Research Article - Neuropsychiatry (2017) Volume 7, Issue 6
Evaluating the Prognosis of Surgical Treatment in Atypical Temporal Lobe Epilepsy-A Study Based on Ictal Onset Patterns and Extratemporal Early Propagations on Stereo-Electroencephalography
- Corresponding Author:
- Guo-ming Luan, M.D., Ph.D.
Epilepsy Center and Department of Functional Neurosurery, Sanbo Brain Hospital
Capital Medical University; Beijing Key Laboratory of Epilepsy; Beijing Institute for Brain Disorders
50, Xiang-shan-yi-ke-song, Haidian District, Beijing 100093, China
Tel: 086-010-62856718
Fax: 086-010-62856902
Abstract
Abstract
Purpose: Our aim is to examine ictal onset patterns (IOPs), extratemporal early propagations (EPs) and inner relationship between them based on stereo-electroencephalography recordings in the patients with atypical temporal lobe epilepsy, and to evaluate their impact on seizure prognosis and cognitive outcomes after epilepsy surgery.
Methods: Forty-three patients were included in this retrospective study. Five to sixteen multilead electrodes were implanted per patient. All of them underwent a standard craniotomy for tailored resection of the hypothetical epileptogenic zone. Post-operative seizure status and cognitive outcomes were evaluated by Engle’s classification, Wechsler intelligence and Wechsler memory scale.
Results: Four types of IOPs were identified in the 43 patients. The patients with low frequency highamplitude periodic spikes (LFPS) and spike or poly-spike fast discharges (SpFD) had better seizure outcomes. There were ten patients with extratemporal EPs, who had worse clinical outcomes than those without extratemporal EPs (Engel’s class I: χ2 =10.689, p=0.001; Engel’s class Ia: χ2 =4.251, p=0.039). Three conditions related to IOPs and extratemporal EPs were combined to predict the seizure prognosis after operation. The sensitivity and specificity were 90.0% and 78.6% for Engel’s class I, and were 87.0% and 55.0% for Engel’s class Ia. The patients with LFPS showed more significant improvement in memory quotient (MQ) but not in full intelligence quotient (FIQ) than those with other IOPs.
Conclusions: IOPs and extratemporal EPs are closely related to seizure status and cognitive outcomes after epilepsy surgery in atypical TLE patients.
Keywords
Temporal lobe epilepsy, Stereo-electroencephalography, Ictal onset patterns, Early propagations, Epileptogenic zone, Surgical outcomes
Introduction
Epileptogenic zone (EZ) represents the minimal amount of cortex that must be resected (inactivated or completely disconnected) to achieve seizure freedom [1]. Therefore, the relationship between EZ and extent of surgical resection is a key factor to determine the prognosis. Obviously, temporal lobe epilepsy (TLE) is the most common type of pharmacoresistant epilepsy in adults, and frequently successfully treated by surgery [2]. The reason may be that the EZs in most of them are within the extent of anterior temporal lobectomy (ATL) which is the most established neurosurgical procedure for TLE.
However, although it primarily affects the temporal lobes, TLE is thought to be a network disease with widespread extratemporal effects [3,4]. The spectrum of TLE includes many subdivisions, from the focal mesiotemporal subtype, the temporopolar subtype, the mesiolateral subtype, the lateral subtype to the widely extended temporal plus epilepsy (TPE) subtype [5]. The patients with the EZs localized in the anterior temporal lobe may have better clinical outcomes after ALT, but those with TPE may not have [6].
There are many studies analyzing the relationship between invasive EEG ictal onset patterns (IOPs) and surgical outcomes in TLE [7-10]. The extent of EZ cannot be accurately estimated by IOPs alone, and the analysis on extratemporal early propagations (EPs) is more important to identify EZ boundaries in patients with atypical TLE. Stereo-electroencephalography (SEEG) has proved to be more suitable for this analysis, however, if the electrodes are not covering the right areas, the final results may be misleading [7].
In this study, we aimed to investigate IOPs, extratemporal EPs and inner relationship between them based on SEEG recordings in the patients with atypical TLE, and to determine their impact on seizure prognosis and cognitive outcomes after epilepsy surgery.
Methods
The study was approved by the ethics committee of Sanbo Brain Hospital, Capital Medical University, Beijing, China, where it was carried out from May 2015 to December 2016.
▪ Patients
Subjects for this retrospective study included patients with drug-resistant focal seizures of suspected temporal lobe origin or temporal origin with extratemporal EPs, who were candidates for epilepsy surgery, but required diagnostic depth electrode studies because results from noninvasive tests were inconclusive.
All patients had a comprehensive evaluation including detailed clinical history, neurological examination, neuropsychological testing, magnetic resonance imaging (MRI, 1.5T Philips MR scanner), scalp electroencephalography (EEG), semiology evaluation, 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) and SEEG. MRI protocols included axial T1- and T2- weighed sequences, axial T2 FLAIR sequence, coronal T2-weighed sequence, coronal T2 FLAIR sequence (hippocampal scan), and sagittal T2 FLAIR sequence. Patients were selected for the present study if they satisfied the following criteria: (i) absence of any detectable lesion at mesial temporal lobe on MRI, with the exception of hippocampal sclerosis (HS); (ii) no history of epilepsy surgery; (iii) SEEG recordings showing that seizures involved at least mesial and/or neocortical temporal lobe (MTL/NTL) structures; (iv) surgery performed according to SEEG results, taking into account anatomical constraints, and (v) at least 1 years of postoperative follow-up. According to the criteria, forty-three patients with drug resistant atypical TLE were selected from a series of 180 cases. Five to sixteen (median=12) multilead electrodes were implanted per patient, in temporal and extratemporal areas depending on the suspected origin and region of early spreading of seizures.
The seizure-onset zone (SOZ) was defined as the depth electrode contacts showing the first unequivocal ictal intracranial EEG change. Seizure EP was defined as a clear seizure discharge, starting 0.5-3s after seizure-onset and recorded outside the SOZ [1].
The candidates were divided into four groups depending on the participation of the temporal regions at seizure onset and extratemporal EPs: (i) MTLE (n = 23): the patients with seizures that initially involved the MTL; (ii) MTLE+ (n=5): the patients with seizures that initially involved the MTL and early spread outside the temporal lobe within 3 seconds; (iii) NTLE (n =10): the patients with seizures that initially involved the NTL; (iv) NTLE+ (n=5): the patients with seizures that initially involved the NTL and early spread outside the temporal lobe within 3 seconds.
In the 25 cases with MTLE/MTLE+, surgery consisted of a tailored resection including at least the temporal pole and MTL structures (amygdala, hippocampus and para-hippocampal gyrus). The posterior limits of the temporal neocortical resection varied according to SEEG results. A selective amygdalo-hippocampectomy was performed in the other 3 cases (CMN, TSY and SXM) with MTLE/MTLE+. In the 15 cases with NTLE/NTLE+, individualized surgical planning was performed according to SEEG and imaging results.
▪ SEEG Recordings
The SEEG exploration was performed using intracerebral multiple-contact electrodes (Huake-Hengsheng Medical Technology, China; 8–16 contacts, length: 2 mm, diameter: 0.8 mm, 1.5 mm apart) placed intracranially with the aid of a stereotactic ROSA robotic device (Medtech). The SEEGs were recorded using a common reference electrode (Nicolet™ system; 128-channels; sampling rate: 512Hz) [11]. The SEEG recording was carried out during long-term video-EEG monitoring in order to record several of the patient’s habitual seizures, following complete or partial withdrawal of antiepileptic drugs.
A multidisciplinary team (MDT) discussion was then held for each individual, after enough seizures recorded (at least 3-5 seizures), to discuss the results and implications of the SEEG study and to decide collectively on a plan for resection. Subsequent to this meeting and approximately 8-12weeks after removal of the SEEG electrodes, patients underwent a standard craniotomy for tailored resection of the hypothetical EZ.
▪ Seizure Analysis and Cognitive Evaluation
An expert electroencephalographist analyzed all the habitual seizures for the identification of the SOZ. In addition, recordings were chosen at least 2 days after the electrode implantation surgical procedure in order to limit possible effect of the general anesthesia. Post-operative seizure outcome was evaluated by Engel’s classification.
Cognitive function evaluation was performed according to Wechsler Adult Intelligence Scale- Revised in China or Wechsler intelligence scale for children-Revised in China (WAIS-RC or WISC-RC) and Wechsler Memory Scale- Revised in China (WMS-RC) before surgery and 1 year after surgery [12]. The antiepileptic drugs were not changed between the two evaluations.
Statistical Analysis
Statistical comparisons were made using Fisher’s exact test or χ2 tests for categorical variables. Significance was accepted where p <0.05.
Results
▪ Demographic Data and Seizure Clinical Semiology
The population consisted of 24 males and 19 females, aged 12.2–39.4 (mean: 24.4±6.9; median: 24.2) years. Epilepsy onset age ranged from 1 to 31 (mean: 12.1±7.3; median: 12.0) years old. Eight patients (35%, 8/23) with MTLE, none with MTLE+ (0%, 0/5), 1 patient (10%, 1/10) with NTLE, and none with NTLE+ (0%, 0/5) had experienced febrile convulsions in childhood (Table 1). The patients with MTLE had higher rate of febrile seizure than the other patients (χ2 =4.075, p=0.044).
| Patient | Sex | Age (yr) | Feb szr | Epi on(yr) | Aura | Epi side | MRI data | Epileptogenic zone | IOPs & SOZ Complete Resection | Intra-and Extratemporal EPs & Complete Resection | Postop class |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HS | NAL | MTL | MTL+ | NTL | NTL+ | |||||||||||
| CMN | F | 26.8 | - | 4 | Vis+Aud | R | RD | - | + | - | - | - | LFPS, Yes | - | IB | |
| FZY | M | 13.7 | - | 4 | Aud+Fear+Drm | L | - | - | - | - | + | - | LVFA, No | - | IVA | |
| GZC | M | 24.9 | - | 12 | Headache +Vis | L | LD | + | + | - | - | - | LFPS, Yes | - | IA | |
| GMC | F | 36.2 | - | 26 | - | L | - | - | + | - | - | - | LVFA, No | - | IIA | |
| HH | F | 23.5 | - | 1 | Fear | L | L | - | + | - | - | - | LFPS, Yes | - | IA | |
| JBY | M | 18.2 | - | 12 | Diz+Aud | R | - | - | - | - | - | + | LVFA, Yes | Parietal, No | IA | |
| JZY | M | 19.6 | - | 11 | Diz | R | - | - | - | - | - | + | SpRA, No | MTL, Yes; parietal, No | IIB | |
| LX | M | 21.5 | + | 6 | Djv | R | RD | + | + | - | - | - | SpFD, Yes | NTL, Yes | IA | |
| LQ | M | 25.8 | - | 21 | - | R | - | - | - | + | - | - | LVFA, Yes | Insula, No | IIA | |
| LRX | M | 25.5 | - | 12 | - | R | R | - | + | - | - | - | SpRA, Yes | - | IA | |
| LWM | M | 26.9 | + | 8 | - | L | L | + | + | - | - | - | SpFD, Yes | NTL, Yes | IA | |
| LWZ | M | 16.4 | - | 7 | Abdominal | R | - | + | - | - | - | + | LVFA, No | Frontal, No | IVB | |
| LCS | M | 39.5 | - | 7 | Fear | L | L | + | + | - | - | - | LFPS, Yes | - | IB | |
| LQS | M | 12.3 | - | 7 | Fear | R | - | + | - | - | + | - | SpFD, Yes | - | IA | |
| LYY | F | 25.4 | - | 21 | - | L | - | - | - | + | - | - | SpRA, Yes | Insula, No | IIIA | |
| MJR | M | 37.4 | + | 15 | Aud | R | - | - | - | - | + | - | SpFD, Yes | - | IA | |
| ML | M | 29.5 | - | 6 | Som | R | - | + | - | - | - | + | SpFD, Yes | Insula, Yes | IIA | |
| MY | F | 21.8 | + | 14 | - | R | R | + | + | - | - | - | LFPS, Yes | - | IB | |
| PXH | F | 29.2 | - | 4 | Abd+Vis | R | R | - | + | - | - | - | SpFD, Yes | - | IA | |
| RF | F | 26.0 | - | 11 | - | R | L | - | - | - | + | - | LVFA, Yes | - | IA | |
| SXM | F | 28.2 | - | 26 | Cephalic | L | - | + | - | + | - | - | SpRA, Yes | Insula, No | IVA | |
| SZY | F | 21.7 | - | 16 | Pal | L | - | - | + | - | - | - | SpFD, Yes | LTL, No | IA | |
| QH | M | 22.6 | + | 12 | Som | R | R | - | + | - | - | - | LFPS, Yes | - | IA | |
| TSY | M | 14.6 | - | 9 | Vis | L | - | - | - | + | - | - | SpFD, Yes | Occipital+cingulate, No | IIA | |
| WH | M | 27.7 | + | 3 | Vis | L | L | + | + | - | - | - | LVFA, Yes | - | IC | |
| WXD | M | 30.9 | - | 19 | - | L | - | + | - | - | + | - | LVFA, Yes | MTL, Yes | IA | |
| WY | F | 21.6 | - | 14 | Diz | R | R | - | + | - | - | - | LVFA, Yes | - | IA | |
| WZH | M | 18.8 | - | 3 | Headache +Vis | R | - | + | - | - | + | - | SpFD, Yes | - | IA | |
| WZP | F | 15.0 | - | 12 | Aud+Som | L | - | - | - | - | + | - | SpRA, Yes | - | IVA | |
| WX | F | 29.0 | + | 17 | Fear | R | R | - | + | - | - | - | LFPS, Yes | - | IA | |
| XLM | F | 32.6 | - | 16 | Djv | R | R | - | + | - | - | - | LFPS, Yes | - | IB | |
| XQY | F | 26.7 | - | 4 | Pal | L | L | + | + | - | - | - | SpFD, Yes | - | IB | |
| YB | M | 37.1 | - | 18 | - | L | - | + | - | - | + | - | SpFD, Yes | - | IA | |
| YC | M | 16.7 | - | 4 | - | L | - | + | - | + | - | - | SpFD, Yes | NTL, Yes; Insula, No | IA | |
| YSX | F | 22.9 | - | 15 | - | L | LD | + | + | - | - | - | LFPS, Yes | - | IA | |
| YY | M | 26.6 | - | 14 | - | L | - | - | - | - | + | - | LVFA, Yes | - | IA | |
| YY | F | 25.0 | - | 10 | Aud | R | - | - | - | - | - | + | LVFA, No | Prietal, No | IVB | |
| YJ | F | 24.8 | - | 12 | Fear | R | - | - | + | - | - | - | LFPS, Yes | - | IA | |
| ZLH | F | 21.0 | - | 5 | Abdominal | R | R | - | + | - | - | - | LFPS, Yes | - | IVB | |
| ZL | M | 31.7 | - | 29 | - | L | - | + | + | - | - | - | SpFD, Yes | - | IA | |
| ZY | M | 27.9 | + | 11 | Pal | L | L | - | + | - | - | - | SpFD, Yes | - | IA | |
| ZY | F | 37.8 | + | 31 | Fear+Pal+Djv+Diz | R | R | - | + | - | - | - | SpFD, Yes | - | IVB | |
| ZHY | M | 12.6 | - | 9 | Aud+Som | L | - | - | - | - | + | - | LVFA, No | - | IVB | |
Table 1: Clinical patient characteristics, presurgical evaluations and surgical outcomes.
Seventy-four per cent of patients with MTLE (17/23), 40% with MTLE+(2/5), 60% with NTLE (6/10) and 100% with NTLE+ (5/5) had epileptic auras. Altogether, auras of varying types were experienced in most of the patients (30/43, 70%). The most common aura in MTLE and NTLE was fear (29%, 5/17) and auditory (67%, 4/6), respectively (Table 2).
| Types of TLE | Auras | Most common aura | |
|---|---|---|---|
| Yes | No | ||
| MTLE (n=23) | 17 | 6 | Fear (n=5) |
| MTLE+ (n=5) | 2 | 3 | - |
| NTLE (n=10) | 6 | 4 | Auditory (n=4) |
| NTLE+ (n=5) | 5 | 0 | - |
Table 2: Distribution of the auras in the types of TLE.
▪MRI Findings
Three patients with MTLE (13%, 3/23), 2 patients with MTLE+ (40%, 2/5), 5 patients with NTLE (50%, 5/10) and 2 patients with NTLE+ (40%, 2/5) had negative MRI. The patients with MTLE had lower rate of negative MRI (χ2 =5.430, p=0.020). HS was identified radio-graphically and later confirmed pathologically in 17 cases with MTLE (74%, 17/23) and 0 cases with the other three types of TLE. One case with MTLE and 1 case with NTLE showed HS on MRI but it could not be confirmed pathologically. One case with MTLE+ showed pathological HS, but not identified radio-graphically.
▪ Ictal Onset Patterns and Histopathology Findings
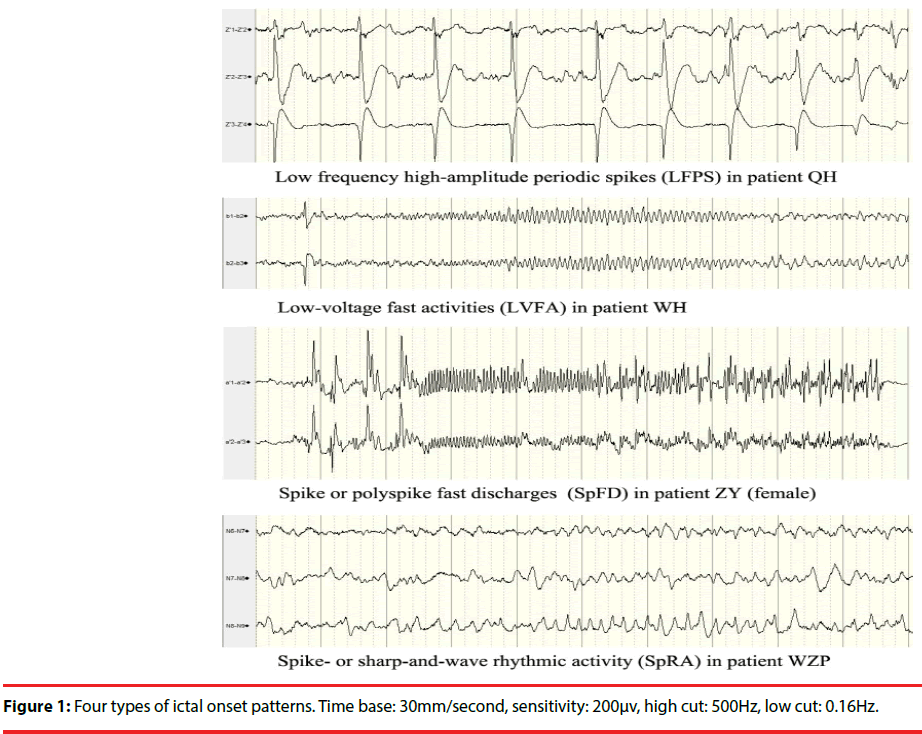
Four seizure-onset patterns were identified in the 43 patients (Figure 1): low-voltage fast activities (LVFA: 12 cases, 28%); low frequency highamplitude periodic spikes (LFPS: 11 cases, 26%); spike or polyspike fast discharges (SpFD: 15 cases, 35%); spike- or sharp-and-wave rhythmic activities (SpRA: 5 cases, 12%). The distribution of IOPs in the types of TLE and the distribution of histopathology in the types of IOPs were shown in Table 3 and Table 4, respectively.
| Types of TLE | Ictal onset patterns | Total | |||
|---|---|---|---|---|---|
| LFPS | LVFA | SpFD | SpRA | ||
| MTLE | 11 | 3 | 8 | 1 | 23 |
| A | 1 | 0 | 2 | 0 | 3 |
| H | 6 | 2 | 2 | 0 | 10 |
| A+H | 4 | 1 | 4 | 1 | 10 |
| MTLE+ | 0 | 1 | 2 | 2 | 5 |
| NTLE | 0 | 5 | 4 | 1 | 10 |
| NTLE+ | 0 | 3 | 1 | 1 | 5 |
| Total | 11 | 12 | 15 | 5 | 43 |
Table 3: Distribution of the ictal onset patterns in the types of TLE.
| IOPs | Types of histopathology | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HS | HS+FCD Ib |
FCD Ia |
FCD Ib |
FCD IIa | FCD IIb |
GG | Others | ||
| LFPS | 7 | 2 | 0 | 1 | 0 | 0 | 0 | 1 (AC) | 11 |
| LVFA | 1 | 1 | 1 | 4 | 1 | 1 | 1 | 2 (NP, BI) | 12 |
| SpFD | 6 | 1 | 1 | 2 | 2 | 1 | 0 | 2 (BI) | 15 |
| SpRA | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 1 (NP) | 5 |
| Total | 14 | 5 | 2 | 10 | 3 | 2 | 1 | 6 | 43 |
Table 4: Distribution of the types of histopathology in the different ictal onset patterns.
▪ Extratemporal Early Propagations
There were 10 patients with extratemporal EPs after seizure onset. In the 5 MTLE+ patients, there were 4 with insular EPs and 1 with occipital and cingulate EPs; in the 5 NTLE+ patients, there were 1 with insular EPs, 1 with frontal EPs, and 3 with parietal EPs. Only 1 patient’s extratemporal EP leads were included in the extent of resection (Table 1).
▪ Seizure Outcomes
Our results showed that 87% (20/23) of MTLE patients were in class I (Ia: 61%, 14/23), whereas only 20% (1/5) of MTLE+ patients were in class I (Ia: 20%, 1/5); 70% (7/10) of NTLE patients were in class I (Ia:70%, 7/10), whereas only 20% (1/5) of NTLE+ patients were in class I (Ia: 20%, 1/5). The patients with class I were more in MTLE than those in the other types of TLE, χ2 =8.576, p=0.001. The patients with extratemporal EPs had worse outcomes than those without extratemporal EPs (class I: χ2 =10.689, p=0.001; class Ia: χ2 =4.251, p=0.039).
Ninety-one percent (10/11) of patients with LFPS (Ia: 55%, 6/11) and 80% (12/15) with SpFD (Ia: 73%, 11/15) were in class I, whereas only 50% (6/12) with LVFA (Ia: 42%, 5/12) and 20% (1/5) with SpRA (Ia: 20%, 1/5) were in class I. There were more patients with LFPS and SpFD in class I group (χ2 =8.833, p=0.003) but not in class Ia group (χ2 =3.741, p=0.053) compared with those with LVFA and SpRA.
▪ Cognitive Outcomes
The improvement in postoperative FIQ and MQ scores was related to the seizure outcomes. In the Engle’s class I group, the patients with LFPS showed more significant improvement in MQ but not in FIQ than those with other IOPs (Table 5).
| Engle’s Class I | Engle’s Class I | |||||
|---|---|---|---|---|---|---|
| Yes | No | P value | LFPS | Other IOPs | P value | |
| FIQ >10 ≤10 |
21 8 |
1 13 |
<0.001 | 9 1 |
12 7 |
0.201 |
| MQ >10 ≤10 |
17 12 |
1 13 |
0.002 | 10 0 |
7 12 |
0.001 |
Table 5: The cognitive improvement more than 10 points 1 year after surgery.
▪ Seizure Prediction
Combining 3 conditions, it was possible to predict the surgical outcomes of the patients with atypical TLE: i. the IOP of the patient must be one of the LFPS, LVFA and SpFD (n=38); ii. no extratemporal EPs within 3s or all the leads with extratemporal EPs within 3s must be included in the extent of resection (n=34); iii. all the onset and intratemporal EP leads must be included in the extent of resection (n=38). The number of patients who fitted into all the above-mentioned conditions was 29. We hypothesize that if a case fits into all the three conditions, better clinical outcome will be predicted. The sensitivity and specificity were 90.0% and 78.6% for Engel’s class I, and were 87.0% and 55.0% for Engel’s class Ia. The positive and negative predictive values were 90.0% and 78.6% for Engel’s class I, and were 69.0% and 78.6% for Engel’s class Ia (Table 6).
| Outcome prediction | Fit all the conditions | Not fit all the conditions | Sensitivity | specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| Class I Yes No |
26 3 |
3 11 |
90.0%(26/29) | 78.6%(11/14) | 90.0%(26/29) | 78.6%(11/14) |
| Class Ia Yes No |
20 3 |
9 11 |
87.0%(20/23) | 55.0%(11/20) | 69.0%(20/29) | 78.6%(11/14) |
Table 6: Prediction of surgical outcomes in TLE combining three given conditions related to IOPs and EPs.
Case Example
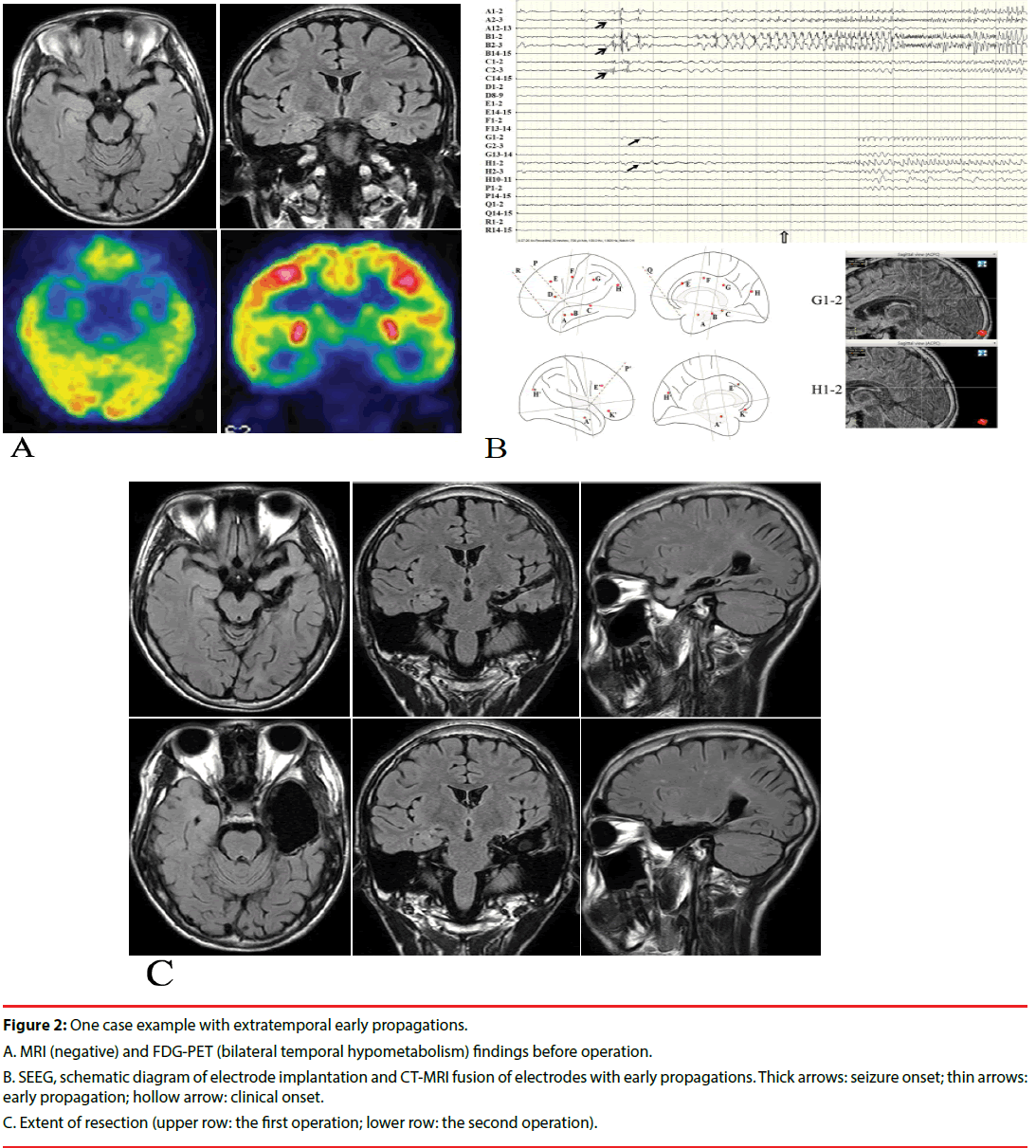
Here, we presented a case (patient TSY) with extratemporal EPs, who underwent operations twice after invasive evaluation. The patient was a 14 years old boy who had two types of seizures for 5 years. The first type of seizure was manifested as loss of consciousness, fearful facial expressions, smacking and both hands fumbling, which lasted for about 20-30 seconds, and occurred about once a week. The second type of seizure was manifested as blurred vision and generalized convulsion, which occurred once a month. He took levetiracetam, valproate acid and oxcarbazepine at the time of visit. Scalp video-EEG monitoring was performed at first. A small number of bilateral temporal spike and waves could be found during the inter-ictal stage. Seven habitual seizures which were manifested as the first type of seizure were available for review, and the ictal onset patterns were diffused. MRI was negative, and inter-ictal FDG-PET showed decreased uptake in the bilateral temporal lobes (Figure 2A). The full intelligence quotient (FIQ) was 82, and the memory quotient (MQ) was 73. Sixteen electrodes were bilaterally implanted. All the seizures originated from the amygdala and hippocampus, and early spread to the cingulate and occipital gyrus (Figure 2B). A selective amygdalo-hippocampectomy was performed, and hippocampal sclerosis was identified pathologically. However, the patient had to undergo re-operation 9 months later, including the whole hippocampus and most of basal temporal and anterior temporal lobes (still not including the extratemporal EP leads), since seizures recurred rapidly, which were manifested as blurred vision and blank stare once a month (Figure 2C). This kind of seizures occurred 3 times in 2 years after the second resection. The FIQ was 90 and the MQ was 79 one year after the re-operation.
Figure 2: One case example with extratemporal early propagations.
A. MRI (negative) and FDG-PET (bilateral temporal hypometabolism) findings before operation.
B. SEEG, schematic diagram of electrode implantation and CT-MRI fusion of electrodes with early propagations. Thick arrows: seizure onset; thin arrows: early propagation; hollow arrow: clinical onset.
C. Extent of resection (upper row: the first operation; lower row: the second operation).
Discussion
It is clear that the notion of focality is too simplistic to suit the variety of temporal lobe seizure generators, and the concept of ‘epileptogenic network’ has been developed to account for the complex spatial organization of several distinct cortical areas generating seizures [5]. On this basis, we aim to analyze anatomoelectrical features of IOPs and extratemporal EPs in atypical TLE, and to evaluate their impact on the seizure status and cognitive outcomes after surgery.
▪ Grouping Method
Here we use the group names of MTLE+ and NTLE+ which are not completely the same as the concept of TPE posed by Ryvlin and Kahane [13]. They used TPE to indicate both an ictal discharges originating simultaneously from the temporal lobe and the neighboured extratemporal structures, or two coexisting seizure types in the same patient, with temporal and extratemporal ictal onset, respectively [5].
▪ Ictal Onset Patterns in Atypical TLE
The relationship between IOPs of intracranial EEG and surgical outcome in atypical TLE has been analysed in several studies [8-10]. IOPs may be related to pathology of epileptogenic lesions [9,14,15]; hence, it can be regarded as a predictor of prognosis. Histopathological analyses demonstrate a strong association between mesial temporal sclerosis and LFPS, especially in severe cases. LVFA may not be always associated with abnormal histopathology in neocortical temporal lobe, and it can be shown in mesial temporal lobe, even in normal histopathology regions unrelated to EZ [14].
LFPS and LVFA are two separate ictal depth EEG onset patterns often recorded in presurgical patients with MTLE [10]. In fact, SpFD can be often found in MTLE, too [16]. In our study, LFPS (11case) was only observed in the patients with MTLE, and highly associated with HS (more than 80%); SpFD (7 cases) was more common than LVFA (3 cases) in MTLE group, and the latter pattern (8cases) was more common in NTLE/NTLE+ group. In addition, an atypical onset pattern, SpRA (5cases) was found in all four TLE groups, and it indicated unfavorable prognosis [1].
▪ Extratemporal Early Propagations in Atypical TLE
Out results showed that the atypical TLE patients with extratemporal EPs had worse seizure prognosis after epilepsy surgery. We defined EP as propagation within 3 seconds because most of the studies showed that a dividing line of 3 seconds could predict the surgical outcome well. Talairach and Bancaud thought that an “early” or a “late” SEEG change cannot be assessed in terms of either seconds or tens of seconds [17]. However, “early spread’ proved to be useful in clinical application [18]. Stimulation elicited enhanced gamma band activity at early spread sites, which was highly coherent with the onset zone [19]. Latencies of spread maybe related to the degree of integrity of inhibition in these areas, with early spread as a possible indicator of secondary epileptogenesis [20].
Our results showed that there were no extratemporal EPs after the onset of LFPS. This has been proved by Perucca, et al. who noted that LFPS was seen as a propagation rhythm in mesial temporal sclerosis. By comparison, the LVFA and <13 Hz sharp activity were seen in regions of seizure spread across all regions [1]. In addition, we found that the extratemporal EPs after the onset of SpFD were similar to that of LVFA. SpRA may not be the real onset pattern, and itself may be a kind of propagation pattern.
▪ Seizure and Cognitive Outcomes
According to the previous studies about surgical outcomes in TLE [6,21], our opinion was that prediction of seizure outcomes after surgery can be phased into two stages: the noninvasive stage and the invasive stage. As the ictal onset and EP are very difficult to distinguish, outcome prediction in the intracranial EEG stage is complicated among some patients.
Most of the studies predicted seizure outcomes in the TLE patients combining many factors, including medical history, seizure semiology, and imaging, scalp EEG, invasive EEG, extent of resection, and histopathology, etc. We only selected three conditions related to IOPs and extratemporal EPs for seizure outcome prediction. This method is very easy to apply; however, its predictive validity should be tested in further clinical practice. Moreover, comparison of the two predictive methods in the noninvasive and invasive stage may be further investigated in future. We have noted that some MTLE patients with LFPS still had auras after operation, which gradually decreased over the time. This may help explain why it is better in the prediction of Engel’s class I but not Ia.
The improvement in MQ and FIQ was closely related to better clinical outcomes in patients [22]. However, after excluding the influence of better prognosis, we found that the patients with LFPS had more significant improvement in MQ but not in FIQ than those with other IOPs. We think that the IOPs and extratemporal EPs should be associated with the cognitive outcomes after surgery. The EEG onset of LFPS is not easy to spread outside MTL which is associated with encoding, storage, and the retrieval of long-term memories. The abnormal epileptic discharges sustainedly localize in the hippocampus, which has shown serious impairment in histopathology and function. Two recent studies showed that the memory of the patients with MTL might be improved after surgery, which was more obvious after right-sided surgery. However, these improvements might be not statistically significant. Instead, the patients demonstrated improved frontal lobe-related cognitive function after surgery [23,24]. We think that it may be caused by a mix of the types of IOPs and EPs in those patients. Further studies are required to analyze the relationship between IOPs, extratemporal EPs and cognitive outcomes in TLE.
Limitations
There are a few limitations in the methodology of this study. First, it was a retrospective study, in which the designs of electrode implantation were not so perfectly standardized. Second, the follow-up data during the past 2 years (2015-2016) of census were not available in 28% of patients; hence, the results of this study need to be confirmed by a long term follow-up.
Conclusions
The determination of the epileptogenicity of brain regions potentially involved in seizure generation is a crucial pre-surgical work in the patients suffering from ‘atypical’ TLE, even if the patients underwent the intracranial EEG recordings. In the current study, three conditions related to IOPs and extratemporal EPs in the SEEG recordings were combined to predict seizure outcomes. It can be easily used, and is better in outcome prediction in the Engel’s class I group but not in the class Ia group. The patients with LFPS showed more significant improvement in MQ but not in FIQ than those with other IOPs.
In brief, IOPs and extratemporal EPs are closely related to seizure status and cognitive outcomes after epilepsy surgery in atypical TLE patients.
Conflict of Interest
The authors declare no competing financial interests.
Acknowledgments
This work was supported in part by China Postdoctoral Science Foundation (2015M571069), Beijing Postdoctoral research Foundation (2015- ZZ-61), Beijing Municipal Science &Technology Commission (Z161100002616016 and Z16100000516131), National Natural Science Foundation of China (81671285), Capital Health Research and Development Special (2016-1-8012), and Basic Clinical Research Cooperation Project of Capital Medical University (15JL90).
References
- Perucca P, Dubeau F, Gotman J. Intracranial electroencephalographic seizure-onset patterns: effect of underlying pathology. Brain 137(Pt 1), 183-196 (2014).
- Coito A, Plomp G, Genetti M, et al. Dynamic directed interictal connectivity in left and right temporal lobe epilepsy. Epilepsia 56(2), 207-217 (2015).
- Barba C, Barbati G, Minotti L, et al. Ictal clinical and scalp-EEG findings differentiating temporal lobe epilepsies from temporal “ plus ” epilepsies. Brain 130(Pt 7), 1957-1967 (2007).
- Haneef Z, Lenartowicz A, Yeh HJ, et al. Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia 55(1), 137-145 (2014).
- Kahane P, Barba C, Rheims S, et al. The concept of temporal “plus” epilepsy. Rev. Neurol 171(3), 267-272 (2015).
- Barba C, Rheims S, Minotti L, et al. Temporal plus epilepsy is a major determinant of temporal lobe surgery failures. Brain 139(Pt 2), 444-451 (2016).
- Singh S, Sandy S, Wiebe S. Ictal onset on intracranial EEG: Do we know it when we see it? State of the evidence. Epilepsia56(10), 1629–1638 (2015).
- Dolezalova I, Brazdil M, Hermanova M, et al. Intracranial EEG seizure onset patterns in unilateral temporal lobe epilepsy and their relationship to other variables. Clin. Neurophysiol 124(6), 1079–1088 (2013).
- Schuh LA, Henry TR, Ross DA, et al. Ictal spiking patterns recorded from temporal depth electrodes predict good outcome after anterior temporal lobectomy. Epilepsia 41(3), 316–319 (2000).
- Memarian N, Madsen SK, Macey PM, et al. Ictal depth EEG and MRI structural evidence for two different epileptogenic networks in mesial temporal lobe epilepsy. PLoS. One 10(4), e0123588 (2015).
- Zhou J, Huang LT, Zhai F, et al. The role of ROSA navigated intracranial electrode implantation technique on precise epileptogenic zone localization. Neuropsychiatry (London) 7(3), 258–264 (2017).
- Wang XF, Gao qing, Zhou J, et al. The Neuropsychological efficacy of vagus nerve stimulation in 56 children with catastrophic Epilepsy. Neuropsychiatry (London) 7(4), 387–392 (2017).
- Ryvlin P, Kahane P. The hidden causes of surgery-resistant temporal lobe epilepsy: extratemporal or temporal plus? Curr. Opin. Neurol 18(2), 125–127 (2005).
- Spanedda F, Cendes F, Gotman J. Relations between EEG seizure morphology, interhemispheric spread, and mesial temporal atrophy in bitemporal epilepsy. Epilepsia 38(12), 1300–1314 (1997).
- Turkdogan D, Duchowny M, Resnick T, et al. Subdural EEG patterns in children with taylor-type cortical dysplasia: comparison with nondysplastic lesions. J. Clin. Neurophysiol 22(1), 37–42 (2005)
- Chabardès S, Kahane P, Minotti L, et al. The temporopolar cortex plays a pivotal role in temporal lobe seizures. Brain 128(Pt 8), 1818–1831 (2005).
- Kahane P, Landré E, Minotti L, et al. The Bancaud and Talairach view on the epileptogenic zone: a working hypothesis. Epileptic. Disord 8(Suppl 2), 16-26 (2006).
- Bartolomei F, Chauvel P, Wendling F. Epileptogenicity of brain structures in human temporal lobe epilepsy: a quantified study from intracerebral EEG. Brain 131(Pt 7), 1818–1830 (2008).
- Lega B, Dionisio S, Flanigan P, et al. Cortico-cortical evoked potentials for sites of early versus late seizure spread in stereoelectroencephalography. Epilepsy. Res 115(1), 17–29 (2015).
- Götz-Trabert K, Hauck C, Wagner K, et al. Spread of ictal activity in focal epilepsy. Epilepsia 49(9), 1594–1601 (2008).
- Menzler K, Thiel P, Hermsen A, et al. The role of underlying structural cause for epilepsy classification: Clinical features and prognosis in mesial temporal lobe epilepsy caused by hippocampal sclerosis versus cavernoma. Epilepsia 52(4), 707–711 (2011).
- Li TF. Epilepsy and associated comorbidities. Neuropsychiatry (London) S(1), 01–03 (2017).
- Gül G, Yandim Kuşcu D, Özerden M, et al. Cognitive Outcome after Surgery in Patients with Mesial Temporal Lobe Epilepsy. Noro. Psikiyatr. Ars 54(1), 43-48 (2017).
- Tang Y, Yu X, Zhou B, et al. Short-term cognitive changes after surgery in patients with unilateral mesial temporal lobe epilepsy associated with hippocampal sclerosis. J. Clin. Neurosci 21(8), 1413-1418 (2014).