Research Article - Neuropsychiatry (2017) Volume 7, Issue 6
Female Wild-Type and APP/PS1 Transgenic Mice Deficient in Sort1 Are Prone to Anxiety-Like Behavior at Older Ages
- Corresponding Author:
- Chun-Sheng Ruan
School of Pharmacy and Medical Sciences
Division of Health Sciences University of South Australia, SA 5000, Australia
Tel: +618-8302-2329
Fax: +618-8302-1087
Xin-Fu Zhou
School of Pharmacy and Medical Sciences
Division of Health Sciences University of South Australia, SA 5000, Australia
Tel: +618-8302-2329
Fax: +618-8302-1087
Abstract
Alzheimer’s disease (AD) and anxiety are two concurrent disorders often co-existing in older adults. Interestingly, women are more likely to experience anxiety than men in both general population and AD. To date, the mechanisms underlying the gender differences in the pathogenesis of AD and anxiety disorder are still not clear. Previously, we have found that the deletion of vacuolar protein sorting 10 protein (VPS10P)-domain containing receptor/sortilin
Keywords
Sortilin, Anxiety, Cognition, Alzheimer’s disease, Sex
Abbreviations
AD: Alzheimer’s Disease; APP: Amyloid Precursor Protein; APP/PS1: Appswe/PS1dE9 Double Transgenic Mice; BDNF: Brain-Derived Neurotropic Factor Precursor; Probdnf: BDNF Precursor; PS1: Presenilin-1; VPS10P: Vacuolar Protein Sorting 10 Protein; WT: Wild Type
Introduction
Alzheimer’s disease (AD) is the most common form of dementia, which is characterized by a number of hallmarks such as amyloid plaque (by abnormal accumulation of beta-amyloid peptide), neurofibrillary tangle (by abnormal accumulation of tau protein), brain atrophy and cognitive decline [1,2]. Patients diagnosed with AD often also show a variety of neuropsychiatric behaviors such as depression and anxiety [3].
The study of depression in connecting with AD has long been conducted, whereas the understanding of the relationship between anxiety and AD is still limited. Anxiety is an unpleasant emotion accompanied with nervous behavior [4]. It is commonly seen in the elderly people and significantly reduces the quality of their life [5]. Up to 70 per cent of AD patients are diagnosed with anxiety disorders [6]; and people with anxiety disorders are more susceptible to develop AD when they get old [7]. Anxiety and AD are concurrent disorders and each one may facilitate the development of another. To date, the molecular mechanism of anxiety in AD is poorly understood. In addition, gender difference in the onset of both AD and anxiety disorder has long been uncovered. Women are more vulnerable to develop AD [8] or anxiety [9] than men, which is most likely due to the involvement of female hormones (i.e. estrogen and progesterone) in the pathogenesis of these two diseases [10,11]. Still, the molecular mechanism of such gender differences in both AD and anxiety disorder is not well known.
Vacuolar protein sorting 10 protein (VPS10P)- domain containing receptors (include sortilin, SorLA and SorCS1/2/3), which are important for intracellular transport and cell signalling [12], have been implicated in regulation of AD [13-15] and mood disorders (such as anxiety and bipolar disorder) [16-21]. Sortilin (encoded by the Sort1 gene) is a 100-kDa type I membrane glycoprotein which is highly expressed in the brain [22]. It is the first receptor identified from the VPS10P family. As a sorting receptor, sortilin modulates the regulated secretion of brain-derived neurotropic factor (BDNF) which has been implicated with important role in anxiety disorder [23-25]. In addition, sortilin also acts as a receptor in signal transduction. It mediates the apoptotic effect of BDNF precursor (proBDNF) by forming a receptor complex with neurotrophin receptor p75 [26]. Moreover, it is also an important receptor to modulate the signalling pathway of neurotensin (Mazella, 2001) [27], which has been well-defined with a role in anxiety disorder [28,29]. Our previous study has shown that Sort1 deficiency results in normal cognitive performance but increased anxiety-like behavior in both sexes of young mice (3 months old); interestingly, our data also suggest that young female mice deficient in Sort1 are more prone to be anxious than males [21]. Given that females are more susceptible to experience anxiety and that anxiety disorders coexist with AD, we propose that Sort1 deficiency may cause more severe anxiety in aged female mice than male mice with or without the transgenes of APP/PS1. In this study, we tested the hypothesis by performing an open field test in 4 types of mice. We have also assessed the cognitive function in these mice by using the Morris water maze test.
Materials and Methods
▪ Animals
To perform this study, wild-type (WT), Sort1 knockout (Sort1-/-; Dr. Morales’s Laboratory, McGill University, Canada), APPswe/PS1dE9 (APP/PS1; Jackson Laboratory) and Sort1-/-APP/ PS1 (generated by Sort1-/- × APP/PS1 crossing) mice (n = 6 each sex and each strain; all animals were C57BL/6 background) were generated at the same time and maintained in 12-h light/12-h dark cycles with free access to food and water in the Reid Animal Facility of University of South Australia.
▪ Behavioral tests
At the age of 9 months old, all animals were subjected to an open field test followed by the Morris water maze test. The open field test was performed as previously described [21]. Animals were brought into an open arena (40 cm long × 40 cm wide × 40 cm high), and allowed to move freely for 5-min while being recorded with a digital camera linked to the ANY-maze software (Stoelting, USA). Traveling distance and percentage of mobility were considered as locomotor activities, and percentage of time in the central zone (central 24 cm × 24 cm area) was considered as anxiety-like behaviour. The Morris water maze test was performed as previously described [21]. Firstly, all animals were introduced to the water maze for acclimatization by allowing them to freely swim for 120 sec. On the second day, a platform was placed in one of the four divided quadrants (target quadrant) at 0.5 cm level above the water surface. Each animal entered the water maze and was given a maximum 60 sec to find the platform with three trials (visible platform training). On the following days, the platform was hidden below the water surface (at 0.5 cm level), and the same approaches in visible platform training were applied every day (hidden platform training). The hidden platform training stopped when the WT mice showed a “well-trained” skill to find the platform within 15 sec. On the last day, the platform was removed and each animal was subjected to a 60-sec probe test. Escape latency to the platform and percentage of time spent in the target quadrant were recorded by ANY-maze software. All data are presented as mean ± SEM and analysed by IBM SPSS Statistics 21. One-way repeated measures ANOVA and two-way ANOVA were used for group comparisons. p<0.05 was considered statistically significant.
Results
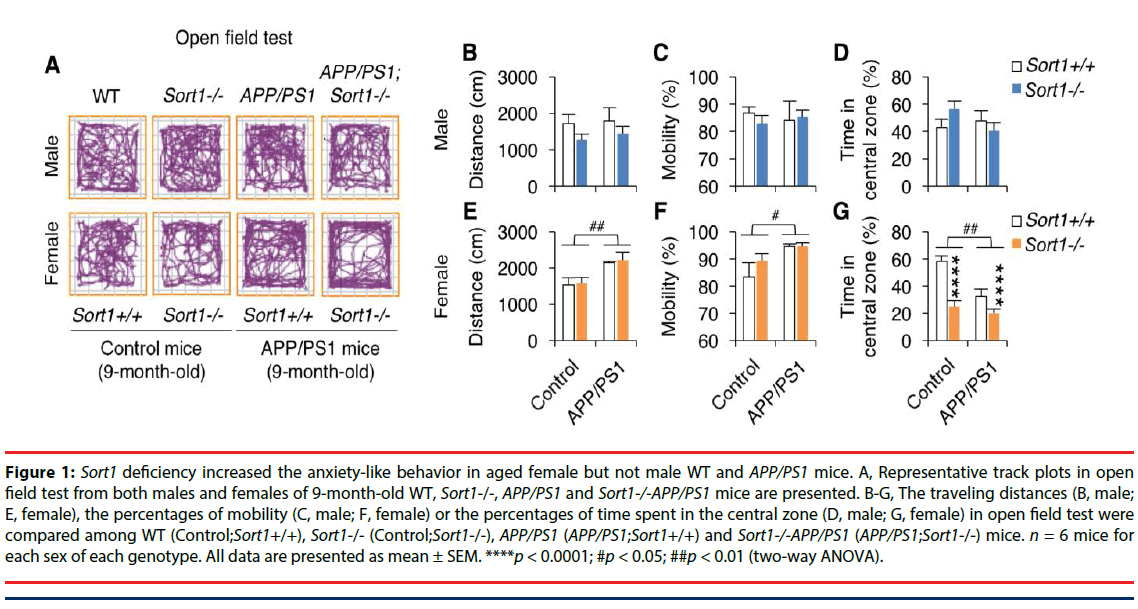
We first compared the locomotion and anxietylike behaviors among WT, Sort1-/-, APP/PS1, and Sort1-/-APP/PS1 mice with both sexes in open field test (Figure 1). Travelling distances were plotted in Figure 1B (males) and Figure 1E (females). Two-way ANOVA analyses showed no main effect of Sort1-/- (F(1,22)=2.43, p=0.14), no main effect of AD transgenes (F(1,22)=0.25, p=0.62) and no Sort1-/- × AD transgene interaction (F(1,22)=0.04, p=0.85) in the travelling distance in male mice; also showed a main effect of AD transgenes (F(1,22)=14.24, p<0.01), but no main effect of Sort1-/- (F(1,22)=0.14, p=0.71) and no Sort1-/- × AD transgene interaction (F(1,22)=0.01, p=0.94) in the travelling distance in female mice. Percentages of mobile time were plotted in Figure 1C (males) and Figure 1F (females). Two-way ANOVA analyses showed no main effect of Sort1-/- (F(1,22)=0.09, p=0.76), no main effect of AD transgenes (F(1,22)=0.00, p=0.98) and no Sort1-/- × AD transgene interaction (F(1,22)=0.39, p=0.54) in the percentage of mobility in male mice; also showed a main effect of the AD transgenes (F(1,22)=7.29, p<0.05), but no main effect of Sort1-/- (F(1,22)=0.99, p=0.33) and no Sort1-/- × AD transgene interaction (F(1,22)=0.88, p=0.36) in the percentage of mobility in female mice. Percentages of time in central zone were plotted in Figure 1D (males) and Figure 1G (females). Two-way ANOVA analyses showed no main effect of Sort1-/- (F(1,22)=0.25, p=0.63), no main effect of AD transgenes (F(1,22)=0.71, p=0.41) and no Sort1-/- × AD transgene interaction (F(1,22)=2.76, p=0.11) in the percentage of time in central zone in male mice; also showed a main effect of Sort1-/- (F(1,22)=29.14, p<0.0001) and a main effect of the AD transgenes (F(1,22)=13.10, p<0.01) and Sort1-/- × AD transgene interaction (F(1,22)=6.06, p<0.05) in the percentage of time in central zone in female mice. These results taken together indicate that Sort1 deficiency specifically increases anxiety-like behavior in aged female mice with or without AD transgenes. Moreover, the results also indicate increases in locomotion activity and anxiety-like behaviors in aged female but not male APP/PS1 mouse model of AD.
Figure 1: Sort1 deficiency increased the anxiety-like behavior in aged female but not male WT and APP/PS1 mice. A, Representative track plots in open field test from both males and females of 9-month-old WT, Sort1-/-, APP/PS1 and Sort1-/-APP/PS1 mice are presented. B-G, The traveling distances (B, male; E, female), the percentages of mobility (C, male; F, female) or the percentages of time spent in the central zone (D, male; G, female) in open field test were compared among WT (Control;Sort1+/+), Sort1-/- (Control;k-/-), APP/PS1 (APP/PS1;Sort1+/+) and Sort1-/-APP/PS1 (APP/PS1;Sort1-/-) mice. n = 6 mice for each sex of each genotype. All data are presented as mean ± SEM. ****p < 0.0001; #p < 0.05; ##p < 0.01 (two-way ANOVA).
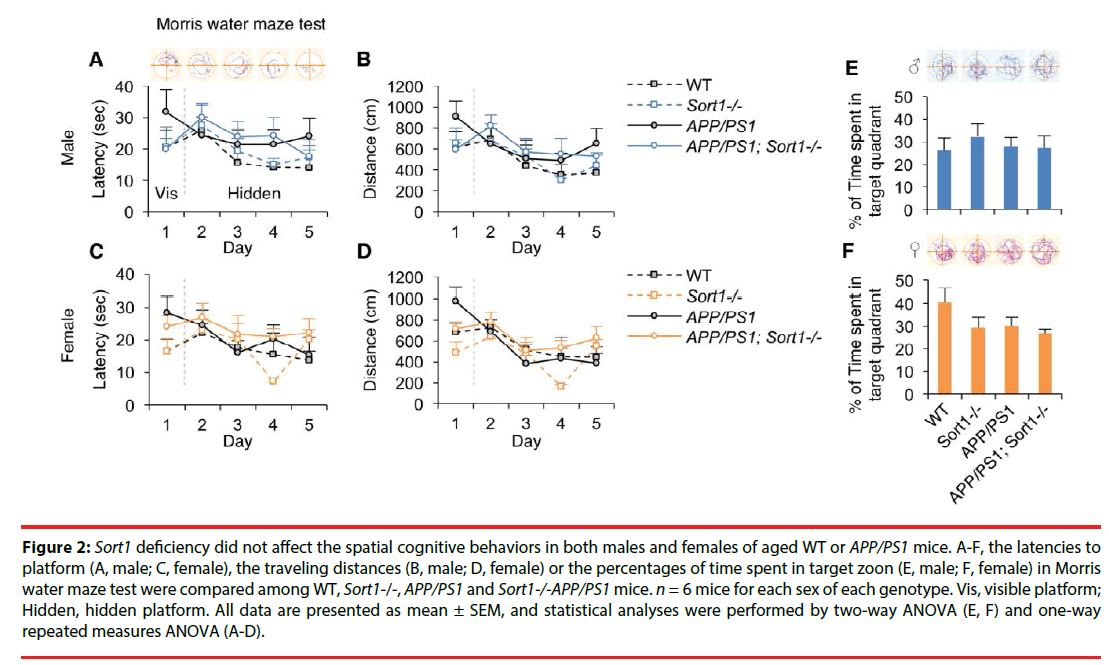
We further compared the spatial cognitive performance among these groups of animals in the Morris water maze test (Figure 2). Escape latencies during training were plotted in Figure 2A (males) and Figure 2C (females); and one-way repeated measures of ANOVA analyses of these data showed no main effect of genotype in males (F(3,19)M=1.73, p=0.20) or females (F(3,19)M=1.44, p=0.26). Swimming distances during training were plotted in Figure 2B (males) and Figure 2 (females); and one-way repeated measures of ANOVA analyses also showed no main effect of genotype in males (F(3,19)M=2.10, p=0.14) or females (F(3,19)M=1.75, p=0.19). Moreover, percentages of time spent in target quadrant during probe test were plotted in Figure 2E (males) and Figure 2F (females). Two-way ANOVA analytic results showed no main effect of Sort1-/- (F(1,22)=0.27, p=0.61, males; F(1,22)=2.64, p=0.12, females), no main effect of AD (F(1,22)=0.12, p=0.73, males; F(1,22)=2.13, p=0.16, females) and no Sort1-/- × AD interaction (F(1,22)=0.39, p=0.54, males; F(1,22)=0.91, p=0.35, females) in the percentage of time spent in target quadrant in males or females.
Figure 2: Sort1 deficiency did not affect the spatial cognitive behaviors in both males and females of aged WT or APP/PS1 mice. A-F, the latencies to platform (A, male; C, female), the traveling distances (B, male; D, female) or the percentages of time spent in target zoon (E, male; F, female) in Morris water maze test were compared among WT, Sort1-/-, APP/PS1 and Sort1-/-APP/PS1 mice. n = 6 mice for each sex of each genotype. Vis, visible platform; Hidden, hidden platform. All data are presented as mean ± SEM, and statistical analyses were performed by two-way ANOVA (E, F) and one-way repeated measures ANOVA (A-D).
Discussion
Overall, the present study examined the role of Sort1 deficiency in the gender-specific anxiety behaviors and cognition in aged mice with or without the AD transgenes. It showed that Sort1 deficiency increases the anxiety-like behaviors in aged female but not male mice in normal aging or in the presence of AD transgenes. It also showed that only female APP/PS1 mouse model of AD showed hyperactivities and anxiety-like behaviors. Finally, it showed that Sort1 deficiency did not change cognitive performance in any gender of aged mice with or without AD transgenes. The present study confirmed the role of sortilin in regulation of anxiety-like behavior and revealed a gender-specific regulation of these behaviors in aged female mice.
The study first showed that the 9-month-old female but not male mice deficient in Sort1 had anxiety-like behaviors. As we previously have discussed [21], the most likely mechanism under ‘Sort1-deficiency induced anxiety-like behavior’ is probably due to Sort1 deficiency-induced dysfunctional regulation of proBDNF [23] or neurotensin [27] which contributes to the pathogenesis of anxiety disorders [24,28]. In regard to the gender-dependent anxiety caused by Sort1 deficiency in aged mice in the open field test, this is consistent with our previous results which showed that young female mice deficient in Sort1 tended to be more anxious than males in both the open field test and elevate plus maze test [21]. The results suggest that deletion of sortilin may alter the metabolism of female hormones (estrogen and progesterone) or their receptors. Given that sortilin is a ‘broad-spectrum’ intracellular sorting receptor, it is likely that lack of sortilin may affect the secretion of the anxiolytic female hormones [30,31] or their uptakes or their cellular transport; and lower level or deregulated locations of these hormones result in the development of anxiety-like behavior. Another possibility is due to the interaction of estrogen (or progesterone) and BDNF (or neurotensin) [32-36]. The deregulated BDNF (or neurotensin) system, which is caused by lack of sortilin, may in turn affect the stabilization of these female hormones. The study also showed that 9-month-old female but not male mice deficient in Sort1 showed anxietylike behavior under Alzheimer’s condition, which is consistent with the above findings in normal aging. In addition, a significant effect of interaction between Sort1 deficiency and AD transgenes suggests that Sort1 deficiency worsens the anxiety-like symptoms in the mouse model of AD. It is known that the prevalence of anxiety disorders declines in patients with age [37], which is likely to occur due to increased capability in coping with negative life impact due to social skills gradually developed over time [38]. Although we did not observe a significant decrease in the anxiety-like behavior in 9-month-old (this study) vs 3-month-old (Ruan et al., previous study [21] WT mice of both males (t(11)=1.08, p=0.30) and females (t(11)=0.84, p=0.42), we observed male but not female Sort1-/- mice showed a significant decline in this behavior (males: t(11)=4.48, p<0.001; females: t(11)=- 1.21, p=0.25; Student t-test), which further confirms the female-specific role of sortilin in the regulation of anxiety-like behaviors. Furthermore, the present study did not show anxiety-like behavior in the aged male APP/PS1 mice in open field test, which is consistent with the studies conducted in 9-month-old [39] or 12-month-old [40] male APP/PS1 mice without showing anxiety-like behavior in both open field test and elevated plus maze test. However, the present study showed hyperactivities and anxietylike behavior in the aged female APP/PS1 mice in open field test. Two other studies which were conducted in 9-month-old or 18-month-old [41] APP/PS1 mice also showed anxiety-like behavior in elevated plus or zero maze test; however, in these studies the mice gender was not specified [42] or female mice included together with male mice [42]. Thus, further studies are required to confirm the increased anxiety-like behaviors in the aged female APP/PS1 mice. Our study also showed that both male and female of 9-monthold mice deficient in Sort1 showed normal cognitive skills in normal aging or Alzheimer’s condition. These findings are consistent with our previous data showing no cognitive deficit in 3-month-old mice with Sort1 deficiency [21]. Why there was no cognitive decline in 9-month-old APP/PS1 mice and Sort1-/-APP/ PS1 mice is not known, but it is most likely because these mice were not very old. The finding is consistent with studies conducted in 9-month-old APP/PS1 mice with both normal learning and memory behaviors [42], and 12-month-old APP/PS1 mice with normal memory behavior [40].
To conclude, the present study shows a gender difference in the development of anxiety disorders due to genetic modifications. Although this study has a limitation as we did not perform other behavioral assessments such as elevated plus/zero maze test, the anxiety-like behavior detected in the open field test in this study is consistent with the behavior in elevated plus maze test as described in our previous study [21]. In addition, open field test has been well examined as a robust paradigm to assess anxiolytics [43]. We will continue to investigate the underlying mechanism involved in the gender-specific regulation of sortilin in anxiety-like behavior in our future studies.
Acknowledgements
This work was supported by grants from National Health and Medical Research Council (APP1021408, APP1020567) and University of South Australia (UniSA) to XFZ, and UniSA President’s Scholarship to CSR. All procedures were approved by the South Australia Pathology Animal Ethics Committee and Animal Ethics Committee of UniSA under the guidelines of the National Health and Medical Research Council of Australia.
References
- Association. 2016 Alzheimer's disease facts and figures. Alzheimer's & Dementia 12(1), 459-509 (2016).
- Clark C, Davatzikos C, Borthakur A, et al. Biomarkers for early detection of Alzheimer pathology. Neurosignals 16(1), 11-18 (2008).
- Lanari A, Amenta F, Silvestrelli G, et al. Neurotransmitter deficits in behavioural and psychological symptoms of Alzheimer's disease. Mechanisms. of. ageing. And. development 127(1), 158-165 (2006).
- Shaikh Z, Roy SP, Patel P. Medicinal value of Mimosa pudica as an anxiolytic and antidepressant: a comprehensive review. WJPPS 5(1), 420-432 (2016).
- De Beurs E, Beekman A, Van Balkom A, et al. Consequences of anxiety in older persons: its effect on disability, well-being and use of health services. Psychological. medicine 29(1), 583-593 (1999).
- Teri L, Ferretti LE, Gibbons LE, et al. Anxiety in Alzheimer's disease: Prevalence and comorbidity. J. Gerontol. A. Biol. Sci. Med. Sci 54(1), M348-M352 (1999).
- Devier DJ, Pelton GH, Tabert MH, et al. The impact of anxiety on conversion from mild cognitive impairment to Alzheimer's disease. Int. J. Geriatr. Psychiatry 24(1), 1335-1342 (2009).
- Andersen K, Launer LJ, Dewey ME, et al. Gender differences in the incidence of AD and vascular dementia The EURODEM Studies. Neurology 53(1), 1992 (1999).
- Altemus M. Sex differences in depression and anxiety disorders: potential biological determinants. Hormones and Behavior 50(1), 534-538 (2006).
- Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Frontiers. In. neuroscience 9 (2015).
- Zandi PP, Carlson MC, Plassman BL, et al. 2002. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. Jama 288(1), 2123-2129 (2002).
- Willnow TE, Petersen CM, Nykjaer A. VPS10P-domain receptors--regulators of neuronal viability and function. Nature reviews. Neuroscience 9(1), 899 (2008).
- Andersson CH, Hansson O, Minthon L, et al. A Genetic Variant of the Sortilin 1 Gene is Associated with Reduced Risk of Alzheimer’s Disease. J. Alzheimers. Dis 53(1), 1353-1363 (2016).
- Dodson SE, Andersen O, Karmali V, et al. Loss of LR11/SORLA enhances early pathology in a mouse model of amyloidosis: evidence for a proximal role in Alzheimer's disease. J. of. Neurosci 28(1), 12877-12886 (2008).
- Lane RF, St George-Hyslop P, Hempstead BL, et al. Vps10 family proteins and the retromer complex in aging-related neurodegeneration and diabetes. J. of. Neurosci 32(41), 14080-14086.
- Alemany S, Ribasés M, Vilor‐Tejedor N, et al. New suggestive genetic loci and biological pathways for attention function in adult attention‐deficit/hyperactivity disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet 168(1), 459-470 (2015).
- Baum A, Akula N, Cabanero M, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol. Psychiatry 13(2), 197 (2008).
- Breiderhoff T, Christiansen GB, Pallesen LT, et al. Sortilin-related receptor SORCS3 is a postsynaptic modulator of synaptic depression and fear extinction. PloS. one 8(9), e75006 (2013).
- Glerup S, Saarma M, Nykjaer A. The role of SorLA in neurotrophic activity and complex behavior. Molecular. Neurodegeneration 8(1), P19 (2013).
- Ollila H, Soronen P, Silander K, et al. Findings from bipolar disorder genome-wide association studies replicate in a Finnish bipolar family-cohort. Molecular. psychiatry 14(1), 351 (2009).
- Ruan CS, Yang CR, Li JY, et al. Mice with Sort1 deficiency display normal cognition but elevated anxiety-like behavior. Exp. Neurol 281, 99-108 (2016).
- Petersen CM, Nielsen MS, Nykjær A, et al. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. Journal Biol. Chem 272(6), 3599-3605(1997).
- Chen ZY, Ieraci A, Teng H, et al. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J. Neurosci 25(26), 6156-6166 (2005).
- Chen ZY, Jing D, Bath KG, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 314(5796), 140-143 (2006).
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nature. neuroscience 10(1), 1089 (2007).
- Teng HK, Teng KK, Lee R, et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci 25(1), 5455-5463 (2005).
- Mazella J. Sortilin/neurotensin receptor-3: a new tool to investigate neurotensin signaling and cellular trafficking? Cellular. signalling 13(1), 1-6 (2001).
- Ollmann T, Péczely L, László K, et al. Anxiolytic effect of neurotensin microinjection into the ventral pallidum. Behavioural. Brain. research 294, 208-214 (2015).
- Prus AJ, Hillhouse TM, LaCrosse AL. Acute, but not repeated, administration of the neurotensin NTS 1 receptor agonist PD149163 decreases conditioned footshock-induced ultrasonic vocalizations in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 49(1), 78-84 (2014).
- Bitran D, Shiekh M, McLeod. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. Journal. of. neuroendocrinology 7(1) 171-177 (1995).
- McCarthy MM, McDonald CH, Brooks PJ. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol. Behav 60(5), 1209-1215 (1996).
- Alexander MJ, Leeman SE. Estrogen‐inducible neurotensin immunoreactivity in the preoptic area of the female rat. J. Comparat. Neurol 345(1), 496-509 (1994).
- McCann S, Vijayan E. Control of anterior pituitary hormone secretion by neurotensin. Ann. NY. Acad. Sci 668(1), 287-297 (1992).
- Singh M, Su C. Progesterone, brain-derived neurotrophic factor and neuroprotection. Neuroscience 239(1), 84-91 (2013).
- Sohrabji F, Lewis DK. Estrogen–BDNF interactions: implications for neurodegenerative diseases. Front. Neuroendocrinol 27(1), 404-414 (2006).
- Warembourg M, Jolivet A. Progesterone receptor and neurotensin are colocalized in neurons of the guinea pig ventrolateral hypothalamus. Neuroendocrinology 60(1), 486-492 (1994).
- Flint AJ. Generalised anxiety disorder in elderly patients. Drug. Aging 22(1), 101-114 (2005).
- Wolitzky‐Taylor KB, Castriotta N, Lenze EJ, et al.. Anxiety disorders in older adults: a comprehensive review. Depression and anxiety 27(1), 190-211 (2010).
- Olesen LØ, Bouzinova EV, Severino M, et al. Behavioural Phenotyping of APPswe/PS1δE9 Mice: Age-Rrelated Changes and Effect of Long-Term Paroxetine Treatment. PloS one 11, e0165144.
- Psotta L, Rockahr C, Gruss M, et al. Impact of an additional chronic BDNF reduction on learning performance in an Alzheimer mouse model. Fron in behavior neurosci 9(1), (2015).
- Biallosterski B, Prickaerts J, Rahnama’i M, et al. Changes in voiding behavior in a mouse model of Alzheimer’s disease. Frontiers. in. aging. neuroscience 7 (2015).
- Lok K, Zhao H, Zhang C, et al. 2013. Effects of accelerated senescence on learning and memory, locomotion and anxiety-like behavior in APP/PS1 mouse model of Alzheimer's disease. Jour of neuro sci 335(1), 145-154 (2013).
- Prut L, Belzung C,. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Euro. jou of pharmacology 463(1), 3-33 (2003).