Research Article - Neuropsychiatry (2016) Volume 6, Issue 5
Gray matter alterations in cirrhosis with and without hepatic encephalopathy: a meta-analysis of voxel-based morphometry studies
- Corresponding Authors:
- Jian-Ping Gu, MD, Ph.D
Department of Vascular and Interventional Radiology, Nanjing First Hospital, Nanjing Medical University, No.68, Changle Road, Nanjing 210006, China
Tel: +862587726268
Fax: +862552236361
Abstract
Voxel-based morphometry (VBM) studies have revealed brain gray matter (GM) abnormalities in cirrhotic patients with and without hepatic encephalopathy. However, these findings are heterogeneous and have not been quantitatively reviewed. Here, we aimed to conduct a meta-analysis that integrated the existing VBM studies, to determine concordant GM alterations in patients with cirrhosis. A systematic search was conducted for the whole-brain VBM studies comparing cirrhotic patients with healthy controls. Coordinates were extracted from clusters with significant GM differences.
Keywords
Cirrhosis, Voxel-based morphometry, Meta-analysis, Gray matter, Activation likelihood estimation
Introduction
Cirrhosis has a critical neuropsychiatric complication of cognitive decline, which will develop gradually into hepatic encephalopathy (HE) [1-3]. HE is regarded as a continuum ranging from minimal HE (MHE) to overt HE (OHE), rather than distinct stages [4]. Patients with HE have a wide spectrum of manifestations, including intellectual impairment, neuromuscular dysfunction, personality changes, and disorientation; even cirrhotic patients with normal mental status may be found to have abnormalities in cognitive function that will cause poor quality of life [4,5]. To date, the methods used to explore the brain function in cirrhotic patients are mainly based on neuropsychometric or neurophysiologic testing, and other clinical findings [4,6,7]. However, there exist no unbiased biomarkers to validate the diagnosis. A growing body of evidence has investigated the key brain regions involved in the pathophysiological markers of cirrhosis through previous neuroimaging studies using conventional magnetic resonance imaging (MRI), MR spectroscopy (MRS), diffusion tensor imaging (DTI), functional MRI (fMRI), and positron emission tomography (PET) [8-12]. Despite extensive research, the neuroanatomical changes and neuropathophysiological mechanisms underlying cirrhosis are not fully understood.
Voxel-based morphometry (VBM) is an automated whole brain-based method which performs a voxel-wise comparison of the local concentration of gray matter (GM) between patients and healthy controls, which has accuracy comparable with manual volumetry and overcomes the technical limitations of region-of-interest (ROI) approaches [13,14]. VBM has been widely applied in characterizing subtle changes of brain structures in diverse neuropsychiatric diseases [14]. Previous VBM studies have identified the GM abnormalities that are associated with cirrhosis [15]. Nonetheless, these studies reported relatively inconsistent results. Several researches demonstrated the increased GM volumes in thalamus between cirrhotic patients and healthy controls [16-20], but not in others [21,22]. Moreover, various GM alterations were revealed in different clinical stages of cirrhosis [17,18]. These reported discrepancies can potentially be attributed to the limited sample size, variable clinical aetiologies and imaging parameters. Thus, there is an urgent need of meta-analysis to confirm concordant results concerning the role of GM anomalies as common markers in patients with cirrhosis.
Activation likelihood estimation (ALE) is the most common coordinate-based meta-analytic method for meta-analyses of neuroimaging literature to identify brain locations showing a consistent response across experiments. This approach is based on the collection of peak coordinates from each study included in the meta-analysis rather than the input of raw images [23-26]. ALE technique has been successfully applied to neuroimaging studies of various neurological or psychiatric disorders, such as schizophrenia [27], epilepsy [28], Parkinson’s disease [29], and narcolepsy [30]; however, to our knowledge, no meta-analysis of VBM studies of cirrhosis has been reported. Therefore, our current study aimed to quantitatively conduct a meta-analysis using the ALE algorithm to determine the GM structural abnormalities underlying cirrhosis with and without HE. We speculated that widespread GM deficits would be detected within the cortical and subcortical regions in cirrhotic patients.
Materials and Methods
▪ Search Strategies and Study Selection
This meta-analysis was performed according to the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) criteria [31]. The search was conducted in February 2016, and no time span was specified for the dates of publication. Studies considered for inclusion in the meta-analysis were identified from exhaustive searches using PubMed, Web of Knowledge, Embase and Science Direct using the terms “cirrhosis,” “cirrhotic,” “hepatic failure,” “liver failure,” or “hepatic encephalopathy,” plus “voxel-based morphometry,” “voxel-based,” “morphometry” or “VBM.” We restricted our search to humans. In addition, we reviewed manually the references cited in articles that were retrieved. We extracted demographic data from each article, including the first author’s name, year of publication, journal title, total patient number, sex distribution, patient mean age and range, the method of matching, and statistic thresholds.
Studies were selected according to the following inclusion criteria: (1) published as an article (and not a letter or an abstract); (2) comparisons of cirrhotic patients with healthy control groups; (3) used the whole-brain VBM procedure to analyze GM alterations; (4) clearly reported Montreal Neurological Institute (MNI) or Talairach coordinates of the activation areas (x, y, z). Studies reporting only findings for specific ROIs were not included in the present metaanalysis. In cases where the overlapping samples were used in separate articles, we only included the data from the analysis of the largest sample. Two independent reviewers (CYC and FY) evaluated the methodology and the risk of bias of the eligible studies. Any disagreements were assessed by the third reviewer (RJ). The majority opinion was used for final analysis.
▪ Data extraction
The x, y, and z peak activation coordinates of all eligible contrasts constituted the metaanalysis input. The data from the originally reported in Talairach spaces were converted to MNI coordinates [32]. The data from MNI coordinates were texted and implemented in GingerALE 2.3.3 (http://brainmap.org/ale/, Research Imaging Institute of the University of Texas Health Science Center, San Antonio, Texas).
▪ ALE meta-analysis
Ginger ALE software was used to compare the GM changes between cirrhotic patients and healthy controls. The reported loci of maximal anatomical differences were modelled as the peaks of three-dimensional Gaussian probability density functions defined by the full-width at half-maximum (FWHM), which was set according to a quantitative uncertainty model [23,25]. ALE values were calculated on a voxelby- voxel basis by measuring the union model activation (MA) maps modelled above. This revised analysis tested for convergence by studies (random effects) instead of foci (fixed effects). These maps were finally threshold at P<0.05, corrected for multiple comparisons using falsediscovery rate (FDR; q) [23,33]. The volume of the minimum cluster threshold was set at 200 mm3. The coordinates of the weighted centre were generated for each cluster. The resulting significant anatomical areas were labeled based on probabilistic cytoarchitectonic maps of the human brain using the SPM Anatomy Toolbox v2.1 [34].
Results were visualized with Mango software (http://ric.uthscsa.edu/mango/mango.html), using the Colin brain template in the MNI space (http://www.brainmap.org/ale).
Results
▪ Study selection
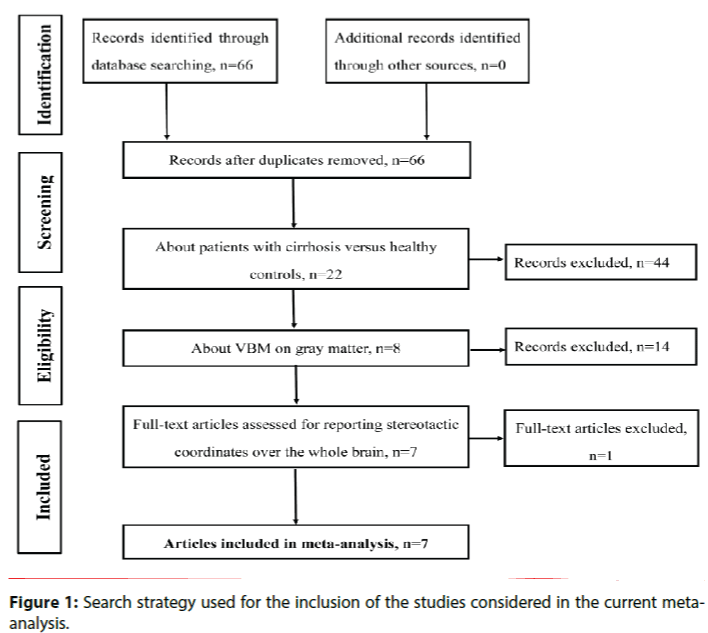
The initial search identified 66 possible articles after the duplicates were removed. Fifty-nine articles were excluded after the abstract and full-text articles selection. Finally, the search strategy identified that a total of 7 VBM studies met the inclusion criterion of comparing GM alterations between patients with cirrhosis and healthy controls [16-22]. No additional articles were found in the reference lists of the selected studies. In 2 studies, the analyses were performed based on 3 different subgroups of patients with cirrhosis, who were then compared with the same healthy control groups [17,18]. Therefore, we treated these studies as unique studies, with each patient subgroup included independently in the meta-analysis; a total of 11 datasets were ultimately included in the meta-analysis.
Our final sample comprised 271 patients with cirrhosis and 215 healthy controls. Figure 1 shows the identification and attrition of the studies. The clinical and demographic data of the participants from all recruited studies are presented in Table 1. The subjects of patients and controls from each study are generally comparable by age, sex, and education.
| Study | Journal | Type of patients | Child-Pugh's class: A/B/C | Sample/male | Mean age ± SD | Matched control | Foci NO. | Scanner | SPM | Smoothing kernel (mm) | Statistical threshold | Measure | Tal or MNI | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| patients | control | patients | control | ||||||||||||
| Guevara,2011 | Journal of Hepatology | cirrhosis without OHE,18 MHE included | 17/13/18 | 48/30 | 51/23 | 61NPY-16-160 (140)9 | 60 ± 11 | age,sex | 22 | 1.5T GE | 2 | NA | P<0.05,FDR corrected | GMD | MNI |
| Iwasa,2012 | Metabolic brain disease | cirrhosis,4 OHE included | 11/4/3 | 18/5 | 16/7 | 65.7 ± 8.6 | 68.7 ± 7.4 | age | 5 | 1.5T Philips | 8 | 8 | p<0.001,uncorrected | GMV | Tal |
| Chen,2012 | European Journal of Radiology | cirrhosis with OHE | 3/8/10 | 21/18 | 20/17 | 51.9 ± 9.8 | 51.2 ± 7.7 | age,sex,education | 24 | 1.5T Toshiba | 5 | 8 | P<0.05,FWE corrected | GMV | MNI |
| cirrhosis with OHE | 11/NA | 14 | |||||||||||||
| *Zhang,2012 | Plos one | cirrhosis with MHE | 32/23/5 | 18/NA | 40/26 | 49.7 ± 10.1 | 49.8 ± 11.8 | age,sex | 13 | 3.0T Siemens | 8 | 8 | P<0.01,FDR corrected | GMV | MNI |
| cirrhosis without HE | 31/NA | 9 | |||||||||||||
| Qi,2013 | European Radiology | cirrhosis with MHE | 12/12/1 | 25/18 | 25/18 | 56.16 ± 6.25 | 53.72 ± 8.51 | age,sex | 15 | 3.0T Siemens | 8 | 8 | P<0.05,FDR corrected | GMV | MNI |
| cirrhosis without HE | 10/8/6 | 24/16 | 47.17 ± 11.43 | 4 | |||||||||||
| *Tao,2013 | European Journal of Radiology | cirrhosis with MHE | 4/6/13 | 23/17 | 33/22 | 44.73 ± 10.34 | 45.18 ± 10.76 | age,sex,education | 7 | 3.0T Siemens | 8 | 8 | P<0.05,FDR corrected | GMV | MNI |
| cirrhosis with OHE | 0/2/22 | 24/17 | 47.08 ± 11.46 | 11 | |||||||||||
| Lin,2014 | Plos one | cirrhosis with MHE | 0/17/9 | 28/24 | 30/19 | 51.1 ± 8.4 | 52.8 ± 9.8 | age,sex | 5 | 3.0T GE | 8 | 8 | P<0.05,FWE corrected | GMV | MNI |
*These two studies have subgroups of cirrhotic patients without HE, with MHE, and with OHE.
Table 1: List of all studies included in the meta-analysis: subjects’ demographic and clinical characteristics. HE, hepatic encephalopathy; MHE, minimal HE;
OHE, overt HE; NA, not available; SPM, statistical parametric mapping; MNI, Montreal Neurological Institute; FDR, false discovery rate; FWE, family-wise error;
▪ Voxel-wise meta-analysis
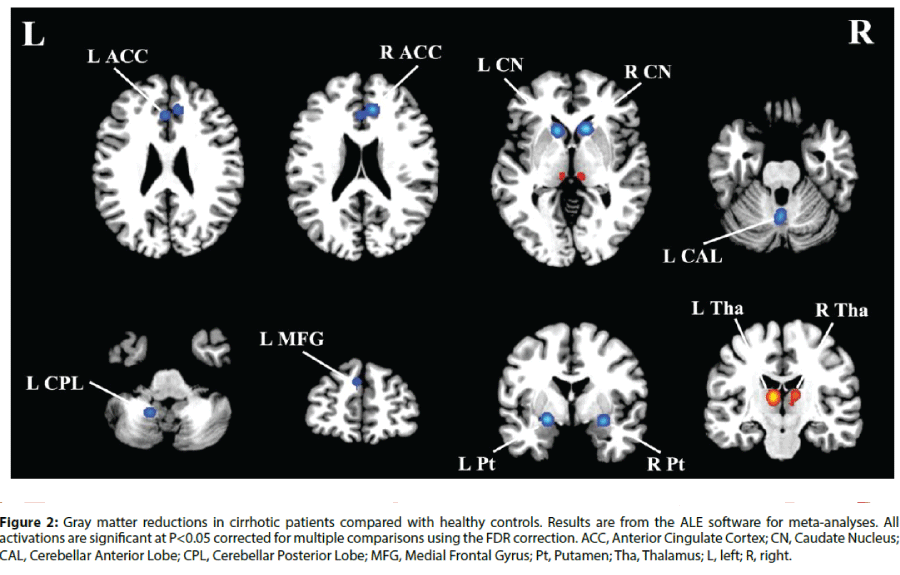
In the voxel-wise meta-analysis, a total of 129 peak foci were reported. Compared with healthy controls, patients with cirrhosis had GM reductions in the bilateral anterior cingulate cortex (ACC), caudate nucleus and putamen. GM decreases were also observed in the left medial frontal gyrus, cerebellar anterior lobe and cerebellar posterior lobe (Figure 2). In addition, GM increases were detected in the bilateral thalamus. Table 2 displays the coordinates of cluster maxima.
Figure 2: Gray matter reductions in cirrhotic patients compared with healthy controls. Results are from the ALE software for meta-analyses. All activations are significant at P<0.05 corrected for multiple comparisons using the FDR correction. ACC, Anterior Cingulate Cortex; CN, Caudate Nucleus; CAL, Cerebellar Anterior Lobe; CPL, Cerebellar Posterior Lobe; MFG, Medial Frontal Gyrus; Pt, Putamen; Tha, Thalamus; L, left; R, right.
| Brain regions | BA | MNI Coordinates x, y, z (mm) |
ALE extrema value | Cluster size (mm3) |
|---|---|---|---|---|
| Cirrhosis<Controls | ||||
| R Anterior Cingulate Cortex | 32 | 10, 36, 20 | 0.0308 | 2168 |
| L Anterior Cingulate Cortex | 32 | -2, 30, 24 | 0.0236 | |
| L CerebellarAnterior Lobe | 0, -62, -28 | 0.0273 | 1928 | |
| L Caudate Nucleus | -12,16,-2 | 0.0337 | 1768 | |
| R Caudate Nucleus | 14,18, -2 | 0.0364 | 2136 | |
| L Putamen | -22, -2, -12 | 0.0367 | 1504 | |
| R Putamen | 28, -2, -14 | 0.0312 | 1160 | |
| L Cerebellar Posterior Lobe | -18, -54, -46 | 0.0249 | 936 | |
| L Medial Frontal Gyrus | 9 | -4, 48, 28 | 0.0171 | 408 |
| Cirrhosis>Controls | ||||
| LThalamus | -8, -18,10 | 0.0330 | 3144 | |
| RThalamus | 12, -18, 12 | 0.0190 | 1936 |
ALE, activation likelihood estimation; BA, Brodmann area; MNI, Montreal Neurological Institute; L, left; R, right.
Table 2: Regions of GM alterations in cirrhosis relative to healthy controls.
Discussions
The current study is the first whole-brain structural meta-analysis exploring the GM changes in cirrhotic patients with and without a complication of HE. By analysing 11 datasets from 7 VBM studies, the voxel-based metaanalysis identified consistent regions of GM reductions within the ACC, basal ganglia, medial frontal gyrus, and cerebellum in cirrhotic patients compared with control subjects; increased GM volumes were only demonstrated in the thalamic regions. Taken together, cirrhosisinduced neuropsychiatric impairment is believed to be associated with diffuse GM deficits involving both the cortical and subcortical structures, which will enhance a more comprehensive understanding of neuropathological mechanisms of this disorder.
▪ Anterior cingulate cortex
The attention deficit is the earliest and most striking finding in cirrhotic patients, which is due in part to the dysfunction of the ACC [35]. The ACC is considered as a critical region involved in attention function that subserves cognitive and emotional processing [36]. Moreover, the ACC also belongs to the default mode network (DMN), which is the most important restingstate network [37]. Previous VBM studies confirmed the GM reduction of the ACC mainly in cirrhotic patients with HE [16,18]. In addition, aberrant metabolites, blood flow, and neural activity have been identified in the ACC in cirrhosis with and without HE [38-42]. Using PET, Kato et al. showed decreased cerebral glucose metabolism of the ACC in subclinical HE [38]. Using MRS, Zhang et al. demonstrated that lower ratio of choline (Cho)/creatine (Cr) and high ratio of glutamine (Glx)/Cr were found in the ACC in MHE patients [40]. Using resting-state fMRI, disrupted functional connectivity in the ACC was observed in cirrhotic patients whom further deteriorated with the increasing severity of HE [41]. These findings suggest that the ACC plays a pivotal role in the neuropathology underlying cirrhosis. Future studies are required to evaluate the relationship between the volumetric and functional abnormalities of the ACC in cirrhotic patients.
▪ Basal ganglia
The basal ganglia, mainly the bilateral caudate nucleus and putamen, exhibited remarkable atrophy in the current meta-analysis, similar to most VBM studies [16-21]. Subcortical structures, especially the basal ganglia, are implicated in brain dysfunction induced by hepatic dysmetabolism [43]. Neuronal cell loss has been proved in basal ganglia samples obtained at autopsy from cirrhotic patients [44]. Likewise, selective loss of the dopamine D2 receptor in the basal ganglia closely links to the development of HE [45]. One PET study revealed the redistribution of ammonia metabolism from cortical regions to basal ganglia during acute HE in cirrhosis [46]. Moreover, patients with cirrhosis have bilateral symmetric hyperintensity of the basic ganglia in conventional T1-weighted MR images [47]. The basal ganglia also showed disrupted spontaneous neural activity and functional connectivity in cirrhosis with or without HE through resting-state fMRI approach [12,48-50]. Together with the functional imaging investigations, our results suggested that GM atrophy in the basal ganglia in cirrhotic patients might serve as a potential imaging biomarker for monitoring its progression.
▪Medial frontal gyrus
As a key region of the dorsal attention network (DAN), the medial frontal gyrus also exhibited GM reduction in cirrhotic patients. DAN is involved in many higher-order cognitive tasks and shows activity increases after presentation of cues indicating where, when, or to what subjects should direct their attention [51]. As discussed above, the attention impairment has been confirmed in cirrhosis, which often in turn leads to learning deficits and dysfunction of working memory [35,52]. Additionally, several resting-state fMRI studies have reported abnormal functional connectivity within the DAN in patients with cirrhosis [53,54]. In this study, there existed GM atrophy in two important regions linked to attention function (i.e. ACC and medial frontal gyrus), indicating the disruption of DAN associated with cirrhosis.
▪ Cerebellum
Interestingly, significant GM reductions were detected not only in the cerebral cortex but in the cerebellum. Previous reported observations using animal models demonstrated that the cerebellum is more likely to be affected by the detrimental effects of hyperammonemia [55,56]. Schmahmann et al. demonstrated that the anterior cerebellum engages in regulating motor controlling as well as the posterior cerebellum involves in cognitive processing and emotion mediation [57]. Besides, the left cerebellum is responsible for attention function and visuospatial skill while the right cerebellum regulates logical reasoning and language processing [58]. Thus, the atrophy in the left cerebellar lobe may reflect the shortened attention span and deficit in visuo-spatial reasoning in cirrhotic patients. Compared to controls, patients with low-grade HE showed decreased amplitude of low-frequency fluctuations (ALFF) in left posterior cerebellar lobe [59]. Using arterial spin labeling (ASL) technique, Felipo et al. detected increased blood flow in cerebellar hemisphere and vermis that correlated with performance in most tests of Psychometric Hepatic Encephalopathy Score (PHES) [60]. Similarly, enhanced regional homogeneity (ReHo) was observed in bilateral anterior cerebellar lobes [61]. On the basis of the aforementioned findings, we suggest that many functions modulated by cerebellum are affected by cirrhosis and that cerebellum is more susceptible than other areas in cirrhotic patients. Nevertheless, the underlying mechanisms by which the cirrhosis results in the aberrant cerebellar function still require further investigation.
▪ Thalamus
By contrast, one important finding in the meta-analysis was the increased GM volume of bilateral thalamus in cirrhotic patients with or without HE. The thalamus, a key component of the cortical-basal ganglia-thalamic circuits, is regarded as a filter for sensory input to the cortex [62]. Moreover, the thalamus may serve as a crucial center for the integration of networks that underlie the ability to modulate behaviors [63]. In this study, we hypothesized that thalamic enlargement, accompanied by reduced GM volumes in basal ganglia and medial frontal gyrus, might represent an activity-dependent hypertrophy of relay nuclei within dysfunctional motor circuits [63]. Previous PET studies have reported increased cerebral blood flow and glucose metabolism in the thalami of cirrhotic patients, supporting that increased thalamic volume might represent a compensatory effect for the basal ganglia dysfunction [64,65]. However, Qi et al investigated the decreased connectivity between thalamus and cerebral cortex, and basal ganglia, indicating the disturbed integrity of thalamic resting-state network in cirrhosis [66]. These results suggest that further work is needed to determine how the cirrhosis disrupts the normal thalamo-cortical interactions.
▪ Limitations
Several practical constraints should be noted in our study. First, the heterogeneity of the included VBM studies could not be entirely ruled out, such as the demographics of patients (e.g. cirrhosis with or without HE, aetiology, and medication treatment), different smoothing kernels and statistical thresholds. Second, voxel-wise meta-analytic approach like ALE provides excellent control of false positive results, but it is more difficult to avoid false negatives [67]. Furthermore, voxel-wise meta-analysis is based on the pooling of peak stereotactic coordinates rather than on raw statistical brain maps from the original studies, which could give rise to less accurate results [67]. Finally, the number of included VBM studies and datasets is relatively small. Further explorations are required to perform the subgroup analyses or metaregression analyses for the control of confounding factors that might influence brain structural changes in cirrhosis, such as the grade of hepatic function and various aetiologies.
Conclusions
In summary, using the ALE method of metaanalysis, our study demonstrated consistent GM reductions in cirrhotic patients with and without HE progression, specifically in the ACC, basal ganglia, medial frontal gyrus, and cerebellum as well as GM increment in the thalamus. Severe GM deficits involving in both cortical and subcortical regions may predict the functional impairments in cirrhotic patients. Future large longitudinal and multicenter VBM studies will investigate whether this GM atrophy pattern is a valuable diagnostic and prognostic biomarker for cirrhosis-induced neuropsychiatric impairment.
Conflict of Interests
The authors declare that there is no potential conflict of interests regarding the publication of this paper.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (Nos. 81601477, 81600638) and Natural Science Foundation of Jiangsu Higher Education Institutions (No. 16KJB320001).
References
- Atluri DK, R Prakash, KD Mullen. Pathogenesis, diagnosis, and treatment of hepatic encephalopathy. J.Clin.Exp.Hepatol1(2), 77-86 (2011).
- Ferenci P, Lockwood A, Mullen K, et al.Hepatic encephalopathy-definition, nomenclature, diagnosis, and quantification. Hepatology35(3), 716-721 (2002).
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. The. Lancet383(9930), 1749-1761 (2014).
- Bajaj JS, WadeJB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: implications for the assessment of hepatic encephalopathy. Hepatology50(6), 2014-2021 (2009).
- Moscucci, F,Nardelli S, Pentassuglio I,et al.Previous overt hepatic encephalopathy rather than minimal hepatic encephalopathy impairs health‐related quality of life in cirrhotic patients. Liver. Int31(10), 1505-1510 (2011).
- Amodio, P,Del Piccolo F, Pettenò E, et al.Prevalence and prognostic value of quantified electroencephalogram (EEG) alterations in cirrhotic patients. J.Hepatol35(1), 37-45 (2001).
- Bajaj JS,Hafeezullah M, Franco J, et al.Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology135(5), 1591-1600 (2008).
- Lockwood AH, Murphy BW, Donnelly KZ, et al.Positron‐emission tomographic localization of abnormalities of brain metabolism in patients with minimal hepatic encephalopathy. Hepatology18(5), 1061-1068 (1993).
- U-King-Im J, Yu E, Bartlett E, et al.Acute hyperammonemic encephalopathy in adults: imaging findings. AJNR. Am. J. Neuroradiol32(2), 413-418 (2011).
- Saraswat VA, Saksena S, Nath K, et al. Evaluation of mannitol effect in patients with acute hepatic failure and acute-on-chronic liver failure using conventional MRI, diffusion tensor imaging and in-vivo proton MR spectroscopy. World J Gastroenterol14(26), 4168 (2008).
- Mardini H, Smith FE, Record CO, et al.Magnetic resonance quantification of water and metabolites in the brain of cirrhotics following induced hyperammonaemia. J.Hepatol54(6), 1154-1160 (2011).
- Zhang LJ, Zheng G, Zhang L, et al.Altered brain functional connectivity in patients with cirrhosis and minimal hepatic encephalopathy: a functional MR imaging study. Radiology265(2), 528-536 (2012).
- Ashburner J, Friston KJ. Voxel-based morphometry-the methods. Neuroimage11(6), 805-821 (2000).
- Davies RR, Scahill VL, Graham A,et al.Development of an MRI rating scale for multiple brain regions: comparison with volumetrics and with voxel-based morphometry. Neuroradiology51(8), 491-503 (2009).
- Zhang XD,Zhang LJ, Wu SY,et al.Multimodality magnetic resonance imaging in hepatic encephalopathy: An update. World. J. Gastroenterol20(32), 11262-11272 (2014).
- Chen HJ, Zhu XQ, Shu H, et al.Structural and functional cerebral impairments in cirrhotic patients with a history of overt hepatic encephalopathy. Eur.J. Radiol81(10), 2463-2469 (2012).
- Tao R, Zhang J, You Z, et al.The thalamus in cirrhotic patients with and without hepatic encephalopathy: a volumetric MRI study. Eur. J. Radiol82(11), e715-e720 (2013).
- Zhang LJ, Qi R, Zhong J, et al.The effect of hepatic encephalopathy, hepatic failure, and portosystemic shunt on brain volume of cirrhotic patients: a voxel-based morphometry study. PLoS. one7(8), e42824 (2012).
- Lin WC, Chou KH, Chen CL, et al.Longitudinal brain white matter alterations in minimal hepatic encephalopathy before and after liver transplantation. PLoS. One9(8), e105887 (2014).
- Qi R, Zhang LJ, Zhong J, et al.Grey and white matter abnormalities in minimal hepatic encephalopathy: a study combining voxel-based morphometry and tract-based spatial statistics. Eur.Radiol23(12), 3370-3378 (2013).
- Guevara M, Baccaro ME, Gómez-Ansón B, et al.Cerebral magnetic resonance imaging reveals marked abnormalities of brain tissue density in patients with cirrhosis without overt hepatic encephalopathy. J. Hepatol55(3), 564-573 (2011).
- Iwasa M, Mifuji-Moroka R, Kuroda M, et al.Regional reduction in gray and white matter volume in brains of cirrhotic patients: voxel-based analysis of MRI. Metab. Brain. Dis27(4), 551-557 (2012).
- Laird AR, Fox PM, Price CJ,et al.ALE meta‐analysis: Controlling the false discovery rate and performing statistical contrasts. Hum. Brain. Mapp25(1), 155-164 (2005).
- Turkeltaub PE, Eden GF, Jones KM, et al.Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage16(3), 765-780 (2002).
- Eickhoff SB, Laird AR, Grefkes C, et al.Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Hum. Brain. Mapp30(9), 2907-2926 (2009).
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage22(4), 1679-1693 (2004).
- Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr.Res117(1), 01-12 (2010).
- Li J, Zhang Z, Shang H. A meta-analysis of voxel-based morphometry studies on unilateral refractory temporal lobe epilepsy. Epilepsy. Res98(2), 97-103 (2012).
- Shao N, YangJ, Shang H. Voxelwise meta-analysis of gray matter anomalies in Parkinson variant of multiple system atrophy and Parkinson’s disease using anatomic likelihood estimation. Neurosci. Lett587(1), 79-86 (2015).
- Weng HH, Chen CF, Tsai YH, et al.Gray matter atrophy in narcolepsy: An activation likelihood estimation meta-analysis. Neurosci. Biobehav.Rev59(1), 53-63(2015).
- StroupDF, Berlin JA, Morton SC, et al.Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA283(15), 2008-2012 (2000).
- Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, et al.Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Hum. Brain. Mapp28(11), 1194-1205 (2007).
- Genovese CR, Lazar NA,Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15(4), 870-878 (2000).
- Eickhoff SB, Stephan KE, Mohlberg H, et al.A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage25(4), 1325-1335 (2005).
- Amodio P, Schiff S, Del Piccolo F, et al. Attention dysfunction in cirrhotic patients: an inquiry on the role of executive control, attention orienting and focusing. Metab. Brain. Dis20(2), 115-127 (2005).
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends. Cogn. Sci 4(6), 215-222 (2000).
- Raichle ME, MacLeod AM, Snyder AZ, et al.A default mode of brain function. Proc. Natl. Acad. Sci. U S A98(2), 676-682 (2001).
- Kato A, Suzuki K, Kaneta H, et al.Regional differences in cerebral glucose metabolism in cirrhotic patients with subclinical hepatic encephalopathy using positron emission tomography. Hepatol. Res17(3), 237-245 (2000).
- Iwasa M, Matsumura K, Nakagawa Y, et al.Evaluation of cingulate gyrus blood flow in patients with liver cirrhosis. Metab. Brain. Dis20(1), 7-17 (2005).
- Zhang LJ, Lu GM, Yin JZ , et al.Metabolic changes of anterior cingulate cortex in patients with hepatic cirrhosis: A magnetic resonance spectroscopy study. Hepatol. Res40(8), 777-785 (2010).
- Zhang LJ, Qi R, Zhong J, et al.Disrupted functional connectivity of the anterior cingulate cortex in cirrhotic patients without overt hepatic encephalopathy: a resting state fMRI study. PLoS One8(1), e53206 (2013).
- Zhang L, Qi R, Wu S, et al.Brain default‐mode network abnormalities in hepatic encephalopathy: A resting‐state functional MRI study. Hum. Brain. Mapp33(6), 1384-1392 (2012).
- Weissenborn K, Kolbe H. The basal ganglia and portal-systemic encephalopathy. Metab. Brain. Dis13(4), 261-272 (1998).
- Finlayson M, Superville B. Distribution of cerebral lesions in acquired hepatocerebral degeneration. Brain104(1), 79-95 (1981).
- Weissenborn K, Berding G, Köstler H. Altered striatal dopamine D2 receptor density and dopamine transport in a patient with hepatic encephalopathy. Metab. Brain. Dis15(3), 173-178 (2000).
- Keiding S, Sørensen M, Bender D, et al.Brain metabolism of 13N‐ammonia during acute hepatic encephalopathy in cirrhosis measured by positron emission tomography. Hepatology43(1), 42-50 (2006).
- McPhail MJ, Patel NR, Taylor-Robinson SD. Brain imaging and hepatic encephalopathy. Clin. Liver. Dis16(1), 57-72 (2012).
- Ni L, Qi R, Zhang LJ, et al.Altered regional homogeneity in the development of minimal hepatic encephalopathy: a resting-state functional MRI study. PLoS One7(7), e42016 (2012).
- Zhu XQ, Chen HJ, Wang Y, et al. Aberrant resting-state corticostriatal functional connectivity in cirrhotic patients with hyperintense globus pallidus on T1-weighted MR imaging. PLoS One7(11), e48886 (2012).
- Qi R, Zhang LJ, Zhong J, et al.Altered effective connectivity network of the basal ganglia in low-grade hepatic encephalopathy: a resting-state fMRI study with Granger causality analysis. PLoS One8(1), e53677(2013).
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci 3(3), 201-215 (2002).
- Bajaj JS, Hafeezullah M, Hoffmann RG, et al.Navigation skill impairment: another dimension of the driving difficulties in minimal hepatic encephalopathy. Hepatology 47(2), 596-604 (2008).
- Qi R, Zhang LJ, Xu Q, et al.Selective impairments of resting-state networks in minimal hepatic encephalopathy. PLoS One7(5), e37400 (2012).
- Chen HJ, Wang Y, Zhu XQ, et al.Classification of cirrhotic patients with or without minimal hepatic encephalopathy and healthy subjects using resting-state attention-related network analysis. PLoS One9(3), e89684 (2014).
- Rodrigo R, Cauli O, Gomez-Pinedo U, et al.Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology139(2), 675-684 (2010).
- Hermenegildo C, Montoliu C, Llansola M, et al. Chronic hyperammonemia impairs the glutamate–nitric oxide–cyclic GMP pathway in cerebellar neurons in culture and in the rat in vivo. Eur. J. Neurosci10(10), 3201-3209 (1998).
- Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain129(2), 290-292 (2006).
- Baillieux H, De Smet HJ, Dobbeleir A, et al.Cognitive and affective disturbances following focal cerebellar damage in adults: a neuropsychological and SPECT study. Cortex46(7), 869-879 (2010).
- Chen HJ, Zhu XQ, Jiao Y, et al.Abnormal baseline brain activity in low-grade hepatic encephalopathy: a resting-state fMRI study. J. Neurol. Sci318(1), 140-145 (2012).
- Felipo V, Urios A, Giménez-Garzó C, et al.Non invasive blood flow measurement in cerebellum detects minimal hepatic encephalopathy earlier than psychometric tests. World. J. Gastroenterol20(33), 11815 (2014).
- Chen HJ, Zhu XQ, Yang M, et al.Changes in the regional homogeneity of resting-state brain activity in minimal hepatic encephalopathy. Neurosci. Lett507(1), 5-9 (2012).
- Herrero MT, Barcia C, Navarro J. Functional anatomy of thalamus and basal ganglia. Childs. Nerv. Syst18(8), 386-404 (2002).
- Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus.Brain. Res. Bull 78(2), 69-74 (2009).
- Ahl B, Weissenborn K, van den Hoff J, et al.Regional differences in cerebral blood flow and cerebral ammonia metabolism in patients with cirrhosis. Hepatology40(1), 73-79 (2004).
- Lockwood AH, Yap EW, Rhoades HM, et al.Altered cerebral blood flow and glucose metabolism in patients with liver disease and minimal encephalopathy. J. Cereb. Blood. Flow. Metab11(2), 331-336 (1991).
- Qi R, Zhang LJ, Zhong J, et al.Disrupted thalamic resting-state functional connectivity in patients with minimal hepatic encephalopathy.Eur. J. Radiol82(5), 850-856 (2013).
- Radua J, Mataix-Cols D, Phillips ML, et al.A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiatry27(8), 605-611 (2012).