Case Report - (2019) Volume 9, Issue 6
Hysteria or Dementia? A Case of Atypical Clinical Onset of Frontotemporal Dementia Associated with a Progranulin Mutation
- Corresponding Author:
- Alexandre Morin, MD, MSc
Neurology Department
Pitié-Salpêtrière Hospital, 47
Boulevard de l’Hôspital 75013, Paris
Tel: +33 (0)6 87 02 75 73
email: [email protected]
Abstract
The behavioral variant of frontotemporal dementia (bvFTD) is one of the main causes of neurodegenerative dementias in adults. Patients present with an early decline in social conduct, impaired regulation of interpersonal conduct, apathy, emotional blunting, and general loss of insight. However, symptoms are highly variable among patients, leading to misdiagnosis or diagnosis delay in particular when psychiatric symptoms are prominent at the onset of the disease. More specifically, personality disorders have been described as either a risk factor for misdiagnosis or as part of the bvFTD phenotype.
Here we report the case of a 52-year-old woman, born from consanguineous parents and with a family history of early-onset dementia, who was first diagnosed with histrionic personality disorder according to the DSM-IV-TR criteria. Nonetheless, atypical symptoms such as apathy, disinhibition, hyperorality, depressive symptoms and psychotic manifestations were described.The clinical exam revealed a frontal lobe dysfunction, along with severe cognitive impairment as revealed by the neuropsychological assessment (alteration of facial emotion recognition and theory of mind, with a relative sparing of executive and instrumental functions). Brain imaging revealed atrophy of frontal lobes and right hemisphere, and a hypometabolism of the right frontotemporal lobe.
Taking into consideration the clinical presentation and her family history, screening for hereditary diseases was performed, revealing a heterozygous mutation of PGRN gene. The diagnosis of bvFTD with progranulin mutation was finally made. This case highlights for the first time a diagnosis of bvFTD associated with a progranulin mutation and a history of late-labeled histrionic personality disorder. It emphasizes the need to actively seek frontal symptoms and consider PGRN mutation in late-labelled patients with personality disorders with a family history of neurodegenerative disorders.
Keywords
Frontotemporal dementia, Progranulin, Histrionic personality disorder, Hysteria
Introduction
The behavioral variant of frontotemporal dementia (bvFTD) is one of the main causes of neurodegenerative dementias among people ages 45 to 65 [1]. Patients show progressive behavioral and/or cognitive impairment and may present with disinhibition, apathy, perseverations, stereotyped behaviors, hyperorality, loss of empathy and/or a dysexecutive syndrome. According to the latest diagnostic criteria consensus, the sensitivity and specificity remain partial, leading to misdiagnosis, although the genetic forms allow for a diagnosis of certainty [2].
In particular, psychiatric onset in bvFTD may lead to a delay in diagnosis [3]. Early symptoms such as psychosis, compulsive behavior, or depression can blur one’s experimented clinician judgement [4]. Apathy, loss of initiative, and reduced speech fluency are known shared features in bvFTD, schizophrenia and depression. Similarly, stereotypical/compulsive behavior or disinhibition may also occur in bipolar disorder, anxiety disorders, obsessive-compulsive disorder, or autism [5]. Furthermore, some authors raised awareness on personality disorders in patients with bvFTD, and its leading risk of delay in diagnosis. Indeed, borderline and narcissistic personalities associated with bvFTD were reported in previous case reports raising this diagnosis difficulty [6,7].
Heterozygous mutations in progranulin (PGRN), C9orf72 and MAPT genes are involved in 20%-30% of patients with frontotemporal dementia [8,9]. The genetic forms of bvFTD open a new path for a better understanding of the specific relationship between psychiatric onset in bvFTD and personality disorder, and its role in misdiagnosis. bvFTD is the most common clinical form of FTD associated with progranulin (PGRN) mutation [10]. Moreover, specific psychiatric features have been described as being more frequent in bvFTD associated with PGRN mutations, such as psychotic symptoms, visual hallucinations and stereotypies [11]. However, personality disorders associated with PGRN bvFTD were not reported nowadays, in particular regarding personality disorders from the dramatic cluster [12].
Herein, we report for the first time the case of a woman with a history of histrionic personality disorder, for whom a diagnosis of bvFTD associated with a progranulin mutation was made a posteriori.
Case Report
A 52 year-old (yo) woman of Moroccan origin, born from consanguineous parents (cousin siblings) was referred to the Behavioral Neuropsychiatry Unit in Pitié- Salpêtrière hospital due to dramatic behavioral changes. She was leader of a professional training company and had to stop her activity at age 51 due to management difficulties. Medical history revealed a depressive episode twenty years ago, after the death of her husband, followed by multiple untreated depressive episodes.
In the unit, she explained having felt depressed for a long time, relating her emotional state to the loss of her husband. She was constantly seeking attention, repeatedly making a moue while looking at doctors during the examination. She exhibited an inappropriate seductive and sometimes provocative behavior, trying to grab the psychiatrist’s coat while notifying she always wanted to become a psychiatrist; and running theatrically at her son when he entered the room. She presented with distractibility, superficiality in the emotional expression, rapid mood swings and high suggestibility, suddenly breaking into tears, claiming being depressed, to eventually switch to a joyful smiling face after having been told by the psychiatrist she may not be that depressed. She acted with a regressive behavior and egocentrism (always wearing pink pyjamas and carrying puppets with her; self-oriented speech, with a lack of empathy). Her child told she always acted this way, even though her behavior had been more prominent for a few years. To that extent, she was first diagnosed with histrionic personality disorder considering the behavioral and emotional symptoms and according to the DSM-IV-TR criteria.
However, some atypical symptoms were eventually found. One of her children described the patient as being extremely apathetic for one year, spending most of the day watching TV. She expressed impaired moral judgment (robbing sweets in a shop), with disinhibition (walking naked in front of her son, shouting at people in the street) and hyperorality. She also reported depressive cognition with sadness, ideas of culpability, anhedonia, social withdrawal along with psychotic symptoms such as visual and auditory hallucinations with delusional interpretation (“I saw and heard a snake eating the right part of my brain”, “a cow was standing by my bed”). There was no sleep disturbance. Those symptoms led to work disruption.
The clinical exam revealed frontal lobe dysfunction such as grasping, utilization behaviour, stereotypies, verbal and motor perseverations without pyramidal symptoms or Parkinsonism. The neuropsychological assessment revealed a severe cognitive deterioration (Mini-Mental State Examination score of 18/30) associated with a relative sparing of executive functions (Frontal Assessment Battery score of 17/18, discrete deficit in mental flexibility (Trail making test (TMT) A=34, B=176) and inhibitory control (Hayling test=10 errors),working memory (digit span forward and backward=3) and instrumental functions (praxis and visuospatial skills) [13,14]. Memory was spared apart and langage examination demonstrated semantic impairment (category fluency below the norm=23 and semantic paraphasias in picture naming). Nonetheless, facial emotion recognition was altered with Ekman faces from Social cognition and emotional assessment (SEA)=23/35 as well as theory of mind (alteration of emotional empathy but preserved cognitive empathy), (Table 1A and 1B) [15]. The patient was peculiarly conscious of her behavioral changes, reporting a feeling of being “weird, different”.
| A) Cognitive domain / Neuropsychological test | Raw score |
| Global cognitive efficiency | |
| Mini-mental state examination (MMSE, [1]) | 18/30* |
| Mattis dementia rating scale (DRS, [2]) | 120/144* |
| Temporo-spatial orientation – MMSE [1] | 3/10* |
| Attention and working memory | |
| Forward digit span (WMS-III, [3]) | 3* |
| Backward digit span (WMS-III, [3]) | 3* |
| Executive functioning | |
| Frontal assessment battery (FAB, [4]) | 17/18 |
| Flexibility | |
| Trail making test [5] – Form A, seconds | 34 |
| Trail making test [5] – Form B - Form A, seconds | 176* |
| Inhibition of interferences – Hayling test [6] (part B) : errors | 10* |
| Verbal fluency | |
| Category fluency 2 min [5] | 23* |
| Letter fluency 2 min [5] | 18 |
| Conceptual elaboration and shifting | |
| Modified Wisconsin card sorting test [5] – Categories | 4/6 |
| Modified Wisconsin card sorting test [5] – Perseverative errors | 6 |
| Brixton test [5] : errors | 16 |
| Apathy – Starkstein scale [7] | 31/42* |
| Verbal learning – Free and cued recall test [8] | |
| Immediate cued recall | 15/16 |
| Total free recall | 28/48 |
| Sum of free and cued recall | 46/48 |
| % sensitivity | 90% |
| Free delayed recall | 12/16 |
| B | |
| Sum of free and cued delayed recall | 16/16 |
| Recognition | 16/16 |
| Visual learning – Rey complex figure recall [9] | 15/36 |
| Visuospatial skills – Rey complex figure copy [9] | 36/36 |
| Picture naming – BECS-GRECO [10] | 34/40* |
| Limb praxis – Mahieux’s battery [11] | 21/23 |
| Social cognition – Emotional facial expression recognition (SEA) [12] | 23/35* |
*In bold: abnormal scores according to each
Table 1: Results of the neuropsychological assessment.
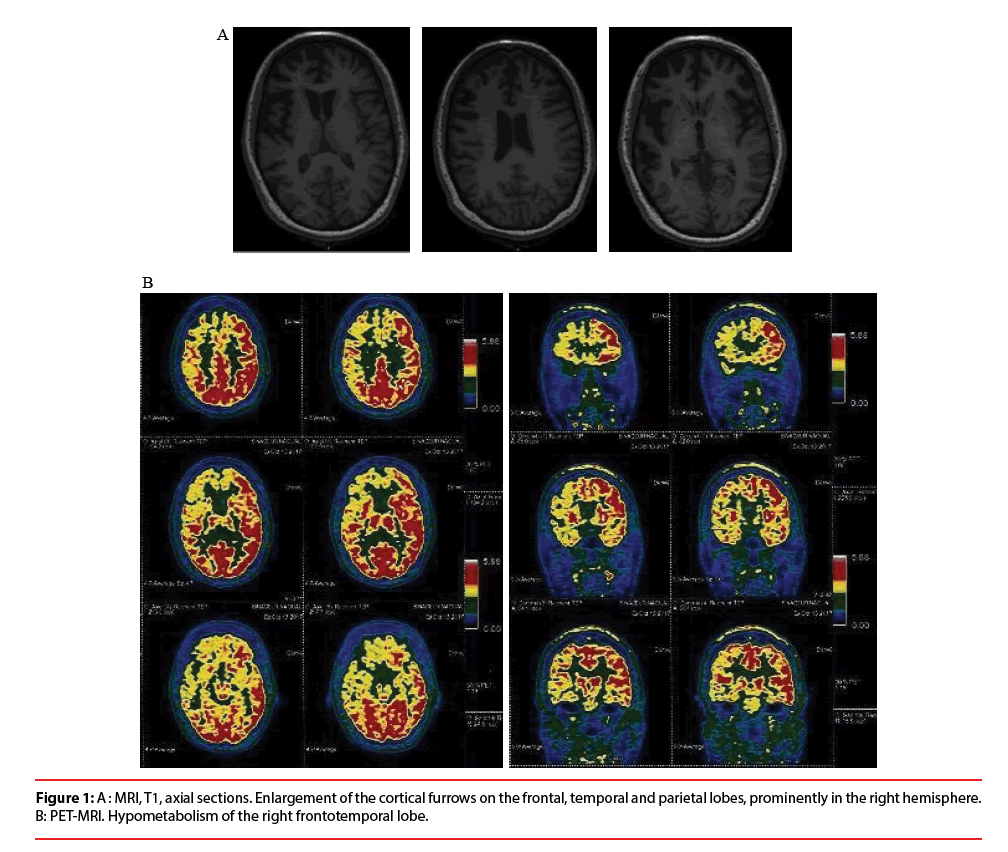
The standard blood tests were normal. Cerebrospinal fluid (CSF) analysis showed no pleiocytosis, no elevated CSF Protein, no oligoclonal bands, no abnormal Alzheimer’s disease CSF biomarkers. Combined Brain 18F-FDG Positron Emission Tomography with Magnetic Resonance Imaging (PET-MRI) revealed an enlargement of the cortical furrows, prominently on the frontal lobes and on the right hemisphere, associated with a hypometabolism of the right frontotemporal lobe (Figure 1A and 1B).
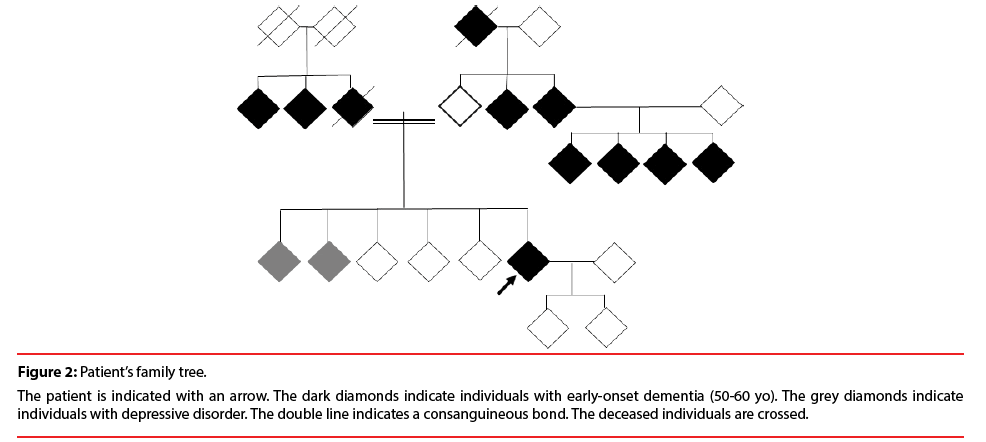
The patient had a positive family history of neuropsychiatric disorders. Two siblings suffered from depression. Her father died from dementia at the age of 68 yo. Her maternal grandfather, two paternal aunts, two maternal uncles and four cousins had early-onset dementia ages 50 to 60 yo (Figure 2).
Figure 2: Patient’s family tree.
The patient is indicated with an arrow. The dark diamonds indicate individuals with early-onset dementia (50-60 yo). The grey diamonds indicate individuals with depressive disorder. The double line indicates a consanguineous bond. The deceased individuals are crossed.
Taking into consideration the clinical presentation and family history, screening for hereditary and metabolic diseases was performed. A decreased level of plasma progranulin (44 μg/L, N=80-300) led to the sequencing of PGRN gene [16]. A heterozygous mutation c.1508del (p.Gln503Argfs*14) was identified in the exon 12 of the gene (transcript NM_002087.2) predicted to lead to a premature stop codon. Written consent for genetic test was obtained from the patient.The diagnosis of definite bvFTD was eventually made.
Discussion and Conclusion
We reported the first case of histrionic clinical presentation of bvFTD associated with PGRN mutation. With this case, we raise some important concerns about misinterpretation of behavioral and cognitive changes in patients. Suggestibility and seductive behavior such as attention-seeking behavior must be interpreted cautiously as it may be part of dorsolateral-prefrontal area dysfunction through disinhibition and lack of inhibitory control. Moreover, distractibility and rapid mood swings may be related to emotional dysregulation through orbitofrontal-prefrontal area dysfunction. In our patient, along with the clinical presentation, neuropsychological assessment confirmed the dorsolateral prefrontal dysfunction (TMT, Hayling test) and orbitofrontal-prefrontal dysfunction (SEA) [15]. Morphologic and metabolic imaging showed both orbitofrontal and dorsolateral prefrontal lobe involvement.
Early psychotic presentations are frequent phenotypic manifestations of C9orf72 expansions, but there are only rare descriptions of personality disorders associated with FTD, particularly in patients carrying FTD mutations. One patient carrying a C9orf72 intermediate allele developed a slowly progressive cerebellar syndrome, mild cognitive impairment, and pyramidal signs at age 64, after a22-year history of a mixed anxiety-depressive disorder associated with somatization disorder and histrionic personality , suggesting the role of C9orf72 or other FTD genes as risk factors for personality disorders [17]. Another patient with bv FTD, previously diagnosed as borderline personality, was also reported [7].
These and our cases lead to discuss such differential diagnosis and the role of preexistent psychiatric diagnosis in the delay in bv FTD diagnosis [7]. Thus, late-labeled patients with personality disorders must raise physicians’ attention to frontal symptoms, even though patients seem highly conscious of their symptoms. Assessment of family history may be required, in addition to behavioral, neuropsychological, structural and functional neuroimaging investigations. The patient’s family history, looking for neurological or psychiatric disease in first and second degree relatives, may address to further genetic analyses [18,19].
Besides, longstanding personality characteristics as a person’s most distinctive features of all may be likely to play a role in how someone presenting with dementia copes with his increasing deficiencies. Thus, the personality traits may have a pathoplastic effect on behavioral personality changes related to neurodegenerative disease [20]. Patients presenting with personality disorders may show some functional frontolimbic abnormalities, most available studies focusing on borderline personality disorder [21-23] In that sense, a patient with a delayed diagnosis of bvFTD and narcissistic personality disorder was reported in 2011 [6]. It raises the question of the role of neurobiological vulnerabilities linked to a premorbid personality disorder on the expression of a neurodegenerative disorder such as bvFTD. Does it predispose to such disorder? Should it be considered as a risk factor or a prodromal stage? Or simply as a confounding factor?
Findings in this domain remain scarce despite a wide available literature on personality and cognitive disorders in FTD, and may represent an interesting field of research. This case emphasizes the need to actively seek frontal symptoms and consider PGRN mutation in latelabeled patients with personality disorders with a family history of neurodegenerative disorders.
Disclosure of Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Ratnavalli E, Brayne C, Dawson K, et al. The prevalence of frontotemporal dementia. Neurology 58(1), 1615–1621 (2002).
- Rascovsky K. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134(1), 2456–2477 (2011).
- Mendez MF, Lauterbach EC, Sampson S, et al. Committee on Research. An evidence-based review of the psychopathology of frontotemporal dementia: A report of the ANPA Committee on Research. J. Neuropsychiatry. Clin. Neurosci 20(1), 130–149 (2008).
- Woolley JD, Khan BK, Murthy NK, et al. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: Rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J. Clin. Psychiatry 72(1), 126–133 (2011).
- Issac TG, Telang AV, Chandra SR. Trichotillomania ranging from “ritual to illness” and as a rare clinical manifestation of Frontotemporal Dementia: Review of literature and case report. Int. J Trichology 10(1), 84–88 (2018).
- Poletti M, Bonuccelli U. From narcissistic personality disorder to frontotemporal dementia: A case report. Behav. Neurol 24(1), 173–176 (2011).
- Salzbrenner LS. Frontotemporal Dementia complicated by comorbid borderline personality disorder. Psychiatry (Edgmont) 6(1), 28–31 (2009).
- van Blitterswijk M. Association between repeat sizes and clinical and pathological characteristics in carriers of C9ORF72 repeat expansions (Xpansize-72): A cross-sectional cohort study. Lancet. Neurol 12(1), 978–988 (2013).
- Snowden JS.Distinct clinical and pathological characteristics of Frontotemporal Dementia associated with C9ORF72 mutations. Brain 135(1), 693-708 (2012).
- Rademakers R. Phenotypic variability associated with progranulin haploinsufficiency in patients with the common 1477C-->T (Arg493X) mutation: An international initiative. Lancet. Neurol 6(1), 857–868 (2007).
- Le Ber I. Phenotype variability in progranulin mutation carriers: A clinical, neuropsychological, imaging and genetic study. Brain 131(1), 732–746 (2008).
- Mendez MF. Manic behavior and asymmetric right frontotemporal dementia from a novel progranulin mutation. Neuropsychiatr. Dis. Treat 14(1), 657–662 (2018).
- Belleville S, Rouleau N, Van der Linden M. Use of the Hayling task to measure inhibition of prepotent responses in normal aging and Alzheimer’s disease. Brain. Cogn 62(1), 113–119 (2006).
- Mahieux-Laurent F. Validation of a brief screening scale evaluating praxic abilities for use in memory clinics. Evaluation in 419 controls, 127 mild cognitive impairment and 320 demented patients. Rev. Neurol. (Paris) 165(1), 560–567 (2009).
- Funkiewiez, A, Bertoux M, de Souza LC, et al. The SEA (Social cognition and Emotional Assessment): A clinical neuropsychological tool for early diagnosis of frontal variant of frontotemporal lobar degeneration. Neuropsychology 26(1), 81–90 (2012).
- Finch N. Plasma progranulin levels predict progranulin mutation status in frontotemporal dementia patients and asymptomatic family members. Brain 132(1), 583–591 (2009).
- Meloni M.C9ORF72 Intermediate repeat expansion in a patient with psychiatric disorders and progressive Cerebellar Ataxia. Neurologist 22, 245–246 (2017).
- Rohrer JD. The heritability and genetics of frontotemporal lobar degeneration. Neurology 73(1), 1451–1456 (2009).
- Goldman JS. Frontotemporal dementia: Genetics and genetic counseling dilemmas. Neurologist 10(1), 227–234 (2004).
- von Gunten A, Pocnet C, Rossier J. The impact of personality characteristics on the clinical expression in neurodegenerative disorders: A review. Brain. Research. Bulletin 80(1), 179–191 (2009).
- Dudas RB.Amygdala and dlPFC abnormalities, with aberrant connectivity and habituation in response to emotional stimuli in females with BPD. J. Affect. Disord 208(1), 460–466 (2017).
- Mak ADP, Lam LC. Neurocognitive profiles of people with borderline personality disorder. Curr. Opin. Psychiatry 26(1), 90–96 (2013).
- Salavert J. Fronto-limbic dysfunction in borderline personality disorder: A 18F-FDG positron emission tomography study. J. Affect. Disord 131(1), 260–267 (2011).