Cross D, Watson J. Meta-analysis of theory-of-mind development: the truth about false belief. Child. Dev72(3), 655-684 (2001).
- Hughes C. Finding your marbles: Does preschoolers’ strategic behavior predict later understanding of mind? Dev. Psychol34(6), 1326-1339 (1998).
- Austin G, Groppe K, Elsner B. The reciprocal relationship between executive function and theory of mind in middle childhood: a 1-year longitudinal perspective. Front. Psychol(2014).
- Bechara A, Damasio H, Tranel D, et al.Deciding advantageously before knowing the advantageous strategy. Science 275(5304), 1293-1295 (1997).
- Goldman-Rakic PS. Working memory and the mind. Sci. Am267(3), 110-117 (1992).
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology18(1), 152-162 (2004).
- Hinson JM, Jameson TL, Whitney P. Somatic markers, working memory, and decision making. Cogn. Affect. Behav. Neurosci 2(4), 341-353 (2002).
- Jameson TL, Hinson JM, Whitney P. Components of working memory and somatic markers in decision making. Psychon. Bull. Rev11(3), 515-520 (2004).
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex.Cereb. Cortex 10(3), 295-307 (2002).
- Hooper CJ, Luciana M, Conklin HM, et al. Adolescents’ performance on the Iowa Gambling Task: implications for the development of decision making and ventromedial prefrontal cortex. Dev. Psychol 40(6), 1148-1158 (2004).
- Toplak ME, Sorge GB, Benoit A, et al. Decision-making and cognitive abilities: A review of associations between Iowa Gambling Task performance, executive functions, and intelligence. Clin. Psychol. Rev 30(5), 562-581 (2010).
- Klingberg T. Training and plasticity of working memory. Trends. Cogn. Sci14(7), 317-324 (2010).
- Holmes J, Gathercole SE, Dunning DL. Poor working memory: impact and interventions. Adv. Child. Dev. Behav39(1), 01-43 (2010).
- Melby-Lervåg M, Hulme C. Is working memory training effective? A meta-analytic review. Dev. Psychol 49(2), 270-291 (2012).
- Shipstead Z, Redick TS, Engle RW. Does working memory training generalize? Psychol. Belg3(4), 245-276 (2010).
- Spencer-Smith M, Klingberg T. Benefits of a working memory training program for inattention in daily life: a systematic review and meta-analysis. PloS. One10(3), e0119522 (2015).
- Brehmer Y, Rieckmann A, Bellander M, et al.Neural correlates of training-related working-memory gains in old age. NeuroImage 58(4), 1110-1120 (2011).
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat. Neurosci7(1), 75-79 (2004).
- Astle DE, Barnes JJ, Baker K, et al.Cognitive training enhances intrinsic brain connectivity in childhood.J. Neurosci. Off. J. Soc. Neurosci35(16), 6277-6283 (2015).
- Stevens MC, Gaynor A, Bessette KL, et al.A preliminary study of the effects of working memory training on brain function. Brain. Imaging. Behav10(2), 387-407 (2015).
- McNab F, Varrone A, Farde L, et al.Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science 323(5915), 800-802 (2009).
- Barnett SM, Ceci SJ. When and where do we apply what we learn?: A taxonomy for far transfer. Psychol. Bull128(4), 612-637 (2002).
- Jonides J. How does practice makes perfect? Nat. Neurosci 7(1), 10-11 (2004).
- Shipstead Z, Redick TS, Engle RW. Is working memory training effective? Psychol. Bull138(4), 628-654 (2012).
- Ulloa RE, Ortiz S, Higuera F, et al.Interrater reliability of the Spanish version of the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime version (K-SADS-PL). Actas. Esp. Psiquiatr34(1), 36-40 (2006).
- Bigorra A, Garolera M, Guijarro S, et al.Long-term far-transfer effects of working memory training in children with ADHD: a randomized controlled trial. Eur. Child. Adolesc. Psychiatry25(8), 853-867 (2016).
- von Bastian CC, Oberauer K. Effects and mechanisms of working memory training: a review. Psychol. Res 78(6), 803-820 (2013)
- Wechsler D. WISC-IV, Escala de Inteligencia de Wechsler para niños. TEA Ediciones (2005).
- Wechsler D. WMS-III. Escala De Memoria De Wechsler-III. Manual de aplicación y puntuación. Madrid: TEA Ediciones (2004).
- Bechara A, Damasio AR, Damasio H, et al.Insensitivity to future consequences following damage to human prefrontal cortex. Cognition50(1-3), 7-15 (1994).
- Happé FGE. An advanced test of theory of mind: Understanding of story characters’ thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. J. Autism. Dev. Disord24(2), 129-154 (1994).
- Shamay-Tsoory SG, Harari H, Aharon-Peretz J, et al.The role of the orbitofrontal cortex in affective theory of mind deficits in criminal offenders with psychopathic tendencies. Cortex 46(5), 668-677 (2010).
- Baron-Cohen S, Wheelwright S, Spong A, et al.Are intuitive physics and intuitive psychology independent? A test with children with Asperger Syndrome. J. Develop. Learn. Disord5(1), 47-78 (2001).
- Baron-Cohen S, Jolliffe T, Mortimore C, et al.Another advanced test of theory of mind: evidence from very high functioning adults with autism or asperger syndrome. J. Child. Psychol. Psychiatry38(7), 813-822 (1997).
- Jenkins JM. Cognitive factors and family structure associated with theory of mind development in young children. Dev. Psychol32(1), 70-78 (1995).
- Plaut DC, Karmiloff-Smith A. Representational development and theory-of-mind computations. Behav. Brain. Sci 16(1), 70-71 (1993).
- Cohen J. Statistical power analysis for the behavioral sciences. Routledge, 594 (1988).
- Fahie CM, Symons DK. Executive functioning and theory of mind in children clinically referred for attention and behavior problems. J. Appl. Dev. Psychol24(1), 51-73 (2003).
- Chacko A, Bedard AC, Marks DJ, et al.A randomized clinical trial of Cogmed Working Memory Training in school-age children with ADHD: a replication in a diverse sample using a control condition. J. Child. Psychol. Psychiatry55(3), 247-255 (2014).
- Gray SA, Chaban P, Martinussen R, et al.Effects of a computerized working memory training program on working memory, attention, and academics in adolescents with severe LD and comorbid ADHD; a randomized controlled trial. J. Child. Psychol. Psychiatry53(12), 1277-1284 (2012).
- Steeger CM, Gondoli DM, Gibson BS, et al.Combined cognitive and parent training interventions for adolescents with ADHD and their mothers: A randomized controlled trial. Child. Neuropsychol1-26 (2015).
- van der Donk M, Hiemstra-Beernink AC, Tjeenk-Kalff A, et al.Cognitive training for children with ADHD: a randomized controlled trial of cogmed working memory training and ‘paying attention in class’. Dev. Psychol1081 (2015).
- Roche JD, Johnson BD. Cogmed working memory training product review. J. Atten. Disord18(4), 379-384 (2014).
- Shipstead Z, Hicks KL, Engle RW. Cogmed working memory training: Does the evidence support the claims? J. Appl. Res. Mem.Cogn1(3), 185-193 (2012).
- Schweizer S, Hampshire A, Dalgleish T. Extending braintTraining to the affective domain: increasing cognitive and affective executive control through emotional working memory training. PLoS. ONE6(9), e24372 (2011).
- Hill AB. The environment and disease: association or causation? Proc. R. Soc. Med58(5), 295-300 (1965).
- Ahmed FS, Stephen Miller L. Executive function mechanisms of theory of mind. J. Autism. Dev. Disord41(5), 667-678 (2011).
- Toplak ME, Jain U, Tannock R. Executive and motivational processes in adolescents with attention-deficit-hyperactivity disorder (ADHD). Behav. Brain. Funct1(1), 8 (2005).
- Rass O, Schacht RL, Buckheit K, et al.A randomized controlled trial of the effects of working memory training in methadone maintenance patients. Drug. Alcohol. Depend156(1), 38-46 (2015).
- Nigg JT. Is ADHD a disinhibitory disorder? Psychol. Bull127(5), 571-598 (2001).
- Sonuga-Barke EJS, Dalen L, Remington B. Do executive deficits and delay aversion make independent contributions to preschool attention-deficit/hyperactivity disorder symptoms? J. Am. Acad. Child. Adolesc. Psychiatry42(11), 1335-1342 (2003).
- Sagvolden T, Johansen EB, Aase H, et al.A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav. Brain. Sci28(3), 397-419 (2005).
- Sonuga-Barke EJS. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol. Psychiatry57(11), 1231-1238 (2005).
- Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends. Cogn. Sci16(1), 17-26 (2012).
- Cardinal RN, Parkinson JA, Hall J, et al.Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev26(3), 321-352 (2002).
- Hardy KK, Willard VW, Allen TM, et al.Working memory training in survivors of pediatric cancer: a randomized pilot study. Psychooncology 22(8), 1856-1865 (2013).
- Fabio RA, Castriciano C, Rondanini A. ADHD auditory and visual stimuli in automatic and controlled processes. J. Atten. Disord19(9), 771-778 (2015).
- Yarmolovsky J, Szwarc T, Schwartz M,et al. Hot executive control and response to a stimulant in a double-blind randomized trial in children with ADHD. Eur. Arch. Psychiatry. Clin. Neurosci1-10 (2016).
- Hutchins TL, Prelock PA, Morris H, et al.Explicit vs. applied theory of mind competence: A comparison of typically developing males, males with ASD, and males with ADHD. Res. Autism. Spectr. Disord 21(1), 94-108 (2016).
- Rubia K. ‘Cool’ inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus ‘hot’ ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biol. Psychiatry69(12), e69-87 (2011).
- Bühler E, Bachmann C, Goyert H, et al. Differential diagnosis of autism spectrum disorder and attention deficit hyperactivity disorder by means of inhibitory control and ‘theory of mind. J. Autism. Dev. Disord 41(12), 1718-1726 (2011).
- Crone EA, van der Molen MW. Development of decision making in school-aged children and adolescents: evidence from heart rate and skin conductance analysis. Child. Dev 78(4), 1288-1301 (2007).