Research Article - Neuropsychiatry (2016) Volume 6, Issue 4
Evaluation of the development of children whose mother is under methadone maintenance treatment during pregnancy
- Corresponding Author:
- Dr. Hung-Yu Chan
Taoyuan Psychiatric Center, Ministry of Health and Welfare, No.71, Longshou St., Taoyuan City, Taoyuan County 33058, Taiwan
Tel: +886-3-3698553
Fax: +886-3-3609498
Abstract
Objective
An ongoing controversial issue is the developmental delay of infants associated with methadone treatment during pregnancy. Previous studies focused on Caucasian population, and most did not use a comprehensive assessment tool. This study used an appropriate assessment tool to evaluate these infants in a Taiwanese population.
Methods
The study proceeded from July 2006 to March 2010 in a large teaching psychiatric hospital in Taiwan. Three domains of the Bayley Scale of Infant Development, 3rd edition (Bayley-III) was used to evaluate the development of infants with histories of prenatal exposure to methadone. The evaluations were performed together by the same child psychiatrist and psychologist at the same month but the social-emotional rating relied on reports of the caregivers.
Results
Eight children and their mothers participated. Five of eight children showed significant developmental delay; three had developmental delay in language composition, three had developmental delay in cognitive composition, but no developmental delay was seen for the social-emotional portion. Parental neglect was noticed in most cases.
Conclusions A substantial number of infants showed developmental delay. The use of methadone and a chaotic life-style associated with illicit drugs use during pregnancy both contributed to this developmental delay. Large studies are needed to clarify the risk of methadone for developmental delay in infants.
Keywords
Methadone, pregnancy, infant, developmental delay, Taiwan
Introduction
Heroin use and related problems have been emerging as an important public health issue. Heroin use is a risk factor of HIV infection usually of intravenous drug users (IDUs) due to needle sharing. Opioid substitution therapy is the medically supervised administration of a psychoactive substance that is similar to the one producing dependence. According to new WHO guidelines confirm that, even after 40 years, substitution therapies such as methadone are still the most promising method of reducing drug dependence [1].
Methadone is a long-acting opioid which is the standard treatment for the management of opioid-dependent pregnant women and its pregnancy category is C [2,3]. Methadone has been used for the treatment of opioid addiction during pregnancy since the 1960s [4,5]. Perinatal methadone substitution therapy provides several potential maternal, obstetrical, and neonatal benefits [6]. Additional benefits include a potential reduction in drug-seeking behaviors, including trading sex for drugs and in engaging in commercial sex to obtain money to buy drugs [7]. Pregnant women enrolled in a substance use disorders treatment program are more likely to receive prenatal care, have infants of higher birth weight and be discharged home with their neonate [8-11].
This treatment has definite advantages for the mother and is currently recommended [12], but in-utero drug exposure is associated with increased risks of perinatal morbidity and mortality [13]. According to previous studies, infants of substance-using mothers have much poorer child protection outcomes than infants of non-substance-using mothers [14,15]. Previous studies also indicate that infants prenatally exposed to opiates are at risk for mild psychomotor developmental impairment [16]. However, there are only a few studies exploring the outcomes of the infants of methadone maintenance treatment (MMT) pregnant mother. The assessment tools of previous related studies are inadequate or not comprehensive enough to assess the outcomes of these infants [13]. Previous studies also focused on the population of North America or West Europe [16-18]. Therefore, we designed a study with an appropriate assessment tool to evaluate the development of these infants prospectively in Taiwanese population. We hope this pilot study may help further research on this topic.
Subjects and Methods
This study was conducted in a large teaching psychiatric hospital in Taiwan from July 2006 to March 2010. The hospital provides methadone treatment to more than 1,000 patients per day and it is also the biggest child psychiatric department in Taiwan. The study protocol was approved by the hospital’s Ethics Committee [IRB number: C20090218-8]. Prior to the study, all of the mothers who received MMT were legally competent and provided informed written consent after full study explanation.
Inclusion criteria included patients who were pregnant woman; met the DSM-IV opioid dependence criteria; were under MMT program during their gestation; and who agreed to join the study and signed an inform consent. There were no exclusion criteria for this study. The eligible patients were invited to participate in our study, and were informed about the necessity and safety of methadone treatment. In order to prevent the possibility of neonatal abstinence syndrome, we also suggested that these women discuss the information about methadone with their obstetricians.
Several studies have suggested that the dosage of methadone should be increased for pregnant women to ensure maintenance concentrations similar to those in non-pregnant patients [19-21]. Otherwise, if the mothers want to tapper methadone dosage, the dose should be monitored closely during their tapering [22]. The usual dosage of methadone for women is 30~60mg/day in Taiwan and we would adjust the dose depending on the patient’s need. We add on the dosage of methadone 5mg per day if patient complained withdrawal symptoms during pregnancy. A principal objective of the methadone maintenance program is to help the patient feel physically comfortable without producing euphoria. We also asked some questions about the breast feeding, motivation to change their life style, and how they felt about the methadone treatment during pregnancy.
Development of all infants of drug-dependent mothers was assessed by the Bayley Scales of Infant Development, third Edition (Bayley-III). The Bayley-III are recognized internationally as one of the most comprehensive tools to assess children from as young as one month old. With Bayley-III, it is possible to obtain detailed information even from non-verbal children as to their functioning. Children are assessed in the five key developmental domains of cognition, language, social-emotional, motor and adaptive behavior. We selected three domains – language scale, cognitive scale, and social-emotional scaleto evaluate these children in this study. These three domains have good discriminative validity when examined in large populations [23], and can identify children ages 1 month to 3.5 years with developmental delay. Bayley-III follows a time-tested tradition of using standardized assessment procedures to provide toddlers and infants with tasks and situations that capture their interest and provide an observable set of behavioral responses [24].
Some studies suggest that Neonatal Intensive Care Unit Network Neurobehavioral Scale Procedures (NNNS) is a good evaluation tool for at risk children, but the reasonable age upper limit of NNNS is 46 to 48 weeks [7]. Several children in our study are not in this scale’s age range. Therefore, we choose Bayley-III instead of NNNS as the assessment tool for this study.
We asked these patients to bring their children back to our outpatient clinic for evaluation, and all tests except social-emotional scale were performed by the same child psychiatrist and psychologist together. The rating of socialemotional scale relied on the reports of their caregivers. Children who had developmental delay were referred to the children’s clinic for further treatment and rehabilitation.
Results
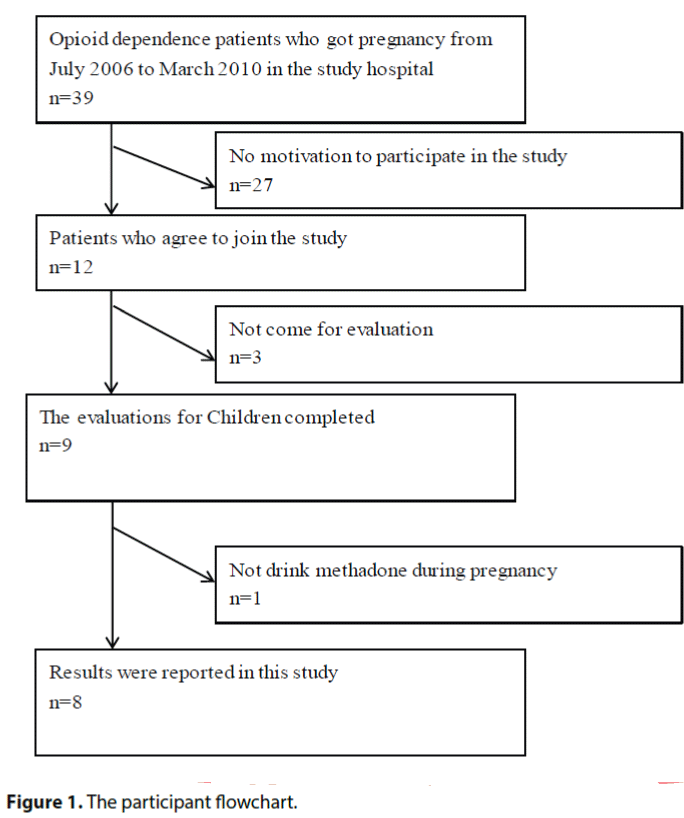
All opioid dependence patients who got pregnancy from July 2006 to March 2010 in the study hospital were invited to join the study. Thirty-nine women were eligible for and 12 agreed to participate in this study. Nine of them completed the evaluation. Only eight results of them were reported in this study because we noticed that one mother didn’t drink methadone during her pregnancy (Figure 1). Reviewing the record of these 4 early terminators, all of their babies are female cases. Their parents do not report the reason why they lose follow up during the study. The demographic data, methadone dose and results of Baley-III of the mothers and babies are shown in the Table 1.
| No. | A | B | C | D | E | F | G | H | median or percentage |
|---|---|---|---|---|---|---|---|---|---|
| Gender(M/F) | M | M | M | M | M | M | M | F | M:87.5% F:12.5% |
| Age (months) | 4 | 38 | 3 | 14 | 17 | 8 | 42 | 34 | 14 |
| Mother’s age when delivery | 34y 11m |
32 y 2 m |
26 y 09m |
28y 05 m |
35 y 02 m |
26 y 02 m |
25 y 11m |
28 y | 28y |
| Methadone dosage before pregnancy (mg/day) | 50 | 50 | 40 | 30 | 30 | 50 | 30 | 40 | 40 |
| Methadone dosage after pregnancy (mg/day) | 50 | 70 | 110 | 80 | 130 | 120 | 70 | 50 | 70 |
| Birth Weight (gm.) | 2350 | 3160 | 2745 | 3410 | 2800 | 2270 | 2200 | 2500 | 2700 |
| Head circumference (cm) | 30◎ | 33 | 34 | 35 | 35 | 31◎ | NA | NA | 33 |
| Height (cm) | 46※ | 50 | 48 | 50 | 48 | 44※ | 47 | NA | 48 |
| Chest circumference (cm) | 29 | 33 | 31.5 | 35 | 32 | 28.5 | NA | NA | 31.75 |
| The way of production | Natural birth | Natural birth | Natural birth | Natural birth | Caesarean section | Caesarean section | Natural birth | Natural birth | Natural:75% Caesarean:25% |
| Premature delivery(Y/N) | Y | N | N | N | N | N | Y | N | 25% |
| G (Gravide) | 3 | 3 | 3 | 2 | 3 | 4 | 10 | 3 | 3 |
| P (Para) | 2 | 1 | 2 | 2 | 3 | 1 | 2 | 3 | 2 |
| A(Abortion) | 1 | 2 | 1 | 0 | 0 | 3 | 8 | 0 | 1 |
| Infant’s age at assessment (mo) | 4 | 38 | 3 | 14 | 17 | 7 | 42 | 34 | 15.5 |
| Cognitive composite score | 65* | 85 | 75* | 110 | 70* | 115 | 95 | 100 | 90 |
| Cognitive composite delay | Obvious | Lower than average | mild | normal | Obvious | normal | normal | normal | Between Lower than average to normal |
| Language composite score | 79* | 86 | 100 | 74* | 62** | 103 | 106 | 97 | 91.5 |
| Language composite delay | mild | Lower than average | Lower than average | mild | Obvious | normal | normal | normal | Between Lower than average to normal |
| Social-emotional portion score | 105 | 100 | 110 | 90 | 110 | NA | 105 | 110 | 105 |
| The education level of mother | Elementary school | Junior high school | Elementary school | Elementary school | Elementary school | Junior high school | Elementary school | Elementary school | |
| Income level of mothers(cut point: 733USD/month) | Lower than the level | Lower than the level | Lower than the level | Lower than the level | Lower than the level | Lower than the level | Lower than the level | Lower than the level | |
| Urine Morphine | Negative | Positive | Negative | Negative | Positive | Negative | Negative | Negative | |
| Urine Amphetamine | Positive | Positive | Positive | Positive | Positive | Positive | Negative | Negative |
*70–84 mild developmental delay **<70 obvious developmental delays, NA: not available ※Lower than 3% ◎Lower than 1%
Table 1: Demographic details and Scores on the Bayley Scales of Infant Development, Third Edition.
Mother’s median methadone dosage was 40 mg (range 30–60 mg) before pregnancy and 70mg during pregnancy (range 50–130 mg). Several patients didn’t have very good compliance due to the stigma of opioid medication. Mother A stopped taking methadone during second trimester. Although Mother H suffered from withdrawal symptoms, she refused to increase methadone dosage. After our psychoeducation, all mothers were breast-feeding their own baby in order to prevent neonatal withdrawal symptom. However, all of them worried about the methadone from the breast-feeding will affect their babies’ health. These mothers need to check urine drug screen when the first time starting a new session of their methadone treatment, and the urine drug screen results were recorded in the Table 1. The socio-economic level and educated level of each mother was also recorded in the Table 1. We use 733USD/month (the minimum wage in Taiwan is 22000 NTD/ month) as the cut-off point of the socio-economic level.
The median age of these drug-exposed children is 14 months (range from 3 to 42). There were more male infants than female ones (M/F: 7/1), and two of them had premature delivery (case A and G). Case A has obvious development delay, but case G doesn’t. Case A and Case F head circumstance are below the average range (33~35cm). The table also demonstrates the scores of each subscale about these eight children, and shows that five children have developmental delay (cases A,B,C,D, and E). Three of these five children have cognitive developmental delay, and three have language developmental delay. All eight children have normal scores on the socialemotional scale.
Because heroin abuse may impact the methods of child rearing used by parents, it is necessary to assess their methods in each case. Although all cases reported having a new child can motivate them to change their life style, we noticed that most parents do not have good skill to rear their offspring. For example, Case C expired one week after our evaluation, and the parents reported that the baby died due to falling down at home. The social worker believed that the parents’ neglect was the main cause of this accident. The mother of case A let her son watch television all day long, and she believed such action was suitable. The mother of case F went to jail during the period of our study. Cases B, D, and G were reared by only one of their parents; the other one was always absent for some reasons, including being in prison. Faulty parenting was noticed in most of the cases.
Discussion
Up to our best knowledge, this is the second study using Bayley-III to evaluate the development of children whose mother is under MMT during pregnancy. There is one similar case series report using Bayley scales was done in 1982 [18]. However, this study focus on several diagnosed physical or mental conditions, such as narcotic abstinence syndrome, elevated systolic blood pressure, and otitis media. That study use Bayley- III to evaluate the development of children only once at 18 months old. Our study focuses on neurodevelopment delay and the age distribution of our cases shows a wider range than previous one. This study is the first one done in Asia and there is no other similar study using Bayley scale was done since 1982. The result of this study is valuable with clinical importance.
In these eight children, the delay in language composition is most obvious, being seen in three of them. Regarding cognitive composition, three of the children have developmental delay. Moreover, height of two cases are lower than 1% of average, and head circumstance of these two cases are lower than 3% of average when birth. Head circumstance is related maternal nutrition status [25].
It is interesting that these eight children’s entire social-emotional portion shows normal development. This may be related to that Social- Emotional Adaptive Behavior Questionnaire is completed by caregivers. All caregivers rated their children have positive representations of self, emotional knowledge and regulatory abilities. However, the validity of their reports and assessments may be not reliable. Furthermore, sometimes there may not be clear markers of impaired functioning in social-emotional domain despite the presence of substance abuse or other disease. This is more likely to occur with young children because caregivers play a more active role in regulating the child’s behavior and emotions [26].
These results are similar to that of previous studies [16,27]. However, there are still some differences between them. First, our study is different from previous studies in gender composition because most of our cases are male children. Second, we evaluate the children within 4 to 42 months old. Previous study was done in 12 month old [16] or in the first 18 months of life born to methadonemaintained mothers [18].
Pregnant women have been treated with methadone for more than 25 years and neither methadone nor other opiates have been shown to directly cause birth defects. However, animal studies indicate detrimental effects on growth, behavior, neuroanatomy and biochemistry, and increased perinatal mortality [28]. Human newborn baby may experience some side effects from methadone. The most common are smallerthan- normal head size, low birth weight, and withdrawal symptoms.
We think there may be two other reasons for the developmental delay of these children. First, two mothers has urine morphine positive record (B, E) and all the other mothers have self-report of heroin use during methadone treatment. That means they used not only the therapeutic methadone but also illegal medications. Illicit drug use in pregnancy is strongly associated with the use of tobacco and heavy drinking [29]. In addition, pregnant women who use any illicit drug typically use multiple substances [30]. Second, these children’s parents can’t take care of them very well. We noticed that most of them are raised by single parent or the child is grandparent-raised child. It is unfortunate that one of the children in the study died due to the neglect of parents at home. Drug effects also appear to be exacerbated in children with multiple risks, including poverty, and non-optimal caregiving environments [31]. Additionally, one study indicated that there is a close relationship between mother’s drug use and child abuse [14]. These children’s outlook is further compromised because of their early childhood deprivation, as their mothers themselves are victims of drug addiction, faulty parenting, and social deprivation [32].
There are some limitations in this study. First, our sample size is small. We tried to collect around 40 cases, but the motivations of those mothers were low. This make the cases of the study may be not representative of this population. Second, this study does not have a control group. Without a control group, you can’t really say, for certain, that the development delay was caused by the methadone treatment during the pregnancy. Third, because heroin or other illicit drugs using during pregnancy is also a risk factor for development deficit in infants and some patients in the study had opioid response in their urine. Therefore, the effects of methadone on infants’ development in the study need further clarification.
Conclusion
In sum, this is a pilot study about the developmental delay of children whose mothers are under MMT during pregnancy. Our result suggests that infants exposed to opiates in-utero are at risk of neuro-developmental impairment. Among the opiate-exposed infants, the language scale and cognitive scale are lower than average. Not only is psycho-education helpful for methadone using mothers, but early intervention from a psychiatrist is necessary for opiate-exposed children. We believe further prospective studies are warranted to confirm the results of this study.
Acknowledgements
The nurse Shu-Hui Peng helped to get into contact with patients during this research. This work was funded by a research grant from Taoyuan Psychiatric Center (TYPC-2009-01) and (TMH-2009-01).
No authors report any financial relationships relevant to the subjects of this article.
References
- Council S. The methadone fix. Bulletin of the World Health Organization 86 (2008).
- Joseph H, Stancliff S, Langrod J. Methadone maintenance treatment (MMT): a review of historical and clinical issues. Mt. Sinai. J. Med 67(5-6), 347-364 (2000).
- Cleary BJ, Donnelly J, Strawbridge J, et al. Methadone dose and neonatal abstinence syndrome-systematic review and meta-analysis. Addiction 105(12), 2071-2084 (2010).
- Dole VP, Nyswander M. A medical treatment for diacetylmorphine (heroin) addiction: a clinical trial with methadone hydrochloride. JAMA 193(1), 646-650 (1965).
- Blinick G, Wallach RC, Jerez E. Pregnancy in narcotics addicts treated by medical withdrawal. The methadone detoxification program. Am. J. Obstet. Gynecol105(7), 997-1003 (1969).
- Dyer KR, White JM, Foster DJ, et al.The relationship between mood state and plasma methadone concentration in maintenance patients. J. Clin. Psychopharmacol21(1),78-84 (2001).
- Seligman NS, Weiner S, Berghella V. Methadone maintenance therapy during pregnancy.10(1), 09.
- Burns L, Mattick RP, Lim K, et al.Methadone in pregnancy: treatment retention and neonatal outcomes. Addiction 102(2), 264-270 (2007).
- Ward J, Hall W, Mattick RP. Role of maintenance treatment in opioid dependence. Lancet 353(9148), 221-226 (1999).
- Kandall SR, Albin S, Lowinson J, et al.Differential effects of maternal heroin and methadone use on birthweight. Pediatrics 58(5), 681-685 (1976).
- Hulse GK, Milne E, English DR, et al.The relationship between maternal use of heroin and methadone and infant birth weight. Addiction 92(11), 1571-1579 (1997).
- Pritham UA, Troese M, Stetson A. Methadone and buprenorphine treatment during pregnancy: what are the effects on infants? Nurs. Womens. Health11(6), 558-567 (2007).
- Hunt RW, Tzioumi D, Collins E, et al. Adverse neurodevelopmental outcome of infants exposed to opiate in-utero. Early. Hum. Dev84(1), 29-35 (2008).
- McGlade A, Ware R, Crawford M. Child protection outcomes for infants of substance-using mothers: a matched-cohort study. Pediatrics124(1), 285-293 (2009).
- Soepatmi S. Developmental outcomes of children of mothers dependent on heroin or heroin/methadone during pregnancy. Acta. Paediatr. Suppl404, 36-39 (1994).
- Bunikowski R, Grimmer I, Heiser A, et al.Neurodevelopmental outcome after prenatal exposure to opiates. Eur. J. Pediatr157(9), 724-730 (1998).
- Aylward GP. Prediction of function from infancy to early childhood: implications for pediatric psychology. J. Pediatr. Psychol29(7), 555-564 (2004).
- Rosen TS, Johnson HL. Children of methadone-maintained mothers: follow-up to 18 months of age. J. Pediatr 101(2), 192-196 (1982).
- Pond SM, Kreek MJ, Tong TG, et al.Altered methadone pharmacokinetics in methadone-maintained pregnant women. J. Pharmacol. ExpTher233(1), 1-6 (1985).
- Wolff K, Boys A, Rostami-Hodjegan A, et al.Changes to methadone clearance during pregnancy. Eur. J. Clin. Pharmacol61(10), 763-768 (2005).
- Hartwig C, Haasen C, Reimer J, et al.Pregnancy and birth under maintenance treatment with diamorphine (heroin): a case report. Eur. Addict. Res14(2), 113-114 (2008).
- Welle-Strand GK, Skurtveit S, Tanum L, et al.Tapering from Methadone or Buprenorphine during Pregnancy: Maternal and Neonatal Outcomes in Norway 1996-2009. Eur. Addict. Res21(5), 253-261 (2015).
- Spittle AJ, Doyle LW, Boyd RN. A systematic review of the clinimetric properties of neuromotor assessments for preterm infants during the first year of life. Dev. Med. Child. Neurol50(4), 254-266 (2008).
- Weiss LG, Oakland T, Aylward GP. Bayley-III clinical use and interpretation. 1st ed. Amsterdam; Boston: Academic Press (2010).
- Lifschitz MH, Wilson GS, Smith EO, et al.Factors affecting head growth and intellectual function in children of drug addicts. Pediatrics75(2), 269-274 (1985).
- Carter AS, Briggs-Gowan MJ, Davis NO. Assessment of young children's social-emotional development and psychopathology: recent advances and recommendations for practice. J. Child. Psychol. Psychiatry 45(1), 109-134 (2004).
- Rosen TS, Johnson HL. Children of methadone-maintained mothers: follow-up to 18 months of age. J. Pediatr 101(2), 192-196 (1982).
- Farid W, Dunlop S, Tait R, et al.The effects of maternally administered methadone, buprenorphine and naltrexone on offspring: review of human and animal data. Curr. Neuropharmacol6(2), 125 (2008).
- Smith IE, Lancaster JS, Moss-Wells S, et al.Identifying high-risk pregnant drinkers: biological and behavioral correlates of continuous heavy drinking during pregnancy. J. Stud. Alcohol48(4), 304-309 (1987).
- Bennett AD. Perinatal substance abuse and the drug-exposed neonate. Adv. Nurse. Pract7(5), 32-36(1999).
- Eyler FD, Behnke M. Early development of infants exposed to drugs prenatally. Clin. Perinatol26(1), 107-150 (1999).
- Hayford SM, Epps RP, Dahl-Regis M. Behavior and development patterns in children born to heroin-addicted and methadone-addicted mothers. J. Natl. Med. Assoc80(11), 1197-1200 (1988).