Research Article - (2018) Volume 8, Issue 4
Gender Differences within the Psychosis Spectrum
- Corresponding Author:
- Lina Díaz-Castro
Psychiatrist, MSP-Epidemiology Health Systems
INSP. Researcher in Medical Sciences “C”
Psychiatric Care Services, Health Secretary, Mexico
Abstract
Subtle gender differences in cognition arise due to the action of sex hormones during brain development, and are shaped by social influences. The sexes also differ with respect to vulnerability to specific classes of psychopathology.
Keywords
Schizophrenia, Psychoses, Brain, Gender differences.
Introduction
Subtle gender differences in cognition arise due to the action of sex hormones during brain development, and are shaped by social influences [1-3]. Sex hormones also have immediate and reversible effects on cognition [4-6]. Women and men differ in specific measures of visuospatial and verbal ability, memory, and processing of emotional information [7,8].
The sexes also differ with respect to vulnerability to specific classes of psychopathology. For example, depressive and anxiety disorders are more prevalent in women, while autism, attention deficit hyperactivity disorder (ADHD) and Tourette Syndrome (TS) are more frequently observed in men [2,3]. Gender differences in psychopathology are most certainly a result of interactions between environmental factors and brain sexual dimorphisms. However, neuropsychiatric disorders also are often strongly influenced by genetic factors [9-11].
Increasingly, it is being appreciated that the neurobiological underpinnings of psychopathology are best understood in terms of dimensional, rather than categorical, terms. Dimensional models of psychopathology assume the existence of many orthogonal symptom dimensions – such as psychotic propensity and negative symptoms – that cross diagnostic boundaries, vary quantitatively with respect to severity, and are continuous with the “normal”, healthy state [12-18]. In the present study, we analyzed a large database of patients (n=1132) with psychotic symptoms, in order to explore gender-related differences in premorbid behavior, familial vulnerability, disease presentation, symptom severity, and DSM-IV diagnosis [19]. This patient sample can be considered to represent the dimensional continuum of clinical psychosis.
Methods
▪ Research setting
Data collection was carried out from mid-2009 until the end if 2010, at the schizophrenia clinic of the Psychiatric Hospital Fray Bernardino Alvarez, Mexico City. This hospital is a federal hospital with 300 beds for inpatient care, providing inpatient and outpatient mental health services to the population without social security. Patients from this hospital were referred by their treating physician to the schizophrenia clinic due to a suspected schizophrenia diagnosis.
▪ Subjects
The final dataset comprised 1,132 patients, and was constructed by the psychiatrists of the schizophrenia clinic as part of their normal clinical activities. The primary criterion for inclusion within the dataset was a suspected diagnosis of schizophrenia, for which they were referred to the schizophrenia clinic. At the time of referral, basic clinical data had already been collected by interviewing the patient and a close family member. These data included: age of first psychotic episode, family history of mental illness, and the patient’s current relationship status. At the time of entry into the schizophrenia clinic, all patients were receiving pharmacological treatment and were symptomatically stable. All patients had given written informed consent for treatment within the hospital.
At the schizophrenia clinic, the patient and family member were further interviewed. The Structured Clinical Interview for DSM-IV (SCID) Axis I Disorders [20] was applied in order to confirm or update the previous DSMIV diagnosis. The patients received a diagnosis of schizophrenia, bipolar disorder (the majority being bipolar I), schizoaffective disorder, or some other disorder (Table 1). Positive and negative symptoms in a subset of patients were assessed using the Positive and Negative Syndrome Scale (PANSS) [21]. PANSS scores were obtained from a total of 879 patients (780/830 schizophrenia, 11/210 bipolar, 61/78 schizoaffective, 5/9 first episode psychosis, 1 schizophreniform, 1 schizotypical).
Additionally, the patient’s family member was questioned on whether the patient showed any of the following behavior characteristics prior to the first psychotic symptoms: schizoid-like (isolated, solitary, without friends, did not like to socialize); hyperactive (restless, motor hyperactivity); irritable (angered very easily and frequently); or other (e.g., timid, withdrawn, inhibited). For the purposes of the present analysis, we considered these to be premorbid behaviors. Due to limitations of the interviewing process, we cannot distinguish between premorbid personality traits and possible prodromal symptoms. Patient identity was coded, and all of this information was entered into a data base.
▪ Statistical analysis
All analyses were done using SPSS software (IBM). In order to compare proportions (e.g., gender-associated differences in prevalence of specific diagnoses, family history, etc.), the Chi2 test was used. Means were compared using the Student’s T test. Univariate General Linear Model (GLM) was applied in order to determine effects of specific categorical and continuous variables on age of symptom onset, positive symptoms severity, and negative symptom severity. In all cases, statistical significance was assumed where p<0.05.
Results
▪ Relationships between categorical variables: diagnosis, gender, family history of psychiatric illness, and premorbid behaviors
The average age of the patients was 35.4 (SD 10.4) for men, and 38.1 (SD 10.55) for women, a difference that was statistically significant (t=- 4.20; p<0.001). The average duration of illness (age at evaluation minus the age of first psychotic episode) did not differ significantly according to gender: 11.9 (SD 9.44) years for men and 11.0 (SD 8.94) years for women (t=1.58; p>0.05). Women were approximately 3 times more likely to report having a romantic partner than were men (132/415 and 79/701, respectively; Fisher’s Exact test, p<0.001) (Table 1).
| Men n=714 | Women N=418 | |||
|---|---|---|---|---|
| CONTINUOUS MEASURES | Mean | SD | Mean | SD |
| Age | 35.4 | 10.4 | 38.1 | 10.5 |
| Duration of illness (M=707; W=409) | 11.9 | 9.4 | 11.0 | 8.9 |
| CATEGORICAL MEASURES | frequency | % | frequency | % |
| Relationship status: with partner (M=701; W=415) | 79 | 11.1 | 132 | 31.8 |
| Diagnosis of patient (M=714; W=418) | ||||
| Schizophrenia (n=830) | 583 | 81.7 | 247 | 59.1 |
| Bipolar disorder (n=210) | 80 | 11.2 | 130 | 31.1 |
| Schizoaffective (n=78) | 40 | 5.6 | 38 | 9.1 |
| Other (n=14) | 11 | 1.5 | 3 | 0.7 |
| Positive family history: any diagnosis (M=699; W=411) | 314 | 44.0 | 200 | 48 |
| Family history: specific diagnoses (M=698; W=404) | ||||
| Schizophrenia | 258 | 37.0 | 123 | 30.4 |
| Bipolar disorder | 40 | 5.7 | 57 | 14.1 |
| Other disorders | 13 | 1.8 | 12 | 3.0 |
| No family history | 387 | 55.4 | 212 | 52.4 |
| PREMORBID BEHAVIOR (M=530; W=266) | ||||
| Schizoid | 246 | 50.2 | 73 | 27.4 |
| Irritable | 15 | 2.8 | 8 | 3 |
| Hyperactive | 15 | 2.8 | 0 | 0 |
| Other | 7 | 1.3 | 8 | 3 |
| None reported | 247 | 46.6 | 177 | 66.5 |
Table 1: Description of the global sample.
Based on DSM-IV criteria, patients were diagnosed with schizophrenia (n= 830; 583 men) or a non-schizophrenia diagnosis (n= 302; 131 men). The latter group comprised bipolar disorder (n=210; 80 men), schizoaffective disorder (n=78, 40 men), 9 patients (6 men) with first-episode psychosis, 3 men diagnosed with delirium, 1 man diagnosed with schizophreniform disorder, and 1 man diagnosed with schizotypical disorder. The prevalence of specific diagnoses differed between men and women (Table 1): men were approximately 1.4 times more likely to have a diagnosis of schizophrenia than were women (583/714 and 247/418, respectively; p=0.001), whereas women were approximately 3 times more likely than men to have a diagnosis of bipolar disorder (130/418 and 80/714, respectively; p<0.001), and approximately 1.6 times more likely than men to have a diagnosis of schizoaffective disorder (38/418 and 40/714, respectively; p=0.03).
Considering those patient families that provided information on family history of psychiatric illness (699 male and 411 female patients, family history unknown for 22 patients; Table 1), 514 patients (314 men) reported that they had a relative that was diagnosed with a psychiatric disorder. Of those patients, 381 (258 men) had a family member diagnosed with schizophrenia, 97 (40 men) had a family member diagnosed with bipolar disorder, 5 (3 men) had a family member with depression, 20 (10 men) had a family member with some other mental illness (information on specific diagnosis was unavailable for 11 individuals; Table 1). A diagnosis of schizophrenia in the patient was most often associated with a family history of schizophrenia. Thus, 235/569 (41%) of men diagnosed with schizophrenia had a relative with schizophrenia, compared to 23/129 (18%) of those diagnosed with a non-schizophrenia diagnosis (p<0.001). Likewise, in the case of women, 103/239 (43%) of those diagnosed with schizophrenia had a relative with schizophrenia, compared to 20/165 (12%) of those with a non-schizophrenia diagnosis (p<0.001). Similarly, a diagnosis of bipolar disorder was most frequently associated with a family history of bipolar disorder. In the case of men, 32/79 (40%) of those diagnosed with bipolar disorder had a relative with bipolar disorder, compared to 8/619 (1%) of those diagnosed with schizophrenia or some other (non-bipolar) disorder (p<0.001). For women, 48/128 (37%) of those diagnosed with bipolar disorder had a relative with bipolar disorder, compared to 9/276 (3%) of those diagnosed with schizophrenia or some other (non-bipolar) disorder (p<0.001).
Considering patients where information on premorbid behavior was available (530 men, 266 women), premorbid characteristics also differed according to gender. Thus, men were more likely to have exhibited some premorbid characteristic – e.g., schizoid, irritable, or hyperactive – than were women: 53% (283/530) of men and 33% (89/266) of women presented some premorbid behavior (p<0.001). For men, premorbid schizoid behaviors were most often associated with a schizophrenia diagnosis: 234/437 (53%) of men with a diagnosis of schizophrenia had shown premorbid schizoid behaviors, compared to 12/93 (13%) of men with a non-schizophrenia diagnosis (p<0.001). This relationship was also significant for women: 66/171 (38%) of women with a diagnosis of schizophrenia had shown premorbid schizoid behaviors, compared to 7/95 (7%) of women with a non-schizophrenic diagnosis (p<0.001). Moreover, men with schizophrenia were approximately 1.4 times more likely to have shown premorbid schizoid behaviors than were women with schizophrenia: 283/530 (53%) of men, compared to 89/266 (33%) women (p=0.001). By contrast, a diagnosis of bipolar disorder was most often associated with the absence of these premorbid behaviors: 45/52 (86%) of men and 66/74 (89%) of women bipolar patients had not shown any of these premorbid behaviors, compared to 202/478 (42%) of men and 111/192 (58%) of women with schizophrenia or other nonbipolar diagnoses (men: p<0.001; women: p<0.001).
Lastly, a family history of schizophrenia was significantly associated with premorbid schizoid behaviors in men, but not in women. For this comparison, we considered only those patients with a non-schizophrenia diagnosis (i.e., bipolar, schizoaffective, other), and for which family history of psychiatric illness was known (91 men, 91 women), in order to eliminate the possible confounding factor of prodromal symptoms that may have been present in patients later diagnosed with schizophrenia. We found that 6/16 (37.5%) of men with a family history of schizophrenia had exhibited premorbid schizoid behaviors, compared to 6/75 (8%) of men that had a family member with a nonschizophrenia diagnosis, or no family history of mental illness (p=0.006). This relationship was not significant for women: 1/10 (10%) of women that had a family history of schizophrenia had shown premorbid schizoid behaviors, compared to 6/81 (7.4%) women that had no family history of mental illness, or that had a family member with a non-schizophrenia diagnosis (p=0.57). Thus, in this sample, men with a family history of schizophrenia were almost 4 times more likely (37.5% compared to 10%) to have shown premorbid schizoid behaviors than were women with a family history of schizophrenia; however the comparison between men and women was not statistically significant (6/16 vs. 1/10; p=0.19).
▪ Effects of gender, family history, and current relationship status on age of onset, PANSS positive, and PANSS negative symptoms
We then applied a 2-way ANOVA, with gender (male, female) and family history of any mental disorder (positive or negative family history) as factors, and age of onset, PANSS positive symptom score, and PANSS negative symptom score as dependent variables. We split the sample according to diagnostic grouping (schizophrenia, or other diagnoses) and current relationship status (in a relationship, or single). Results of analyses done on the subgroup of patients that were in a romantic relationship are provided as supplementary material.
In the subsample of single patients with schizophrenia, the 2-way ANOVA indicated significant main effects of gender and family history on age of onset, with no gender by family history interaction. This model explained a small, though statistically significant, proportion of the corrected variance in age of onset (adjusted R2 = 0.033; df=3, F=8.798), with gender and family history respectively accounting for 1.3% and 2.1% of this variance. Likewise, in single patients with a non-schizophrenia diagnosis, this model was statistically significant (adjusted R2=0.047; df=3, F=4.162, p=0.007). Gender and family history each showed significant main effects, but the interaction was not significant. Gender accounted for 4.2% of the variance, and family history accounted for 2.2% (Table 2).
| No family history | Family history | Family history (no vs. yes) | |
|---|---|---|---|
| Men, non-schiz | 23.57, 7.0 (n=53) | 21.3, 6.5 (n=44) | P = 0.11, 0.33 |
| Men, schiz | 23.71, 7.5 (n=278) | 21.49, 6.2 (n=236) | P < 0.001, 0.32 |
| Women, non-schiz | 26.9, 8.9 (n=49) | 24.49 8.32 (n=47) | P = 0.174, 0.28 |
| Women, schiz | 26.02, 9.9 (n=88) | 23.25, 7.31 (n=88) | P = 0.036, 0.32 |
| Gender, non-schiz | P = 0.037, 0.42 | P = 0.046, 0.42 | |
| Gender, schiz | P = 0.021, 0.28 | P = 0.032, 0.27 |
Table 2: Effects of family history and gender on age of first psychotic episode (mean, SD).
With PANSS positive symptoms as the dependent variable, this model was significant only for single patients with schizophrenia (R2=0.014, df=3, F=4.114, p=0.007). Family history (p=0.085) and the gender by family history interaction (p=0.063) showed trends toward significance, potentially explaining 0.45% and 0.5% of the variance. Men with a family history of schizophrenia showed significantly greater PANSS positive symptom scores than men with no family history. This relationship was not observed for women with a schizophrenia diagnosis, nor for men or women with a non-schizophrenia diagnosis (Table 3).
| No family history | Family history | Sig. (T-test), Hedge’s g | |
|---|---|---|---|
| Men, non-schiz | 2.76, 1.05 (n=25) | 2.57, 0.76 (n=14) | P = 0.559, 0.19 |
| Men, schiz | 2.58, 0.99 (n=264) | 2.91, 1.06 (n=223) | P < 0.001, 0.25 |
| Women, non-schiz | 2.45, 0.93 (n=11) | 2.77, 1.30 (n=13) | P = 0.511, 0.31 |
| Women, schiz | 2.74, 1.11 (n=87) | 2.72, 1.12 (n=83) | P = 0.941, 0.02 |
Table 3: Effect of family history on severity of PANSS positive symptoms.
Considering PANSS negative symptoms as the dependent variable, the model was significant for single patients with a schizophrenia (R2=0.019, df=3, F=5.29, p=0.001) and for those with a non-schizophrenia diagnosis (R2=0.13, df=3, F=4.023, p=0.011). Gender, but not family history or the interaction between these factors, showed a significant main effect in samples, respectively explaining 1.8% and 15.7% of the total corrected variance (Table 4).
| Men | Women | Sig. (T-test), Hedge’s g | |
|---|---|---|---|
| Non-schiz, no family history | 2.96, 0.72 (n=26) | 2.24, 0.66 (n=17) | P= 0.002, 1.03 |
| Schiz, no family history | 3.08, 0.84 (n=295) | 2.84, 0.99 (n=122) | P = 0.014, 0.27 |
| Non-schiz, family history | 3.05, 1.07 (n=21) | 2.53, 0.92 (n=15) | P=0.14, 0.51 |
| Schiz, family history | 3.23, 0.90 (n=239) | 2.88, 1.00 (n=105) | P = 0.001, 0.37 |
Table 4: Effect of gender on severity of PANSS negative symptoms.
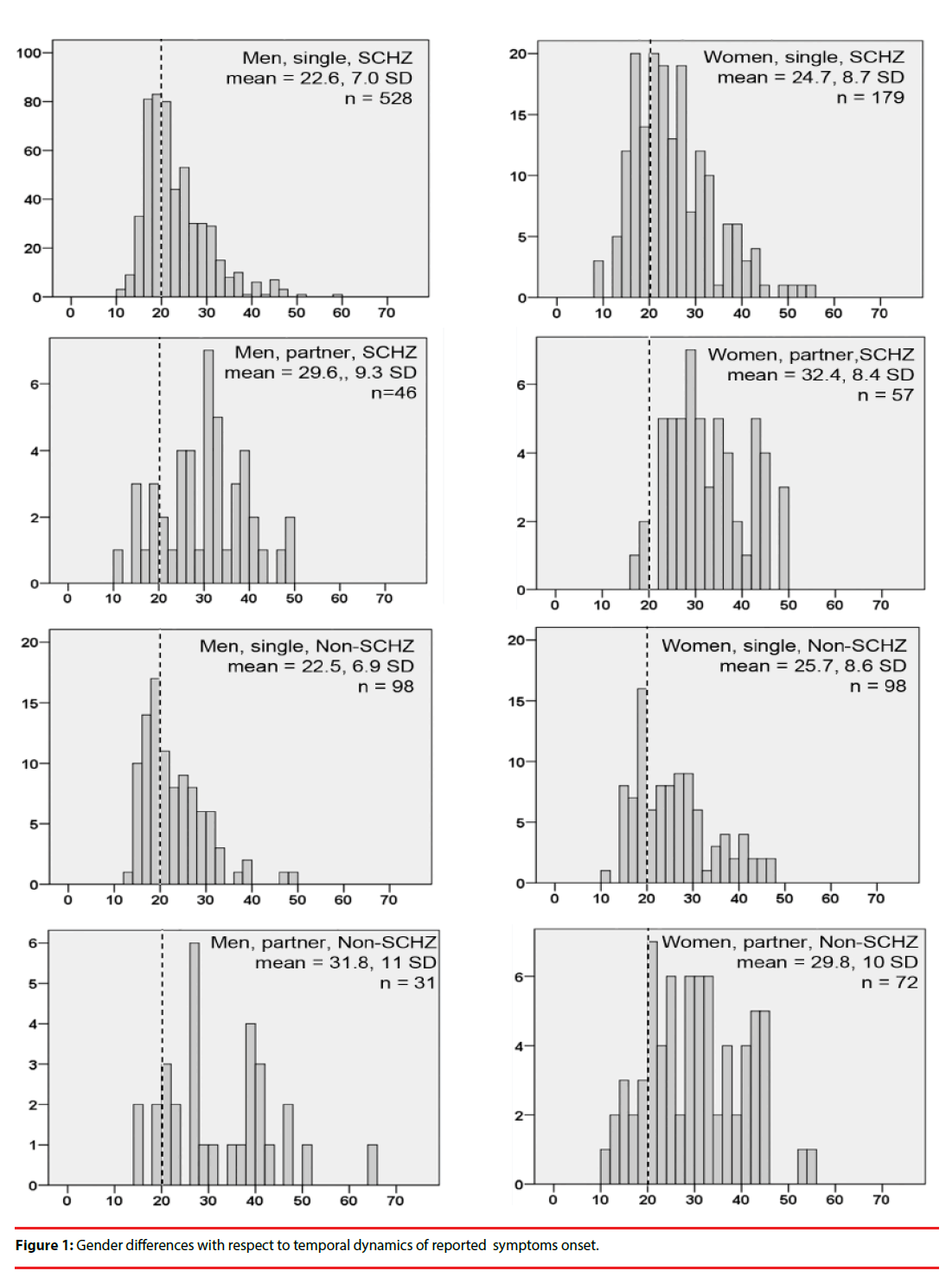
▪ Gender differences with respect to temporal dynamics of reported symptom onset.
We plotted frequency distribution histograms for age of symptom onset (Figure 1). For single men with schizophrenia, symptom onset showed a sharp peak in the late teens, and thereafter sharply declined into the 30’s. By symptom onset in women showed a broader peak encompassing the late teens to the late 20’s – early 30’s, and then a plateau during the late 30’s and 40’s. Remarkably, the temporal characteristics of symptom onset for schizophrenia and non-schizophrenia diagnoses (the majority of which were bipolar disorder, in both sexes) were practically identical.
▪ Relationship of premorbid behaviors to symptom severity and age of onset
We explored the effects of premorbid behaviors on PANSS positive and PANSS negative symptom severity and on age of first psychotic episode. Considering all patients for which premorbid characteristics were known (530 men, 266 women; Table 1), premorbid schizoid behaviors in single men were associated with an 11% increase in negative symptom scores (3.43 ± 0.88 SD vs. 3.1 ± 0.83 SD; t =-3.76, p<0.001; Hedge’s g=0.39). Likewise, in single women, premorbid schizoid behaviors were associated with a 17% increase in negative symptom scores (3.23 ± 1.1 SD vs. 2.77 ± 1.1; t = -2.49, p = 0.014; Hedge’s g=0.43). PANSS positive symptom severity did not differ according to premorbidity. Age of symptom onset in single men did not differ significantly according to premorbidity, while in single women premorbid schizoid behaviors were associated with a significantly earlier age of symptom onset (25.2 ± 8.8 SD vs. 21.8 ± 6.9 SD, t=2.60, p = 0.01; Hedge’s g=0.41). In both men and women that were in a relationship, neither negative nor positive symptoms differed according to premorbid schizoid characteristics. Patients (men and women, single and partnered, combined) with premorbid irritability or hyperactivity did not differ from those with no premorbidity with respect to PANSS positive or PANSS negative symptoms severity, but they did show a significantly earlier mean age symptom onset, by almost 5 years (irritable characteristics: 25.0 ± 8.6 SD vs. 20.5 ± 5.9 SD; t=2.47, p=0.014; Hedge’s g=0.53; hyperactive characteristics: 25.0 ± 8.6 SD vs. 20.1 ± 3.8 SD, t=2.19, p=0.029; Hedge’s g=0.57). Men and women, and single and partnered patients could not be analyzed separately due to low sample sizes.
Discussion
▪ Relationships between gender and family history, premorbid behaviors, and diagnosis
Our results suggest that, regardless of gender, there are familial factors that are independently associated with diagnosis: a family history of schizophrenia or bipolar disorder was significantly associated, respectively, with a diagnosis of schizophrenia or bipolar disorder. This finding is consistent with Goldstein et al. [22], although in that study possible gender affects were not tested. Likewise, in men and women, a schizophrenia diagnosis was more often associated with premorbid schizoid behaviors, while a diagnosis of bipolar disorder was more often associated with the absence of premorbid behaviors.
In the present patient sample, men were 1.4 times more likely than women to be diagnosed with schizophrenia, while women were 3 times more likely to receive a bipolar diagnosis, and 1.6 times more likely to receive a schizoaffective diagnosis. With respect to schizophrenia and schizoaffective disorder, this gender difference is consistent with published data [23-25], but gender differences have not been reported with respect to bipolar disorder. Taken together, these studies indicate that psychosis in men more often occurs in the context of schizophrenia, while psychosis in women may be more frequently associated with disorders of mood.
▪ Pan-diagnostic gender effects
In subgroups of single patients with schizophrenia and non-schizophrenia diagnoses, the mean age of first psychotic episode was approximately 3 years earlier in men than in women. Overall, this result is in agreement with published literature pertaining to the schizophrenia diagnosis [25-28]. However, some studies have failed to show a significant gender effect on age of onset of schizophrenia [24] or psychosis associated with other diagnoses, such as major depressive or bipolar disorder [24,29]. Our results suggest that discordant results in the literature may be due to confounding factors, such as relationship status.
The frequency distribution histograms for age of symptom onset differed markedly between men and women: men showed a sharp peak in symptom onset during the late teens, followed by a sharp decline into the late twenties. Symptom onset in women was more broadly distributed across the late teens into the thirties, with a “late-life plateau” that encompassed the forties. Notably, the temporal characteristics of symptom onset were practically identical for schizophrenia and non-schizophrenia diagnoses (Figure 1), implying that gender-associated factors affect this variable a similar manner across the psychotic disorder spectrum.
The frequency distribution curves of age of symptom onset in the present study were similar to those reported by Häfner [27], where it was suggested that these gender differences are due to protective effects of estradiol in women, which supposedly wane across the peri-menopause period [27,28], resulting in a second wave of symptom onset in women. In the present study, the “late-life plateau” of symptom onset probably encompassed the perimenopausal period: the mean age of menopause in women of Mexico City was determined to be 46.5 ± 5 years [30]. However, during the perimenopausal period, circulating estradiol and follicle stimulating hormone (FSH) levels are typically erratic and high (not low or declining), while progesterone and Inhibin B levels are decreased [31,32]. In approximately 30% of healthy perimenopausal women [33], these hormonal changes were associated with mood symptoms; such typical perimenopausal mood alterations could add to vulnerability to psychotic symptoms during this time.
Our analyses showed that gender and family history of mental illness were independent factors that affected age of first psychotic episode. By contrast, other studies have reported that this gender difference disappeared when genetic load for schizophrenia was high, for example when the subject’s sibling was affected [27,34,35]. These diverging results could be due to differences in sample characteristics, including relationship status (in the present study, only the non-partnered subgroup showed a significant gender effect on age of onset), and specific characteristics of the familial genetic risk; for example, high familial risk involving a few rare genetic variants, or lower familial risk involving many common genetic variants [10].
Across diagnoses, women were 3 times more likely to be involved in a romantic relationship than were men. This result agrees with the published literature [24,27,36]. Moreover, men were more likely than women to have exhibited premorbid behaviors, and the most commonly reported premorbid behavior was the schizoid type. In other studies, men with first-episode psychosis showed poorer premorbid adjustment in the social realm than did women, particularly during late adolescence [27,37,38]. The apparent vulnerability of men to schizoid characteristics might partially explain the increased negative symptom severity, earlier age of symptom presentation, and worse overall social functioning in men [27,38,39].
Our data suggest that some gender associations might be related to male vulnerability to genetic factors: men with a non-schizophrenia diagnosis but with a family history of schizophrenia were almost 4 times more likely to have exhibited premorbid schizoid behaviors, compared to women with a family history of this disorder. Moreover, men with schizophrenia were 1.4 times more likely to have shown premorbid schizoid behaviors than were schizophrenic women. A recent study found sex-dependent effects of the COMT (catechol-o-methyl transferase) Val allele in a general non-clinical adult population: male, but not female, carriers of this allele showed higher negative schizotypy and negative psychotic experiences [40]. Moreover, in schizophrenic patients, a significant association between the COMT Val allele and a dopamine D1 receptor risk allele was detected in men, but not in women [41].
▪ Gender effects associated selectively with the schizophrenia diagnosis
Our data suggest that gender and family history interact to increase positive symptom severity (PANSS positive scores). Thus, men with schizophrenia and a positive family history of any mental illness showed a 13% increase in positive symptoms, compared to schizophrenic men with no family history of mental illness. This relationship was not present in women, nor was it observed in men or women with non-schizophrenia diagnoses. Thus, although familial history of mental illness was related to an earlier age of symptom presentation in both sexes (Table 2), it was associated with increased positive symptom severity only in men diagnosed with schizophrenia (Table 3). This latter finding again suggests that men may be more sensitive than are women to some genetic factors that impact on symptom severity.
▪ Gender as a dimensional, rather than categorical, factor in mental illness
The present study demonstrates significant gender effects on psychosis presentation, but these gender differences were associated with modest effect sizes (Hedge’s g = 0.2 – 0.5), much like those observed for gender-associated differences in cognition. For example, healthy men show poorer performance than healthy women on certain tasks related to processing emotional information [7,42]. Such gender differences are influenced by developmental defeminization/masculinization of brain and behavior, in addition to acute levels of circulating sex hormones [6,43]. Gender differences due to developmental brain defeminization/ masculinization are quantitative rather than qualitative [44], and we propose that such quantitative measures of brain defeminization/ masculinization might better explain gender differences in psychosis.
There is reason to suspect that typical gender differences in emotion recognition specifically might influence the expression of psychotic symptoms. Impaired emotion recognition has been associated with a high family risk for schizophrenia, prodromal symptoms, firstepisode psychosis, and schizophrenia [45-47]. Moreover, a positive relationship between premorbid schizoid behaviors and negative symptoms has been reported for first-episode psychosis [48], as well as a positive relationship between emotion recognition deficits and negative symptoms in first-episode schizophrenia and bipolar psychosis [49]. Together, emotion recognition deficits and negative symptoms predicted the transition to schizophrenia in a high-risk cohort [50].
In sum, the present results point to complex interactions between gender and familial factors in determining premorbid characteristics, age of first psychotic episode, symptom severity, social functioning, and ultimate diagnosis. We propose that these gender differences may be related to quantitative variation in masculinization/ defeminization of brain and cognition, as well as from acute effects of circulating estrogens and testosterone. Future studies on gender differences in the manifestation of psychosis spectrum disorders should consider these possibilities.
References
- Auyeung B, Knickmeyer R, Ashwin E, et al. Effects of fetal testosterone on visuospatial ability. Arch. Sex. Behav 41(3), 571-81 (2012).
- Baron-Cohen S, Lombardo MV, Auyeung B, et al.Why are autism spectrum conditions more prevalent in males? PLoS. Biol 9(6), e1001081 (2011).
- Viveros MP, Mendrek A, Paus T, et al. A comparative, developmental, and clinical perspective of neurobehavioral sexual dimorphisms. Front. Neurosci 6(84), 1-21 (2012).
- Guapo VG, Graeff FG, Zani AC, et al. Effects of sex hormonal levels and phases of the menstrual cycle in the processing of emotional faces. Psychoneuroendocrinology 34(7), 1087-94 (2009).
- Solis-Ortiz S, Corsi-Cabrera M. Sustained attention is favored by progesterone during early luteal phase and visuo-spatial memory by estrogens during ovulatory phase in young women. Psychoneuroendocrinology 33(7), 989-998 (2008).
- Sundstrom-Poromaa I, Gingnell M. Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front. Neurosci 8(1), 380 (2014).
- Donges US, Kersting A, Suslow T. Women's greater ability to perceive happy facial emotion automatically: gender differences in affective priming. PLoS. One 7(7), e41745 (2012).
- Herlitz A, Lovén J. Sex differences in cognitive functions. Acta. Psychologica. Sinica; 41(1), 1081-1090 (2009).
- International Schizophrenia Consortium. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature 455(7210), 237-241 (2008).
- Mulle JG. Schizophrenia genetics: progress, at last. Curr. Opin. Genet. Dev 22(3), 238-244 (2012).
- Schizophrenia Psychiatric Genome-Wide Association Study Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat. Genet 43(10), 969-976 (2011 ) .
- Cohen AS, Davis TE. Quality of life across the schizotypy spectrum: findings from a large nonclinical adult sample. Compr. Psychiatry 50(5), 408-414 (2009).
- Hanssen M, Bak M, Bijl R, et al. The incidence and outcome of subclinical psychotic experiences in the general population. Br. J. Clin. Psychol 44(Pt 2), 181-191 (2005).
- Lyne J, Renwick L, O'Donoghue B, et al. Negative symptom domain prevalence across diagnostic boundaries: The relevance of diagnostic shifts. Psychiatry. Res 228(3), 347-354 (2015).
- Rosa A, van Os J, Fañanás L, et al. Developmental instability and schizotypy. Schizophr. Res 43(2), 125-134 (2000).
- van Os J, Linscott RJ, Myin-Germeys I, et al. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol. Med 39(2), 179-195 (2009).
- Wigman JTW, Wardenaar KJ, Wanders RBK, et al. Dimensional and discrete variations on the psychosis continuum in a Dutch crowd-sourcing population sample. Eur. Psychiatry 42(1), 55-62 (2017).
- Loch AA, Chianca C, Alves TM, et al. Poverty, low education, and the expression of psychotic-like experiences in the general population of Sao Paulo, Brazil. Psychiatry. Res 253(1), 182-188 (2017).
- American Psychiatric Association. Diagnostic and Statiscal Manual of mental Disorder Fourh Edition, Text Revision. Washington, DC; APA, USA (2000).
- First MB, Spitzer RL, Gibbon M. Structured clinical interview for DSM-IV Axis I disorders, Clinial Version (SCID-CV). Washington, D.C; American Psychiatric Press, USA (1996).
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull 13(1), 261–276 (1987).
- Goldstein JM, Buka SL, Seidman LJ, et al. Specificity of familial transmission of schizophrenia psychosis spectrum and affective psychoses in the New England family study's high-risk design. Arch. Gen. Psychiatry 67(5), 458-467 (2010).
- Kirkbride JB, Errazuriz A, Croudace TJ, et al. Incidence of schizophrenia and other psychoses in England, 1950-2009: a systematic review and meta-analyses. PLoS. One 7(3), e31660 (2012).
- Morgan VA, Castle DJ, Jablensky AV. Do women express and experience psychosis differently from men? Epidemiological evidence from the Australian National Study of Low Prevalence (Psychotic) Disorders. Aust. N. Z. J. Psychiatry 42(1), 74-82 (2008).
- Owoeye O, Kingston T, Scully PJ, et al. Epidemiological and clinical characterization following a first psychotic episode in major depressive disorder: comparisons with schizophrenia and bipolar I disorder in the Cavan-Monaghan First Episode Psychosis Study (CAMFEPS). Schizophr. Bull 39(4), 756-765 (2013).
- Gureje O, Bamidele RW. Gender and schizophrenia: association of age at onset with antecedent, clinical and outcome features. Aust. N. Z. J. Psychiatry 32(3), 415-423 (1998).
- Häfner H. Gender differences in schizophrenia. Psychoneuroendocrinology 28(1), 17-54 (2003).
- Häfner H, an der Heiden W, Behrens S, et al. Causes and consequences of the gender difference in age at onset of schizophrenia. Schizophr. Bull 24(1), 99 (1998).
- Thorup A, Petersen L, Jeppesen P, et al. Gender differences in young adults with first-episode schizophrenia spectrum disorders at baseline in the Danish OPUS study. J. Nerv. Ment. Dis 195(5), 396-405 (2007).
- Garrido-Latorre F, Lazcano-Ponce EC, López-Carillo L, et al. Age of natural menopause among women in Mexico City. Int. J. Gynaecol. Obstet 53(2), 159-66 (1996).
- Prior JC. Perimenopause: The complex endocrinology of the menopausal transition. Endocrine. Rev 19(4), 397-428 (1998).
- Prior JC, Hitchcock CL. The endocrinology of perimenopause: need for a paradigm shift. Front. Biosci 3(1), 474-486 (2011).
- Prior JC. 2011. Progesterone for symptomatic perimenopause treatment – Progesterone politics, physiology and potential for perimenopause. Facts. Views. Vis. Obgyn; 3(2), 109-120 (2011).
- Albus M, Maier W. Lack of gender differences in age at onset in familial schizophrenia. Schizophr. Res 18(1), 51-57 (2005).
- Könnecke R, Häfner H, Maurer K, et al. Main risk factors for schizophrenia: increased familial loading and pre-and peri-natal complications antagonize the protective effect of oestrogen in women. Schizophr. Res 44(1), 81-93 (2000).
- Pang S, Subramaniam M, Abdin E, et al. Gender differences in patients with first-episode psychosis in the Singapore Early Psychosis Intervention Programme. Early. Interv. Psychiatry 10(6), 528-534 (2016).
- Foerster A, Lewis S, Owen M, et al. Pre-morbid adjustment and personality in psychosis. Effects of sex and diagnosis. Br. J. Psychiatry 158(1), 171-176 (1991).
- Preston NJ, Orr KG, Date R, et al. Gender differences in premorbid adjustment of patients with first episode psychosis. Schizophr. Res 55(3), 285-290 (2002).
- Salokangas RK, Stengård E. Gender and short-term outcome in schizophrenia. Schizophr. Res 3(5), 333-345 (1990).
- de Castro-Catala M, Barrantes-Vidal N, Sheinbaum T, et al. COMT-by-sex interaction effect on psychosis proneness. Biomed. Res. Int 829237 (2015).
- Hoenicka J, Garrido E, Ponce G, et al. Sexually dimorphic interaction between the DRD1 and COMT genes in schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet 153B(4), 948-954 (2010).
- Hoffmann H, Kessler H, Eppel T, et al. Expression intensity, gender and facial emotion recognition: Women recognize only subtle facial emotions better than men. Acta. Psychol 135(3), 278-283 (2010).
- Bos PA, Hofman D, Hermans EJ, et al. Testosterone reduces functional connectivity during the 'Reading the Mind in the Eyes' Test. Psychoneuroendocrinology 68(1), 194-201 (2016).
- Schwarz JM, McCarthy MM. The role of neonatal NMDA receptor activation in defeminization and masculinization of sex behavior in the rat. Horm. Behav 54(5), 662-668 (2008).
- Allott KA, Rice S, Bartholomeusz CF, et al. Emotion recognition in unaffected first-degree relatives of individuals with first-episode schizophrenia. Schizophr. Res 161(2-3), 322-328 (2015).
- Eack SM, Mermon DE, Montrose DM, et al. Social cognition deficits among individuals at familial high risk for schizophrenia. Schizophr. Bull 36(6), 1081-1088 (2010).
- Erol A, Putgul G, Kosger F, et al. Facial emotion recognition in schizophrenia: the impact of gender. Psychiatry. Investig 10(1), 69-74 (2013).
- Cuesta MJ, Gil P, Artamendi M, et al. Premorbid personality and psychopathological dimensions in first-episode psychosis. Schizophr. Res 58(2), 273-280 (2002).
- Daros AR, Ruocco AC, Reilly JL, et al. Facial emotion recognition in first-episode schizophrenia and bipolar disorder with psychosis. Schizophr. Res 153(1-3), 32-37 (2014).
- Corcoran CM, Keilp JG, Kayser J, et al. Emotion recognition deficits as predictors of transition in individuals at clinical high risk for schizophrenia: a neurodevelopmental perspective. Psychol. Med 45(14), 2959-73 (2015).