Research Article - Neuropsychiatry (2017) Volume 7, Issue 5
Gray Matter Reduction in Currently Depressed Patients of Major Depressive Disorder: A Meta-Analysis
- Corresponding Author:
- Dachun Chen, MD
Beijing Huilongguan Hospital, Peking University, Beijing 100096, PR China
Tel: +86 13520506068, +86 10 62715511
Fax: 843-792-0664
Abstract
Aim:
Existing studies have shown that gray matter changes may be involved in the pathophysiology of major depression disorder (MDD). However, whether the severity of the disease can affect the change of gray matter was still controversial. The aim of the present study is to identify the changes of gray matter on patients with currently depressed MDD (cMDD) using metaanalysis based on the reported studies with VBM.
Methods:
A systematic retrieval of PubMed, Web of Science, Embase, Medline, and Science Direct was conducted to identify VBM studies that compared the gray matter differences between MDD patients (untreated or not recovered) and healthy controls. A meta-analysis was then carried out to quantitatively evaluate the gray matter changes of patients with cMDD.
Results:
Seventeen data sets from 14 studies were used in the present meta-analysis. The results showed reduced gray matter in the left superior temporal gyrus, right superior temporal gyrus, right insula, left median network, and left inferior parietal gyrus in patients with cMDD. It was also shown that the percentage of female patients and the mean age did not have a significant relationship with gray matter changes.
Conclusion:
The meta-analysis in the present study showed that the gray matter reduction not only exists in the frontal lobe and the hippocampus of patients with cMDD as reported in previous studies, but also exists in the temporal lobe, which may also be important in the pathophysiology basis of MDD.
Keywords
Major depressive disorder, Meta-analysis, Seed-based Mapping, Voxel-based morphometry
Introduction
Major depressive disorder (MDD), which is a common mental disorder with a lifetime prevalence of 13-16%, has become one of the major causes of the global burden of disease and worldwide disability [1]. The main clinical manifestations are depression, slow thinking, loss of interest, and lack of energy. During the past few decades, numerous studies have been focused on the investigation of the neurobiological basis of depression; however, our understanding of the underlying pathophysiological mechanism of depression is still at the initial stage. Although there are a lot of magnetic resonance imaging results to clarify the edge loop hypothesis of depression [2-4], the accurate brain structure change of MDD patients and the repeatable test results are still in suspense. The possible reasons include the heterogeneity of the disease, the complexity of clinical features and brain morphology interaction, and the differences of the samples, magnetic resonance and statistical methods. Most of the existing studies on brain morphology of MDD patients have used the traditional volume measurement, namely, the method based on the region of interest (ROI), to calculate the volume difference between the patients and the healthy controls [5-7] . More recently, the measurement method named voxel-based morphometry (VBM) has been used frequently in the brain structure research of schizophrenia and mood disorders. This method can estimate the total brain volume, and quantitatively detect the density difference of the brain tissue. The process of this method is repeatable, making the results more accurate and automated than the traditional methods [8].
Although VBM has been widely adopted in study of neuroanatomical abnormalities in patients with MDD, there have been many contradictory results using this method. For example, some researchers found that MDD patients had decreased cingulate gray matter [9,10], but a contrary result was found by others [11]. Some researchers reported that the hippocampal gray matter of MDD patients decreased compared with that of the healthy controls [12,13], while some researchers did not find a significant difference in this region of gray matter between the MDD patients and the healthy controls [9,14]. In addition, some researchers found that MDD patients had different brain structures at different stages of the disease. Arnone [15] and Salvadore [16] et al. found that the currently depressed MDD (cMDD) patients had different gray matter volumes compared with the remitted depressed MDD (rMDD) patients. In the classic study of fluorodeoxyglucose F 18, Baxter [17] found that the severity of the disease was positively correlated to the lower levels of the metabolic rate in the left frontal lobes the patients’ metabolism of left frontal lobe dorsal cortex returned to being normal after treatment. Using the PET technology, Drevets [18] found that only patients with depressive episodes had the increase of blood flow and glucose metabolism in the left frontal cortex, whereas no change was found in the patients in the remission of the disease. Using functional magnetic resonance imaging (fMRI), Kalin [19] found that the more serious the depression symptoms, the greater the degree of brain activation increase. The amygdala activation increased in patients with depression, which was associated with the severity of depression. The metabolic rate of some brain areas in patients with depression was reduced, and was related to the degree of depression and the cerebral metabolic function recovery after the improvement of this disease [20,21] . The above evidences show that the brain structure is distinct in different periods of depression. The changes with brain structure in currently depressed patients are worth of studying. So we assume that the cMDD patients had more gray matter structural abnormalities than the rMDD patients, as compared with healthy controls. As mentioned above, the different results of studies showed different parts of gray matter changes in cMDD patients. Therefore a meta-analysis of published studies (including 583 cMDD patients and 727 healthy controls) was conducted in the present study to comprehensively assess the structural abnormalities of gray matter in cMDD patients. All the patients in these studies were in the currently depressed periods. The VBM method was used to assess the gray matter abnormalities of MDD patients.
Methods
▪ Literature search
A systematic and comprehensive literature search was carried out for relevant studies in the published literature before May 2016. The database of PubMed, Web of Science, Embase, Science Direct, and Medline were included. The search used the following key words: “major depressive disorder” OR “depression” AND “voxel - based morphometry” OR “voxel*” OR “morphometry”. Furthermore, we retrieved the references of the paper of each study, in order to reduce the probability of missing. Based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [22] , we included studies that met the following criteria: (1) the study included both MDD patients and healthy controls; (2) the VBM method was used in the study to analyze the overall brain gray matter changes of the MDD patients; (3) the study clearly reported the coordinates of the activation areas and the coordinates were normalized into a stereotaxic standardized space [e.g. the Montreal Neurological Institute (MNI) or Talairach space]; (4) the standards of cMDD [23] : HAMD-17(17-item Hamilton Depression Rating Scale)≥24], including those patients with the scales still higher than 17 points after adequate treatment; Montgomery and Asberg Depression Rating Scale ≥ 30, and including those patients that the scales are still higher than 22 points after adequate treatment; Center for Epidemiological Studies Depression Scale ≥ 20. The studies were excluded if: (1) the study did not include healthy controls; (2) they are reviews, animal studies, case reports, etc.; (3) the patients in the study did not reach the standard of cMDD; (4) the MDD patients recovered after treatment, in which the changes of gray matter caused by drugs cannot be completely excluded.
All analyses in this study were based on previously published studies, and thus no ethical approval and patient consent are required.
▪ Quality assessment
We evaluated the relevant studies according to the literature quality evaluation standard of Newcastle-Ottawa Scale (NOS) which is designed for quality evaluation of the case-control study (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). The literature was evaluated on the following three perspectives: the choosing of the objects (4 points for 4 items), the comparability of the cases and controls (2 points for 2 items), and the measurement of the research factors (3 points for 3 items). The highest score is 9 points. The higher the score of the evaluation means the better the quality of the documents. Those with a score larger than 7 points are defined as high quality documents; those with 4 ~ 7 points are medium quality documents; and those with a score less than 4 points are low quality documents. The scoring system provided an objective indication of the conscientiousness of each study, while it was not an instrument to assess the quality of the research. A unanimous quality score was achieved by at least 2 authors reviewing each paper and discussing the different viewpoints.
▪ Meta-analysis
The regional gray matter differences between the MDD patients and healthy control groups were analyzed using ES-SDM (Effect Size Seedbased d Mapping) [11] (http://www.sdmproject.com). The ES-SDM combines the advantages of activation likelihood estimate (ALE) [24] and multilevel kernel density analysis (MKDA) [25] . In the present study, a pooled meta-analysis with all studies included was first performed, followed by 2 subgroup meta-analysis with studies which are methodologically homogenous. The methodologically homogenous studies included those that utilized 3.0 T MRI and those of firstepisode MDD patients without drug treatment.
The meta-analysis consisted of the following steps [26] :
The peak coordinates of all gray matter differences were first identified from the studies. The differences were statistically significant at the whole brain level. For each study, the same statistical threshold was adopted throughout the brain in order to avoid any potential bias related to the methods using the liberal thresholds.
The peak coordinates of each study were then rebuilt with a map of the anisotropic effect size of group differences. The peak t value was converted to Hedges’ effect size and an unnormalized Gaussian Kernel was adopted to provide an indicator of proximity to the reported coordinates [12]. A wide FWHM (full-width at half-maximum) of 20 mm was adopted in order to control for false-positive results [27] .
Some studies reported no group difference and these studies were recreated with variance maps and anisotropic effect size. Different from existing studies, a null anisotropic effect size was assumed herein for all voxels in the anisotropic effect size group.
The mean study map was obtained weightedly by accounting for the heterogeneity between the study and the inverse of each study variance. And we assessed the statistical significance using a permutation test.
We determined the statistical significance by Monte Carlo randomization tests, and thus created the whole-brain null distributions; after that the p values were obtained directly. In order to balance the sensitivity and specificity, a p value of 0.005 was used as the main threshold, and the additional peak height z equaled 1 and the cluster extent was 10 voxels [11].
Meta-regression analysis: The mean age of patients and the percentage of female MDD patients were explored with simple linear regression using SDM [28] . The probability threshold was reduced to 0.005. The abnormalities were required to be detected in the slope and in one of the extremes of the regressor. In order to minimize the possibility of false relationships, the findings which were not detected in the major analysis were discarded. Finally, based on the regression plots, the fittings based on too few studies were discarded [29] .
Analysis of sensitivity, heterogeneity and publication bias
Jack-knife sensitivity analysis was conducted to test the replicability of results in different studies [29] . A random-effects model with Q statistics was used to examine the statistical heterogeneity of clusters between the studies. The χ2 distribution was first converted to z values. A permutation approach (p<0.005) was then used to test the distribution. The permutation approach was uncorrected for FDR (false discovery rate) with a peak height z of 1 and a cluster extent of 10 voxels. And then we used the Egger test to examine the possibility of publication bias for each changed brain region [30] .
Results
▪ Sample characteristics
The literature search identified 749 studies, and ultimately 14 studies [12,13,15,16,31-40] that met the inclusion criteria were used. Figure 1 shows the flow chart of document screening. These studies included 583 cMDD patients (227 male and 356 female with a mean age of 35.79 years old) and 727 healthy controls (176 male and 551 female with a mean age of 35.55 years old). In each study, no significant difference in age or gender existed between the patient group and the healthy controls. The demographic and clinical characteristics of the patients and healthy controls from all the 14 studies are summarized in Table 1.
| Study | Subjects (Female) | Mean Age | Illness duration (months) |
Severity (scale type) |
Education Level (years) |
Medication status | MRI | Quality Score | ||
|---|---|---|---|---|---|---|---|---|---|---|
| MDD | HC | MDD | HC | |||||||
| Jia, et al. [32] | 36(16) | 52(28) | 34.7 | 37.1 | 21 | 22.3 HAMD | 12.8 | N.A | 3 | 8 |
| Jia, et al. [32] | 16(11) | 52(28) | 34.2 | 37.1 | 80 | 24.6 HAMD | 12.8 | N.A | 3 | 8 |
| Li, et al. [31] | 25(20) | 25(19) | 46.5 | 40.6 | 21 | 21.9 HAMD | 10.5 | Treated | 1.5 | 8 |
| Zou, et al. [13] | 23(13) | 23(13) | 31.1 | 36.6 | 7.6 | 24.4 HAMD | 11.9 | Untreated | 3 | 8 |
| Scheurecker, et al. [40] | 13(3) | 15(5) | 37.9 | 35.5 | 52.3 | 20.5 HAMD | N.A | Untreated | 3 | 8 |
| Perico, et al. [38] | 20(15) | 94(41) | 29.9 | 30.2 | 8.2 | 19.6 HAMD | 9.4 | N.A | 1.5 | 7 |
| Lee, et al. [33] | 47(42) | 51(46) | 46 | 45.7 | 46.7 | 20.1 HAMD | N.A | Treated | 1.5 | 7 |
| Wagner, et al. [12] | 15(11) | 30(25) | 41 | 35.1 | 106.8 | 23.9 HAMD | 10.4 | N.A | 1.5 | 8 |
| Wagner, et al. [12] | 15(11) | 30(25) | 34.1 | 35.1 | 36 | 25.7 HAMD | 10.5 | N.A | 1.5 | 8 |
| Salvadore, et al. [16] | 58(37) | 107(60) | 38.8 | 36.2 | 220.8 | 26 MADRS | N.A | Untreated | 3 | 8 |
| Zhang, et al. [36] | 33(16) | 32(15) | 20.52 | 21.03 | N.A | 37.67 CESD | 13.85 | Untreated | 1.5 | 8 |
| Machino, et al. [39] | 29(13) | 29(13) | 39.57 | 38.66 | 52.55 | 19.9 HAMD | N.A | N.A | 1.5 | 8 |
| Arnone, et al. [15] | 39(27) | 66(46) | 36.3 | 32.1 | 5.35 | 27.2 MADRS | N.A | Untreated | 1.5 | 8 |
| Lai, et al. [37] | 38(20) | 27(15) | 36.57 | 38.29 | 4.68 | 22.26 HAMD | 15.68 | Untreated | 3 | 7 |
| Guo, et al. [35] | 44(22) | 44(24) | 27.52 | 29.39 | 19.61 | 25.18 HAMD | 12.52 | Untreated | 3 | 7 |
| Stratmann, et al. [34] | 35(21) | 132(74) | 34.86 | 37.82 | 14.66 | 19.46 HAMD | N.A | Treated | 3 | 8 |
| Stratmann, et al. [34] | 97(55) | 132(74) | 38.94 | 37.82 | 121.75 | 20.85 HAMD | N.A | Treated | 3 | 8 |
Table 1: Summary of demographic and clinical characteristics of participants in the 14 VBM studies (17 data sets).
▪ Pooled meta-analysis
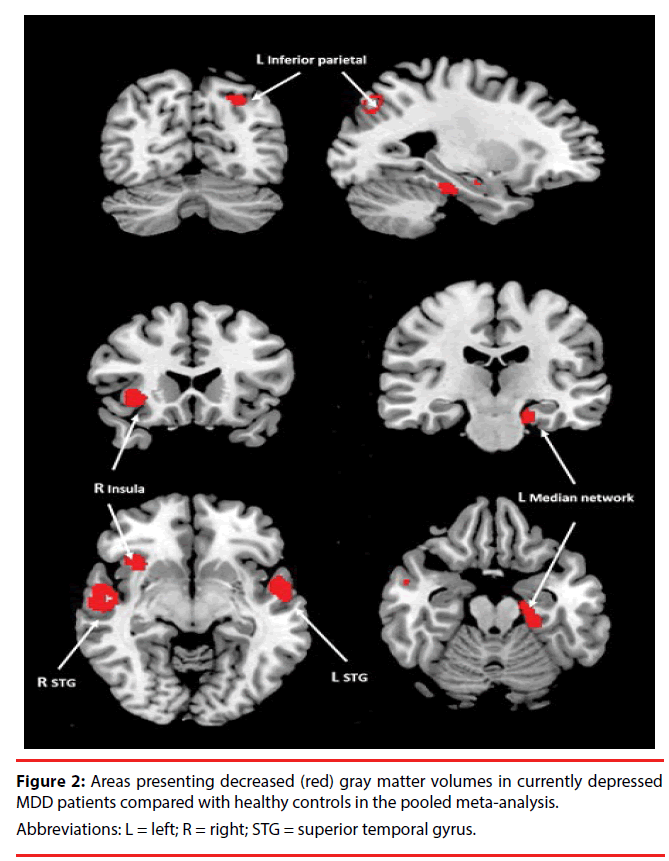
A pooled meta-analysis including all included studies was performed. The pooled meta-analysis revealed that the cMDD patients presented decreased gray matter volumes in the left superior temporal gyrus, right superior temporal gyrus, right insula, left median network, and left inferior parietal gyrus compared with healthy controls (Figure 2, Table 2). No gray matter volume increases were indicated in the pooled meta-analysis.
| Region | MNI coordinates | SDM-Z score | P Value uncorrected |
Voxels | Cluster breakdown(voxels) | Jackknife Sensitivity | Egger test p value |
||
|---|---|---|---|---|---|---|---|---|---|
| x | y | Z | |||||||
| Left superior temporal gyrus | -54 | 2 | 0 | -2.627 | 0.000149667 | 802 | Left superior temporal gyrus (395) | 13/14 | 0.312 |
| Left inferior frontal gyrus (127) | |||||||||
| Left rolandic operculum (171) | |||||||||
| Left insula (59) | |||||||||
| Corpus callosum (29) | |||||||||
| Left frontal aslant tract (21) | |||||||||
| Right superior temporal gyrus | 54 | -8 | -14 | -2.439 | 0.000361264 | 503 | Right superior temporal gyrus (246) | 13/14 | 0.534 |
| Corpus callosum (92) | |||||||||
| Right middle temporal gyrus (143) | |||||||||
| Right inferior network (22) | |||||||||
| Right insula | 36 | 18 | -4 | -2.541 | 0.000206411 | 211 | Right insula (126) | 13/14 | 0.283 |
| Right inferior frontal gyrus (46) | |||||||||
| Right lenticular nucleus (19) | |||||||||
| Right inferior network (20) | |||||||||
| Left median network, cingulum | -22 | -22 | -20 | -2.145 | 0.001543105 | 161 | Left median network (64) | 10/14 | 0.416 |
| Left parahippocampal gyrus (66) | |||||||||
| Left hippocampus (21) | |||||||||
| Left fusiform gyrus (10) | |||||||||
| Left inferior parietal gyri | -28 | -68 | 44 | -2.119 | 0.001718521 | 97 | Left superior parietal gyrus (39) | 13/14 | 0.837 |
| Left inferior parietal gyrus (27) | |||||||||
| Left superior occipital gyrus (21) | |||||||||
| Left middle occipital gyrus (10) | |||||||||
Table 2: Decreased gray matter volumes in major depressive disorder patients compared with the healthy controls.
▪ Subgroup meta-analysis
Seven studies with first-episode MDD patients without drug treatment were used in a subgroup meta-analysis (Table 3). The results showed that the gray matter volume decreased in the left insula, right fusiform gyrus, left inferior temporal gyrus, and left anterior thalamic projections. While for the 3.0 T MRI subgroup of the 7 studies, the results revealed that the gray matter volumes decreased in the left rolandic operculum, left inferior parietal gyrus, right superior temporal gyrus, left median network, and right inferior frontal gyrus in cMDD patients.
| Region | MNI coordinates | SDM-Z score | P Value uncorrected |
Voxels | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Subgroup meta-analysis of studies with first-episode drug-naive | ||||||
| Left insula | -34 | -8 | 12 | -1.963 | 0.000268340 | 1462 |
| Right fusiform gyrus | 38 | -18 | -28 | -2.328 | 0.000036120 | 611 |
| Left hippocampus | -22 | -18 | -20 | -2.306 | 0.000036120 | 581 |
| Left inferior temporal gyrus | -38 | -14 | -36 | -1.763 | 0.001114726 | 68 |
| Left anterior thalamic projections | -6 | -10 | -2 | -1.641 | 0.002188206 | 56 |
| Subgroup meta-analysis of studies with 3.0 T MRI | ||||||
| Left rolandic operculum | -56 | 6 | 4 | -2.617 | 0.000103235 | 1048 |
| Left inferior parietal gyrus | -32 | -66 | 44 | -2.298 | 0.000485122 | 231 |
| Right superior temporal gyrus | 58 | -14 | -8 | -2.007 | 0.001847565 | 154 |
| Left median network, cingulum | -24 | -30 | -16 | -2.131 | 0.001073420 | 137 |
| Right inferior frontal gyrus | 30 | 24 | -6 | -1.911 | 0.002910674 | 38 |
Table 3: Areas presenting decreased gray matter volumes in MDD patients compared with HC in two subgroup meta-analysis.
▪ Meta-regression analyses
Because there were no adequate datasets and consistent depression scale data, illness duration and depressive severity could not be explored using meta-regression [29]. Therefore, we selected the variables of the mean age of patients and the percentage of female patients for regression. There variables were available for all 583 patients in the 14 studies. The analyses indicated no significant effects of the female patient percentage and the mean age of patients.
▪ Analysis of reliability, heterogeneity and publication bias
A jack-knife sensitivity analysis was performed on the pooled meta-analysis and the results showed that the gray matter volume reduced in the left superior temporal gyrus, right superior temporal gyrus, right insula, and left inferior parietal gyrus. The reductions were found to be highly replicable, as it was found throughout all the 14 studies but one study. The results in the left median network were significant in all except four studies (Table 2). The heterogeneity analysis revealed that the gray matter volume reduced in the right superior temporal gyrus, left superior temporal gyrus, right insula, and left median network. A significant heterogeneity among studies exists in the reductions (p<0.005) (Table 2). The Egger test showed that it was not significant for each brain area in the publication bias analysis (Table 2).
Discussions
Our results have showed that the gray matter alterations of the cMDD patients are in the left superior temporal gyrus, right superior temporal gyrus, right insula, left median network, and left inferior parietal gyrus.
Existing studies have shown that gray matter changes are mostly concentrated in the frontal cortex and limbic system; however, based on our meta-analysis, we found that the patients with MDD have gray matter changes also in the temporal lobe. Patients with MDD mainly show emotion adjustment disorders, which also affect cognition, will and behavior. The neural circuits, which the temporal and parietal lobes connected with the frontal lobe through the nerve fiber bundle, plays an important role in the emotion regulation [41]. It was found that the anatomical deficits of brain were obvious in severe period of depression [42], and the functional alterations became normal as the illness recovered [43]. Tang, et al. found that the first episode, MDD patients without drug treatment showed reduced gray matter in the right precentral gyrus, left temporal lobe temporal gyrus, right postcentral gyrus, left parietal paracentral lobule, and left posterior cingulate [44]. In addition, patients with schizophrenia have been reported to have similar anatomical and functional abnormalities [45,46], while the patients with major depressive disorder often have the psychotic symptoms, such as hallucinations, which may be a reason to support the results of our study.
Our study has also found that the cMDD patients have reduced gray matter in the right insula. The insula is the source of social mood such as sexual desire, shame, guilt, etc. The insula can perceive the body state, which is an essential area in the pathogenesis of depression. There are numerous studies which are consistent with our result [47,48]. And the fMRI studies on the MDD have also found the abnormal activity in insula [49,50].
The anterior cingulated cortex (ACC) is mainly composed of cognitive and affective sub-regions [51]. ACC is closely related with the affection. Several meta-analyses of ROI-based imaging studies have revealed a consistent observation with volumetric reduction in the ACC of MDD patients [4,7]. Compared with the control subjects, drug-naive MDD patients have been found to have a decreased connectivity between the subgenual ACC and the precuneus according to functional MRI [52]. Some researchers found a consistent gray matter reduction in rostral ACC based on the Seed-based d Mapping (SDM) technique. Some scholars have found that male and elderly patients would be correlated with the gray matter deficits of ACC, indicating that old and male MDD patients are more specific to this type of gray matter reduction. It was also found that the drug-naive patients of MDD are prone to have a greater gray matter deficit in the right ACC [53]. Anand [54] found that the structural and functional abnormalities of the amygdala and anterior cingulate gyrus in patients with depression are closely related, and these structural and functional abnormalities are closely related to the mood and emotion regulation [55,56]. The gray matter volume decreased in the anterior cingulate and amygdala in the first episode and drug-free male MDD patients, and there is a study showing that the structure damage of neural circuits in anterior cingulated- amygdale may be involved in the pathophysiology of depression [57]. Our study did not find any significant change in this area; the possible reasons may be due to the diverse analysis method and the different patients included in the research, which are needed to be further studied by enlarging the samples.
The frontal lobe, which accounts for 40%-50% of the whole brain volume, is closely related to the high level of human mental activity, especially in emotional activities and the cognitive function. A significant number of neuroimaging studies have indicated that MDD patients have structure and functional abnormalities in frontal lobe. There are some functional MRI studies showing that the MDD patients have abnormal functional activity in the inferior and right middle frontal gyrus which extends to the anterior temporal area [58]. A structural MRI study on MDD showed significant gray matter volume deficits in bilateral superior frontal gyrus, left medial frontal gyrus and left middle frontal gyrus, which were correlated with the attention deficit and emotional bias [37]. In addition, a functional study showed that patients with early onset depression (EOD) and late onset depression (LOD) exhibited reversal patterns of abnormal amplitude of low-frequency fluctuations (ALFF) in bilateral SFG, which might indicate that the patients with different onset ages would have diverse functional activity in the SFG [59]. Besides, MFG changes were found to be also important in the pathophysiology of MDD [60]. A study suggests that the dysfunction in the left middle frontal gyrus (I-MFG) may be an imaging endophenotype which is an indicator of a risk for MDD [47]. It also suggested that the right dorsal medial frontal gyrus (r-DMFG) may play an important role in depressive symptomatology, and it may reveal therapeutic target for MDD [61]. A longitudinal study on selective serotonin reuptake inhibitor (SSRI)-treated MDD patients has shown that effective antidepressant treatment with sertraline is related with left dorsolateral prefrontal cortex (DLPFC) volume increase, and this volume increase may reflect cortical architectural changes related to topdown neuronal modulation of emotion [62]. As mentioned in the preceding sections, the metaanalysis in the present study also demonstrated significant gray matter decreases in the inferior frontal gyrus and the frontal aslant tract.
Our study has found that the cMDD patients showed reduced gray matter volumes in the left median network, left parahippocampal gyrus, and left hippocampus. The hippocampus and parahippocampus are two key regions for the limbic system which are essential in regulating the motivation, emotions, memory, and affective dimension of pain [63]. They play important roles in connecting with the cortical and subcortical structures of the frontal and temporal lobes in the cognitive processes of MDD [64]. The hippocampus has been extensively studied in MDD. The reduction of gray matter volume in the hippocampus was found in MDD patients by some researchers [65]. The possible mechanism is that a negative event causes an increase in glucocorticoid in the human body, while the hippocampus is an easily damaged area of the brain and is very sensitive to the reactivity of hormone. The hippocampal neurons and the volume of hippocampal shrink due to the reduction in the neuropeptides and synapses in the hippocampus [66]. Natalia Jaworska also found that MMD patients had smaller right hippocampi compared with healthy controls [50]. And he also found that an older depressed participant had a smaller hippocampal volume than a younger one [49]. However, some scholars have pointed out that the antidepressant treatment itself may affect the results of the study. Animal and human studies have shown that the therapy of grey matter has a significant effect. He found that adult MDD patients had a region with significantly increased gray matter volume in the left DLPFC after 12 weeks of SSRI treatment [62]. An animal study on tree shrews has shown that stressed animals treated with 28 days of tianeptine had an increased hippocampal volume above the decrease caused by stress alone [67]. As a result, there have been many studies on the gray matter of drug-free MDD patients. It has also been reported that gray matter volumes in the bilateral hippocampus and parahippocampal gyrus reduce in MDD patients without drug treatment [65,68].
Our study found that the MDD patients in severe episodes had significantly abnormal gray matter volumes in the temporal lobe. The role of the rest neocortex, such as the temporal lobe, parietal lobe, and occipital lobe, on the pathogenesis and pathophysiology of depression is worthy of our further research.
Conclusions
By using the SDM method, we identified that the currently depressed MDD (cMDD) patients have significantly decreased gray matter volumes in the temporal lobe, the right insula, the left median network, and left inferior parietal gyrus. We identified the most crucial brain anatomical structural changes directly in MDD. The findings in this study provide evidence that the gray matter abnormality may be affected by the different episodes of the disease, and the gray matter decrease in the temporal lobe and right insula may also be the basic pathophysiology of MDD.
Disclosure
The authors have no conflicts of interest to declare. The authors alone are responsible for the content and writing of the paper.
Funding
The authors acknowledge the support received from Beijing Municipal Administration of Hospitals’ Youth Programme (QML 20162002).
Author Contributions
C. Lin and D.C. Chen conceived and designed the study. C. Lin drafted the manuscript. Y. Bian and C. Lin did the literature search and review. Y. Bian and D.C. Chen extracted the data from the literature. C. Lin, Y. Bian, X.L. Han and L. Chen performed the study. Y. Bian and X.L. Han conducted the statistical analyses. Y. Zhu participated in the processing of brain images and the tables. All authors reviewed the manuscript. C. Lin, Y. Bian and D.C. Chen revised the manuscript.
References
- Rizvi SJ, Kennedy SH. Emerging drugs for major depressive disorder: an update. Expert. Opin. Emerg. Drugs 17(3), 285-294 (2012).
- MacQueen GM. Magnetic resonance imaging and prediction of outcome in patients with major depressive disorder. J. Psychiatry. Neurosci 34(5), 343-349 (2009).
- Wu F, Tang Y, Xu K et al. Whiter matter abnormalities in medication-naive subjects with a single short-duration episode of major depressive disorder. Psychiatry. Res 191(1), 80-83 (2011).
- Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, et al. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum. Brain. Mapp 30(11), 3719-3735 (2009).
- Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol. Psychiatry 13(11), 993-1000 (2008).
- McKinnon MC, Yucel K, Nazarov A, et al. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J. Psychiatry. Neurosci 34(1), 41-54 (2009).
- Hajek T, Kozeny J, Kopecek M, et al. Reduced subgenual cingulate volumes in mood disorders: a meta-analysis. J. Psychiatry. Neurosci 33(2), 91-99 (2008).
- Guo XJ, Yao L, Jin Z. A method of voxel-based morphometry and its applications to brain image processing. J. Beijing. Normal. Univ (Natural Science). 42(2), 213-216 (2006).
- Treadway MT, Grant MM, Ding Z, et al. Early adverse events, HPA activity and rostral anterior cingulate volume in MDD. PLoS. ONE 4(3), e4887 (2009).
- Vasic N, Walter H, Höse A, et al. Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: a voxel-based morphometry study. J. Affect. Disord 109(1-2), 107-116 (2008).
- Yuan Y, Zhu W, Zhang Z et al. Regional gray matter changes are associated with cognitive deficits in remitted geriatric depression: an optimized voxel-based morphometry study. Biol. Psychiatry 64(6), 541-544 (2008).
- Wagner G, Koch K, Schachtzabel C, et al. Structural brain alterations in patients with major depressive disorder and high risk for suicide: evidence for a distinct neurobiological entity. Neuroimage 54(2), 1607-1614 (2011).
- Zou K, Deng W, Li T, et al. Changes of brain morphometry in first-episode, drug-naïve, non-late-life adult patients with major depression: an optimized voxel-based morphometry study. Biol. Psychiatry 67(2), 186-188 (2010).
- Tang Y, Wang F, Xie G, et al. Reduced ventral anterior cingulate and amygdala volumes in medication-naïve females with major depressive disorder: A voxel-based morphometric magnetic resonance imaging study. Psychiatry. Res 156(1), 83-86 (2007).
- Arnone D, McKie S, Elliott R, et al. State-dependent changes in hippocampal grey matter in depression. Mol. Psychiatry 18(12), 1265-1272 (2013).
- Salvadore G, Nugent AC, Lemaitre H, et al. Prefrontal cortical abnormalities in currently depressed versus currently remitted patients with major depressive disorder. Neuroimage 54(4), 2643-2651 (2011).
- Baxter LR, Phelps ME, Mazziotta JC, et al. Cerebral metabolic rates for glucose in mood disorders. Studies with positron emission tomography and fluorodeoxyglucose F 18. Arch. Gen. Psychiatry 42(5), 441-447 (1985).
- Drevets WC, Videen TO, Price JL, et al. A functional anatomical study of unipolar depression. J. Neurosci 12(9), 3628-3641 (1992).
- Kalin NH, Davidson RJ, Irwin W, et al. Functional magnetic resonance imaging studies of emotional processing in normal and depressed patients: effects of venlafaxine. J. Clin. Psychiatry 58(1), 1632-1639 (1997).
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann. N. Y. Acad. Sci 877614-877637 (1999).
- Konarski JZ, McIntyre RS, Kennedy SH, et al. Volumetric neuroimaging investigations in mood disorders: bipolar disorder versus major depressive disorder. Bipolar. Disord 10(1), 01-37 (2008).
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med 151(4), 264-269, W64 (2009).
- Huijbrechts IP, Haffmans PM, Jonker K, et al. A comparison of the 'Hamilton Rating Scale for Depression' and the 'Montgomery-Asberg Depression rating Scale'. Acta. Neuropsychiatr 11(1), 34-37 (1999).
- Turkeltaub PE, Eden GF, Jones KM, et al. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16(3 Pt 1), 765-780 (2002).
- Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Soc. Cogn. Affect. Neurosci 2(2), 150-158 (2007).
- Peng W, Chen Z, Yin L, et al. Essential brain structural alterations in major depressive disorder: A voxel-wise meta-analysis on first episode, medication-naive patients. J. Affect. Disord 199114-199123 (2016).
- Radua J, Mataix-Cols D, Phillips ML, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiatry 27(8), 605-611 (2012).
- Radua J, Rubia K, Canales-Rodríguez EJ, et al. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front. Psychiatry 513 (2014).
- Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br. J. Psychiatry 195(5), 393-402 (2009).
- Chen ZQ, Du MY, Zhao YJ, et al. Voxel-wise meta-analyses of brain blood flow and local synchrony abnormalities in medication-free patients with major depressive disorder. J. Psychiatry. Neurosci 40(6), 401-411 (2015).
- Li CT, Lin CP, Chou KH, et al. Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. Neuroimage 50(1), 347-356 (2010).
- Jia Z, Huang X, Wu Q, et al. High-field magnetic resonance imaging of suicidality in patients with major depressive disorder. Am. J. Psychiatry 167(11), 1381-1390 (2010).
- Lee HY, Tae WS, Yoon HK, et al. Demonstration of decreased gray matter concentration in the midbrain encompassing the dorsal raphe nucleus and the limbic subcortical regions in major depressive disorder: an optimized voxel-based morphometry study. J. Affect. Disord 133(1-2), 128-136 (2011).
- Stratmann M, Konrad C, Kugel H, et al. Insular and hippocampal gray matter volume reductions in patients with major depressive disorder. PLoS. ONE 9(7), e102692 (2014).
- Guo W, Liu F, Yu M, et al. Functional and anatomical brain deficits in drug-naive major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 541-546 (2014).
- Zhang X, Yao S, Zhu X, et al. Gray matter volume abnormalities in individuals with cognitive vulnerability to depression: a voxel-based morphometry study. J. Affect. Disord 136(3), 443-452 (2012).
- Lai CH, Wu YT. Frontal-insula gray matter deficits in first-episode medication-naïve patients with major depressive disorder. J. Affect. Disord 16074-16079 (2014).
- de Azevedo-Marques Périco C, Duran FL, Zanetti MV, et al. A population-based morphometric MRI study in patients with first-episode psychotic bipolar disorder: comparison with geographically matched healthy controls and major depressive disorder subjects. Bipolar. Disord 13(1), 28-40 (2011).
- Machino A, Kunisato Y, Matsumoto T, et al. Possible involvement of rumination in gray matter abnormalities in persistent symptoms of major depression: an exploratory magnetic resonance imaging voxel-based morphometry study. J. Affect. Disord 168229-168235 (2014).
- Scheuerecker J, Meisenzahl EM, Koutsouleris N, et al. Orbitofrontal volume reductions during emotion recognition in patients with major depression. J. Psychiatry. Neurosci 35(5), 311-320 (2010).
- Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb. Cortex 15(6), 854-869 (2005).
- Bora E, Fornito A, Pantelis C, et al. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J. Affect. Disord 138(1-2), 9-18 (2012).
- Wang L, Hermens DF, Hickie IB, et al. A systematic review of resting-state functional-MRI studies in major depression. J. Affect. Disord 142(1-3), 06-12 (2012).
- Tang YQ, Wu F, Kong LT, et al. Gray matter volume changes in first-episode,medication naive patients with major depressive disorder: a voxel-based morphometric 3.0 T MRI study. Chin. J. Clinicians 05(10), 2926-2929 (2011).
- Ren W, Lui S, Deng W, et al. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am. J. Psychiatry 170(11), 1308-1316 (2013).
- Rubinov M, Bassett DS. Emerging evidence of connectomic abnormalities in schizophrenia. J. Neurosci 31(17), 6263-6265 (2011).
- Jung J, Kang J, Won E, et al. Impact of lingual gyrus volume on antidepressant response and neurocognitive functions in Major Depressive Disorder: a voxel-based morphometry study. J. Affect. Disord 169179-169187 (2014).
- Liu CH, Jing B, Ma X, et al. Voxel-based morphometry study of the insular cortex in female patients with current and remitted depression. Neuroscience 262190-262199 (2014).
- Jaworska N, Yücel K, Courtright A, et al. Subgenual anterior cingulate cortex and hippocampal volumes in depressed youth: The role of comorbidity and age. J. Affect. Disord 190726-190732 (2016).
- Kaiser RH, Andrews-Hanna JR, Wager TD, et al. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA. Psychiatry 72(6), 603-611 (2015).
- Yücel M, Wood SJ, Fornito A, et al. Anterior cingulate dysfunction: implications for psychiatric disorders. J. Psychiatry. Neurosci 28(5), 350-354 (2003).
- Connolly CG, Wu J, Ho TC, et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol. Psychiatry 74(12), 898-907 (2013).
- Lai CH. Gray matter volume in major depressive disorder: a meta-analysis of voxel-based morphometry studies. Psychiatry. Res 211(1), 37-46 (2013).
- Anand A, Li Y, Wang Y, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol. Psychiatry 57(10), 1079-1088 (2005).
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J. Comp. Neurol 453(2), 116-130 (2002).
- Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat. Neurosci 8(6), 828-834 (2005).
- Tang YQ, Kong LT, Wu F, et al. Anterior cingulate and amygdala volume changes in first-episode, medication naive females with major depressive disorder: a voxel-based morphometric MRI study. Chin. J. Behav. Med. Brain. Sci 20(6), 525-527 (2011).
- Garrett A, Kelly R, Gomez R, et al. Aberrant brain activation during a working memory task in psychotic major depression. Am. J. Psychiatry 168(2), 173-182 (2011).
- Guo WB, Liu F, Xun GL, et al. Reversal alterations of amplitude of low-frequency fluctuations in early and late onset, first-episode, drug-naive depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 40153-401159 (2013).
- Abe O, Yamasue H, Kasai K, et al. Voxel-based analyses of gray/white matter volume and diffusion tensor data in major depression. Psychiatry. Res 181(1), 64-70 (2010).
- Liu CH, Ma X, Wu X, et al. Resting-state brain activity in major depressive disorder patients and their siblings. J. Affect. Disord 149(1-3), 299-306 (2013).
- Smith R, Chen K, Baxter L, et al. Antidepressant effects of sertraline associated with volume increases in dorsolateral prefrontal cortex. J. Affect. Disord 146(3), 414-419 (2013).
- Fossati P. Neural correlates of emotion processing: from emotional to social brain. Eur. Neuropsychopharmacol 22(1), 3S487-3S491 (2012).
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology 35(1), 192-216 (2010).
- Zhao YJ, Du MY, Huang XQ, et al. Brain grey matter abnormalities in medication-free patients with major depressive disorder: a meta-analysis. Psychol. Med 44(14), 2927-2937 (2014).
- MacMaster FP, Mirza Y, Szeszko PR, et al. Amygdala and hippocampal volumes in familial early onset major depressive disorder. Biol. Psychiatry 63(4), 385-390 (2008).
- Czéh B, Michaelis T, Watanabe T, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci. U.S.A. 98(22), 12796-12801 (2001).
- Kempton MJ, Salvador Z, Munafò MR, et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch. Gen. Psychiatry 68(7), 675-690 (2011).