Research Article - (2018) Volume 8, Issue 6
Epi-Aortic Trunk Evaluation in Elderly Patients with and Without Depression: A Cross-Sectional Study
- *Corresponding Author:
- Grazia D’Onofrio,
Fondazione Casa Sollievo della Sofferenza, Complex Unit of Geriatrics, Department of Medical Sciences, San Giovanni Rotondo, Foggia, Italy - Tel and Fax: +39 0882-410271
Abstract
Background:
In older patients depression and atherosclerosis can occur. The aim of the present study was to determine whether late-life depression (LLD) is associated with presence of carotid atherosclerosis, and to assess the direct proportionality between carotid atherosclerosis and depression severity.
Methods:
456 patients [333 with LLD and 123 without LLD (noLLD)] attending the Ageing Evaluation Unit and Vascular disease Evaluation Unit were recruited. All patients were assessed by a standardized Comprehensive Geriatric Assessment (CGA), Mini Mental State Examination (MMSE), Clock Drawing Test (CDT), Frontal Assessment Battery (FAB), and Hamilton Rating Scale for Depression – 21 items (HDRS-21). All patients had made a B-Mode Ultrasound scan and Color Doppler ultrasound scan of the epi-aortic trunks.
Results:
LLD patients showed significantly a higher grade of cognitive impairment (MMSE:p=0.003), a major impairment in any CGA domains (ADL:p<0.0001; IADL:p<0.0001; MNA:p<0.0001; ESS:p<0.0001; social support network distribution: p=0.017), and more frequent white matter lesions (WMLs:p<0.0001) than noLLD patients. Very severe LLD patients had a higher grade of cognitive impairment (MMSE:p=0.009; FAB:p=0.026; CDT:p=0.006), and a major impairment in any CGA domains (ADL:p=0.006; IADL:p=0.001; MNA:p<0.0001; ESS:p=0.003). WMLs were more frequent in Severe and Very severe LLD patients (p<0.0001). The patients with atherosclerosis were mainly more depressed (p<0.0001), smokers (p=0.002) and with WMLs (p=0.001) than patient without atherosclerosis. Patients with LLD demonstrated significantly a higher frequency in Moderate-severe atherosclerosis (p<0.0001). The severity of LLD seems increasing progressively in patients with Mild and Moderate-severe atherosclerosis, showing that the patients with Very severe LLD were significantly more frequent in Moderate-severe atherosclerosis (p=0.002).
Conclusions:
Subjects with atherosclerosis were more likely to be depressed. Moreover the severity of LLD seems increasing progressively in patients with Mild and Moderate-severe atherosclerosis.
Keywords
Depression, Atherosclerosis, Cerebral white matter lesions, Carotid B-Mode Ultrasound scan, Comprehensive geriatric assessment.
Introduction
Depression is not a normal component of aging [1]. It is one of the most common diseases in elderly patients worldwide [2]. Depressive syndromes that arise from 65 years and over are tagged as Late-life depression (LLD) [3]. Moreover, there is a general consensus on a syndromal approach to LLD to identify symptom clusters such as late-life major depressive disorder (MDD) [2]. Late-life MDD has a pooled prevalence of 7% [4] and accounts for 5.7% of years lived with disability among over 60 year olds [4].
The serious consequences of persistent depressive symptoms in older persons include relapse and recurrence [5], functional disability [6], increasing of health care utilization [7] and cognitive decline owing in part to the impact of long periods of untreated depression on hippocampal volume [8]. Persisting of depression is also associated with an increased mortality [9].
Depression pathophysiology is complex and implicates mechanisms involved in vascular disease, especially in LLD patients [10]. It has been shown that cerebral white matter lesions (WMLs) are more common in individuals with LLD than in healthy controls [11,12]. WMLs are focal or confluent areas in the cerebral white matter that display high signal intensity on T2- and proton density–weighted magnetic resonance imaging (MRI) [10]. The development of WMLs is allowed by carotid atherosclerosis through inducing cerebral hypoperfusion [10]. This last condition may be explained by decreased compliance of the carotid artery wall that leads to a higher pulsatile pressure in the cerebral vasculature and, in response, adaptive vascular remodeling inducing hypotensive conditions and localized brain tissue ischemia [13, 14]. This feasible hemodynamic effect of carotid atherosclerosis could be an important mechanism behind WMLs in depression, not the least because patients with LLD [15, 16] show signs of increased arterial stiffness.
On one side WMLs may lead to mood disorders, their progression predicts incident depression [17] and poorer depression outcomes [18], and tract-specific localization of WMLs correlates with depression severity [19]. On the other side, depression may affect WML progression through behavioral or genetic mechanisms, h y p o t h a l a m i c - p i t u i t a r y - a d re n a l - a x i s dysregulation, vascular endothelial dysfunction, or autonomic dysregulation, which have all been observed in depression patients [20]. Evidence shows that the co-morbidity between depression and atherosclerosis can occur because people with depression have a high risk of developing atherosclerosis and, vice versa people with atherosclerosis are at risk of depression [21-24]. Therefore, starting from the assumption that there is a probable bidirectional relationship between depression and carotid atherosclerosis, the aim of the present study was to determine whether LLD is associated with presence of carotid atherosclerosis, and to assess the direct proportionality between carotid atherosclerosis and depression severity.
METHODS
̢̻̉ Subjects
This cross-sectional study was conducted on the basis of the guidelines for Good Clinical Practice, the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) and was approved by the local ethics committee. Written informed consent for research was obtained from each patient or from.
Patients and healthy controls consecutively were evaluated from May 2007 to March 2017 in two different and indipendent evaluation units: 1) Ageing Evaluation Unit of the Geriatrics Unit, and 2) Vascular disease Evaluation Unit performed by two experienced physicians (M. P. of the Cardiologic Unit and M. G. L. of the Geriatric Unit) of the IRCCS “Casa Sollievo della Sofferenza”, San Giovanni Rotondo (FG), Italy.
Patients were eligible for study inclusion if they had reached the age ≥ 55 years, the ability to provide an informed consent or availability of a relatives or a legal guardian in the case of severe demented patients, the diagnosis of LLD according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM 5) [25], a complete CGA, a clinical and cognitive-affective assessment.
Controls were eligible if they were without any history of mental disorders according to the DSM 5 criteria
Exclusion criteria were imminent suicide intent, ongoing compulsory treatment, a history of head trauma causing more than 2 minutes of unconsciousness, mental disorders according to sections F00–F29 of the Inter national Classification of Diseases, Tenth Revision (e.g., organic mental disorders, disorders due to psychoactive substance use, and schizophrenia), epilepsy, history of vascular diseases, or body mass index > 35 kg/m2.
̢̻̉ Ageing Evaluation Unit: LLD diagnosis, affective, clinical and cognitive evaluation
The diagnostic criteria for major depression in the DSM-5, require the presence of either sadness or anhedonia with a total of five or more symptoms over a 2-week period [25].
Depressive symptoms were evaluated using the Hamilton Rating Scale for Depression with 21 items (HDRS-21) [26]. The scoring is based on the first 17. It generally takes 15-20 minutes to complete the interview and score the results. Eight items are scored on a 5-point scale, ranging from 0 = not present to 4 = severe. Nine are scored from 0-2. The grades of severity depression were considered as shown below: no depression (HDRS-21 score = 0-7), mild depression (HDRS-21 score = 8-13), moderate depression (HDRS-21 score = 14-18), severe depression (HDRS-21 score = 19-22), very severe depression (HDRS-21 score ≥ 23).
Clinical history was achieved through a semistructured interview. Clinical assessment was performed through the Comprehensive Geriatric Assessment (CGA). The CGA was carried out using assessment instruments widely employed in geriatric practice and comprehend eight domains: 1) Activities of Daily Living (ADL) [27] and 2) Instrumental Activities of Daily Living (IADL) scales [28] to evaluate the functional status, 3) Short Portable Mental Status Questionnaire (SPMSQ) [29] to screen the cognitive status, 4) Cumulative Illness Rating Scale Comorbidity Index (CIRS-CI) [30] to examine the comorbidity, 5) Mini Nutritional Assessment (MNA) [31] to explore nutritional status, 6) Exton-Smith Scale (ESS) to evaluate the risk of developing pressure sores [32], 7) medication use is defined according to the Anatomical Therapeutics Chemical Classification code system, and the number of drugs used by patients is recorded, and finally 8) social aspects that include household composition, home service, and institutionalization.
In all patients, cognitive status was assessed with the Mini Mentale State Examination (MMSE) [33], Clock Drawing Test (CDT) [34], and Frontal Assessment Battery (FAB) [35].
Moreover, all patients had performed a neuroimaging examination (CT scan) in order to highlight the presence of WML.
̢̻̉ Vascular disease Evaluation Unit: risk factor assessment, laboratory test and Ultrasound scan
Through a semi-structured interview medical history and milestones from the patient’s life were performed as below shown: 1) life time tobacco use, 2) psychoactive substance use and abuse, 3) history of vascular disease (stroke, myocardial infarction, and significant cardiac arrhythmia), 4) blood pressure, and 5) height and weight.
According to the Guideline for the diagnosis and management of hypertension in adults, hypertension was defined as systolic blood pressure > 140 mmHg, diastolic blood pressure > 90 mmHg, or current antihypertensive treatment [36].
Hyperlipidemia was defined according to the Guidelines for management of dyslipidemia and prevention of cardiovascular disease [37].
Diabetes mellitus was defined according to the Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm [38].
̢̻̉ Body mass index was defined as weight (in kilograms) divided by height (in meters) squared
The diagnosis of carotid stenosis in the selected cases was performed through clinical examination, including B-Mode Ultrasound scan and Color Doppler ultrasound scan of the epi-aortic trunks using ATL HDI 5000 (Philips, Amsterdam, Netherlands) [39]. Intima-media thickness was measured by recording ultrasonographic images of both the left and right carotid arteries. The lumen intima interface and the media-adventitia interface of the distal common carotid artery were measured offline. The common carotid intima-media thickness was determined as the average of near and far wall measurements of both left and right sides. The presence of plaques in the carotid artery was assessed by evaluating the ultrasonographic images of the common, internal, and bifurcation sites of the carotid artery for the presence of atherosclerotic lesions [40]. According to European Mannheim consensus, plaques were defined as a focal widening relative to adjacent segments and composed of calcified or non-calcified components: the plaques encroach into the lumen by 0.5 mm or by 50% of the surrounding intima-media thickness or where intima-media thickness is >1.5 mm [41].
Moreover, regarding the atherosclerosis diagnosis and severity, we used American Heart Association (AHA) criteria that provides for the following 4 atherosclerosis categories [42]: 1) Normal (type I/ II: near-normal wall thickness, no calcification), 2) Mild (Type III: diffuse intimal thickening or small eccentric plaque, no calcification), 3) Moderate (Type IV/V: plaque with lipid or necrotic core surrounded by fibrous tissue with possible calcification), and 4) Severe (Type VI: complex plaque with possible surface defect, haemorrhage or thrombus; Type VII: calcified plaque; Type VIII: fibrotic plaque without lipid core and with possible small calcification).
̢̻̉ Statistical analyses
For dichotomous variables, hypotheses regarding differences between the groups were tested using the Fisher’s exact test. This analysis was made using the 2-Way Contingency Table Analysis available at the Interactive Statistical Calculation Pages (The R Project for Statistical Computing; available at URL http://www.r-project.org/). For continuous variables, normal distribution was verified by the Shapiro-Wilk normality test and the one-sample Kolgomorov-Smirnov test. For normally-distributed variables, hypotheses regarding differences among the groups were compared by means of the Welch two sample t-test or by means of the analysis of variance (ANOVA) under general linear model. For non-normally-distributed variables, hypotheses regarding differences among the groups were compared by means of the Wilcoxon rank sum test with continuity correction or by means of the Kruskal-Wallis rank sum test. Risks will be reported as odds ratios (OR) along with their 95% confidence interval (CI). All the statistical analyses were made with the R Ver. 2.8.1 statistical software package (The R Project for Statistical Computing; available at URL http://www.r-project.org/). Tests in which the p value was smaller than the type I error rate α = 0.05 were declared significant.
Results
During the enrolment period, 4653 elderly patients were screened for the inclusion in the study.
Of these, 735 patients were excluded because they were younger than 55 years, 1211 patients had an incomplete examination, 1423 patients had a history of vascular diseases (544 patients had a history of stroke, and 879 patients had myocardial infarction and/or a significant cardiac arrhythmia), and 828 patients had a body mass index > 35 kg/m2. Thus, the final population included 456 patients, 236 men (51.8%) and 220 women (48.2%) with a mean age of 77.98 years ± 7.49 (range=55-93 years).
Therefore, the patients were examined according to the presence/absence of LLD, depression severity, and atherosclerosis severity.
̢̻̉ Patients with and without LLD
Of all patients 333 had a diagnosis of LLD and 123 had not diagnosis of LLD (noLLD). Demographic, affective, cognitive and clinical characteristics of LLD patients and noLLD patients are summarized in Table 1. The two groups of patients did not differ in following parameters: age (p = 0.733), FAB (p = 0.062), and CDT (p = 0.219). LLD patients were significantly more woman (56.2% vs. 26.8%, p < 0.0001), and had a lower educational level (5.27 vs. 7.10, p < 0.0001) than noLLD patients.
| ALL N=456 |
LLD n=333 |
noLLD n=123 |
P-value | |
|---|---|---|---|---|
| Sex | <0.0001 | |||
| Males/Females | 236/220 | 146/187 | 90/33 | |
| Males (%) | 51.80 | 43.80 | 73.20 | |
| Age (years) | 0.733 | |||
| Mean ± SD | 77.98 ± 7.49 | 77.91 ± 7.69 | 78.18 ± 6.95 | |
| Range | 55 – 93 | 55 – 93 | 59 – 93 | |
| Educational level (years) | <0.0001 | |||
| Mean ± SD | 5.77 ± 4.21 | 5.27 ± 3.83 | 7.10 ± 4.88 | |
| Range | 0 – 18 | 0 – 18 | 0 - 18 | |
| HRSD-21* | <0.0001 | |||
| Mean ± SD | 13.67 ± 7.85 | 17.36 ± 5.67 | 3.68 ± 2.23 | |
| Range | 0 – 35 | 8 – 35 | 0 – 7 | |
| MMSE† | 0.003 | |||
| Mean ± SD | 21.71 ± 5.13 | 21.28 ± 4.88 | 22.88 ± 5.61 | |
| Range | 0 – 30 | 0 - 30 | 0 - 30 | |
| FAB‡ | 0.062 | |||
| Mean ± SD | 11.08 ± 5.33 | 10.57 ± 5.14 | 12.09 ± 5.57 | |
| Range | 0 – 18 | 0 – 18 | 0 – 18 | |
| CDT§ | 0.219 | |||
| Mean ± SD | 3.55 ± 2.02 | 3.67 ± 1.99 | 3.30 ± 2.05 | |
| Range | 0 – 6 | 0 – 6 | 1 – 6 | |
| ADLâÃâ¬Ãâ | <0.0001 | |||
| Mean ± SD | 4.66 ± 1.64 | 4.49 ± 1.63 | 5.12 ± 1.57 | |
| Range | 0 – 6 | 0 – 6 | 0 - 6 | |
| IADL# | <0.0001 | |||
| Mean ± SD | 4.13 ± 3.15 | 3.68 ± 3.08 | 5.34 ± 3.02 | |
| Range | 0 – 8 | 0 – 8 | 0 - 8 | |
| SPMSQ** | 0.754 | |||
| Mean ± SD | 2.83 ± 2.38 | 2.86 ± 2.37 | 2.68 ± 2.48 | |
| Range | 0 – 10 | 0 – 10 | 0 - 7 | |
| MNA†† | <0.0001 | |||
| Mean ± SD | 23.56 ± 4.09 | 22.75 ± 4.07 | 25.76 ± 3.25 | |
| Range | 7 – 20 | 7 – 29 | 12 – 30 | |
| EES‡‡ | <0.0001 | |||
| Mean ± SD | 17.47 ± 2.37 | 17.20 ± 2.38 | 18.18 ± 2.20 | |
| Range | 10 – 20 | 10 – 20 | 10 - 20 | |
| CIRS-CI§§ | 0.159 | |||
| Mean ± SD | 2.36 ± 1.56 | 2.44 ± 1.56 | 2.16 ± 1.53 | |
| Range | 0 – 10 | 0 – 10 | 0 – 6 | |
| N of medications | 0.149 | |||
| Mean ± SD | 4.67 ± 2.72 | 4.84± 2.76 | 3.44 ± 2.13 | |
| Range | 0 – 12 | 0 – 12 | 0 – 6 | |
| Social support network | ||||
| Living with family N (%) | 318 (69.7) | 220 (66.1) | 98 (79.7) | 0.017 |
| Institutionalized N (%) | 27 (5.9) | 21 (6.3) | 6 (4.9) | |
| Living alone N (%) | 111 (24.3) | 92 (27.6) | 19 (15.4) |
Abbreviations
*HRSD-21: Hamilton Rating Scale for Depression with 21 items
†MMSE: Mini Mental State Examination
‡FAB: Frontal Assessment Battery
§CDT: Clock Drawing Test
âÃâ¬ÃâADL: Activities of Daily Living
#IADL: Instrumental Activities of Daily Living
**SPMSQ: Short Portable Mental Status Questionnaire
††MNA: Mini Nutritional Assessment
‡‡EES: Exton-Smith Scale
§§CIRS-CI: Cumulative Illness Rating Scale-Comorbidity Index
Table 1. Demographic, affective, cognitive and clinical characteristics of older patients with Late-life depression (LLD) and without LLD (noLLD).
LLD patients had a higher grade of cognitive impairment (MMSE: 21.28 vs. 22.88, p = 0.003), and showed a major impairment in any domains of CGA than noLLD patients, as shown below: 1) ADL (4.49 vs. 5.12, p < 0.0001), 2) IADL (3.68 vs. 5.34, p < 0.0001), 3) MNA (22.75 vs. 25.76, p < 0.0001), 4) ESS (17.20 vs. 18.80, p < 0.0001), and 5) social support network distribution (p = 0.017). The two group did not differ in other CGA domains, as shown below: SPMSQ score (p = 0.754), CIRC-CI score (p = 0.159), and number of medications (p = 0.149).
Vascular risk assessment is summarized in Table 2. The two groups of patients did not differ in the following parameters, as shown below: tobacco use (p = 0.634), hypertension (p = 0.091), dyslipidemia (p = 0.809), diabetes (p = 0.887), and BMI (p = 0.833). WMLs were more frequent in LLD patients than noLLD patients (68.8% vs. 26.8%, p < 0.0001).
| ALL N=456 |
LLD n=333 |
noLLD n=123 |
P-value | |
|---|---|---|---|---|
| Tobacco use | 0.634 | |||
| Smoker – N (%) | 92 (20.2) | 66 (19.8) | 26 (21.1) | |
| Ex smoker - N (%) | 89 (19.5) | 62 (18.6) | 27 (22.0) | |
| No smoker – N (%) | 275 (60.3) | 205 (61.6) | 70 (56.9) | |
| WML* | <0.0001 | |||
| Yes – N (%) | 262 (57.5) | 229 (68.8) | 33 (26.8) | |
| No - N (%) | 194 (42.5) | 104 (31.2) | 90 (73.2) | |
| Hypertension | 0.091 | |||
| Yes – N (%) | 169 (37.1) | 130 (39.0) | 39 (31.7) | |
| No – N (%) | 287 (62.9) | 203 (61.0) | 84 (68.3) | |
| Dyslipidemia | 0.809 | |||
| Yes – N (%) | 82 (18.0) | 59 (17.7) | 23 (18.7) | |
| No – N (%) | 374 (82.0) | 274 (82.3) | 100 (81.3) | |
| Diabetes | 0.887 | |||
| Yes – N (%) | 76 (16.7) | 55 (16.5) | 21 (17.1) | |
| No – N (%) | 380 (83.3) | 278 (83.5) | 102 (82.9) | |
| BMI† | 0.833 | |||
| Mean ± SD | 26.91 ± 4.18 | 26.77 ± 4.32 | 26.93 ± 3.80 | |
| Range | 15 – 35 | 15 - 35 | 20 - 34 |
Abbreviations
*WML: White matter lesion
†BMI: Body Mass Index
Table 2: Vascular risk assessment of older patients with Late-life depression (LLD) and without LLD (noLLD).
̢̻̉ Patients with LLD according to depression severity
According to depression severity, 98 patients had mild LLD, 100 patients had moderate LLD, 63 patients had severe LLD and 72 patients had very severe LLD.
Demographic, affective, cognitive and clinical characteristics LLD patients according to depression severity are summarized in Table 3. The four groups of patients did not differ in following parameters: gender distribution (p = 0.260), age (p = 0.954), and educational level (p = 0.051). Very severe LLD patients had a higher grade of cognitive impairment (MMSE: 21.98 vs. 21.70 vs. 21.49 vs. 19.58, p = 0.009; FAB: 10.60 vs. 12.00 vs. 10.84 vs. 7.58, p = 0.026; and CDT: 3.49 vs. 3.06 vs. 4.29 vs. 4.62, p = 0.006), and showed a major impairment in any domains of CGA, as shown below: 1) ADL (4.80 vs. 4.63 vs. 4.41 vs. 3.94, p = 0.006), 2) IADL (4.31 vs. 4.09 vs. 3.29 vs. 2.57, p = 0.001), 3) MNA (23.21 vs. 23.40 vs. 23.28 vs. 20.61, p < 0.0001), and 4) ESS (17.75 vs. 17.25 vs. 17.22 vs. 16.36, p = 0.003). The four groups did not differ in other CGA domains, as shown below: SPMSQ score (p = 0.550), CIRC-CI score (p = 0.876), number of medications (p = 0.476), and social support network distribution (p = 0.774).
| Mild LLD n=98 |
Moderate LLD n=100 |
Severe LLD n=63 |
Very severe LLD n=72 |
P-value | |
|---|---|---|---|---|---|
| Sex | 0.260 | ||||
| Males/Females | 40/58 | 50/50 | 30/33 | 26/46 | |
| Males (%) | 40.80 | 50.00 | 47.60 | 36.10 | |
| Age (years) | 0.954 | ||||
| Mean ± SD | 78.17 ± 7.21 | 78.01 ± 8.09 | 77.73 ± 8.06 | 77.54 ± 7.55 | |
| Range | 55 – 93 | 58 – 90 | 55 – 93 | 55 – 94 | |
| Educational level (years) | 0.051 | ||||
| Mean ± SD | 5.56 ± 4.07 | 5.82 ± 4.22 | 5.16 ± 3.63 | 4.25 ± 2.86 | |
| Range | 0 – 18 | 0 – 18 | 0 - 18 | 0 - 18 | |
| HRSD-21* | <0.0001 | ||||
| Mean ± SD | 10.76 ± 1.47 | 16.05 ± 1.49 | 20.56 ± 1.23 | 25.38 ± 2.43 | |
| Range | 8 – 13 | 14 – 18 | 19 – 22 | 23 – 35 | |
| MMSE† | 0.009 | ||||
| Mean ± SD | 21.98 ± 4.58 | 21.70 ± 4.76 | 21.49 ± 5.36 | 19.58 ± 4.74 | |
| Range | 12 - 30 | 9 - 30 | 0 - 30 | 9 - 30 | |
| FAB‡ | 0.026 | ||||
| Mean ± SD | 10.60 ± 4.63 | 12.00 ± 4.57 | 10.84 ± 5.47 | 7.58 ± 5.97 | |
| Range | 2 – 18 | 0 – 18 | 1 – 18 | 0 – 17 | |
| CDT§ | 0.006 | ||||
| Mean ± SD | 3.49 ± 1.90 | 3.06 ± 1.95 | 4.29 ± 2.05 | 4.62 ± 1.80 | |
| Range | 1 – 6 | 0 – 6 | 1 – 6 | 1 – 6 | |
| ADLâÃâ¬Ãâ | 0.006 | ||||
| Mean ± SD | 4.80 ± 1.59 | 4.63 ± 1.55 | 4.41 ± 1.49 | 3.94 ± 1.79 | |
| Range | 0 – 6 | 1 – 6 | 2 - 6 | 0 - 6 | |
| IADL# | 0.001 | ||||
| Mean ± SD | 4.31 ± 2.99 | 4.09 ± 2.99 | 3.29 ± 3.01 | 2.57 ± 3.08 | |
| Range | 0 – 8 | 0 – 8 | 0 - 8 | 0 - 8 | |
| SPMSQ** | 0.550 | ||||
| Mean ± SD | 2.86 ± 2.72 | 3.09 ± 2.67 | 2.16 ± 1.92 | 3.03 ± 1.94 | |
| Range | 0 – 10 | 0 – 10 | 0 - 5 | 0 - 7 | |
| MNA†† | <0.0001 | ||||
| Mean ± SD | 23.21 ± 3.64 | 23.40 ± 3.59 | 23.28 ± 4.14 | 20.61 ± 4.63 | |
| Range | 10 – 29 | 11 – 28 | 7 – 29 | 10 – 28 | |
| EES‡‡ | 0.003 | ||||
| Mean ± SD | 17.75 ± 2.08 | 17.25 ± 2.31 | 17.22 ± 2.24 | 16.36 ± 2.76 | |
| Range | 11 – 20 | 12 – 20 | 13 - 20 | 10 - 20 | |
| CIRS-CI§§ | 0.876 | ||||
| Mean ± SD | 2.51 ± 1.72 | 2.37 ± 1.41 | 2.55 ± 1.84 | 2.33 ± 1.33 | |
| Range | 0 – 9 | 0 – 5 | 0 – 10 | 0 – 6 | |
| N of medications | 0.476 | ||||
| Mean ± SD | 4.64 ± 3.29 | 5.13 ± 2.50 | 3.85 ± 2.08 | 5.28 ± 2.98 | |
| Range | 1 – 12 | 1 – 11 | 0 – 7 | 0 – 10 | |
| Social support network | |||||
| Living with family N (%) | 64 (65.3) | 61 (61.0) | 45 (71.4) | 50 (69.4) | 0.774 |
| Institutionalized N (%) | 8 (8.2) | 7 (7.0) | 3 (4.8) | 3 (4.2) | |
| Living alone N (%) | 26 (26.5) | 32 (32.0) | 15 (23.8) | 19 (26.4) |
Abbreviations
*HRSD-21: Hamilton Rating Scale for Depression with 21 items
†MMSE: Mini Mental State Examination
‡FAB: Frontal Assessment Battery
§CDT: Clock Drawing Test
âÃâ¬ÃâADL: Activities of Daily Living
#IADL: Instrumental Activities of Daily Living
**SPMSQ: Short Portable Mental Status Questionnaire
††MNA: Mini Nutritional Assessment
‡‡EES: Exton-Smith Scale
§§CIRS-CI: Cumulative Illness Rating Scale-Comorbidity Index
Table 3. Demographic, affective, cognitive and clinical characteristics of older patients with Late-life depression (LLD) according to the depression severity.
Vascular risk assessment are summarized in Table 4. The four groups of patients did not differ in the following parameters, as shown below: tobacco use (p = 0.115), hypertension (p = 0.081), dyslipidemia (p = 0.243), diabetes (p = 0.123). The patients with mild LLD had significantly a lower BMI (25.18 vs. 27.46 vs. 28.12 vs. 26.64, p = 0.049). WMLs were more frequent in Severe and Very severe LLD patients (53.1% vs. 56.0% vs. 90.5% vs. 88.9%, p < 0.0001).
| Mild LLD n=98 |
Moderate LLD n=100 |
Severe LLD n=63 |
Very severe LLD n=72 |
P-value | |
|---|---|---|---|---|---|
| Tobacco use | 0.115 | ||||
| Smoker – N (%) | 16 (16.3) | 21 (21.0) | 17 (27.0) | 12 (16.7) | |
| Ex smoker - N (%) | 24 (24.5) | 22 (22.0) | 8 (12.7) | 8 (11.1) | |
| No smoker – N (%) | 58 (59.2) | 57 (57.0) | 38 (60.3) | 52 (72.2) | |
| WML* | <0.0001 | ||||
| Yes – N (%) | 52 (53.1) | 56 (56.0) | 57 (90.5) | 64 (88.9) | |
| No - N (%) | 46 (46.9) | 44 (44.0) | 6 (9.5) | 8 (11.1) | |
| Hypertension | 0.081 | ||||
| Yes – N (%) | 41 (41.8) | 45 (45.0) | 16 (25.4) | 28 (38.9) | |
| No – N (%) | 57 (58.2) | 55 (55.0) | 47 (74.6) | 44 (61.1) | |
| Dyslipidemia | 0.243 | ||||
| Yes – N (%) | 20 (20.4) | 21 (21.0) | 6 (9.5) | 12 (16.7) | |
| No – N (%) | 78 (79.6) | 79 (79.0) | 57 (90.5) | 60 (83.3) | |
| Diabetes | 0.123 | ||||
| Yes – N (%) | 19 (19.4) | 21 (21.0) | 5 (7.9) | 10 (13.9) | |
| No – N (%) | 79 (80.6) | 79 (79.0) | 58 (92.1) | 62 (86.1) | |
| BMI† | 0.049 | ||||
| Mean ± SD | 25.18 ± 3.21 | 27.46 ± 4.23 | 28.12 ± 4.39 | 26.64 ± 4.97 | |
| Range | 17 – 31 | 19 – 35 | 18 - 35 | 15 - 35 |
Abbreviations
*WML: White matter lesion
†BMI: Body Mass Index
Table 4: Vascular risk assessment of older patients with Late-life depression (LLD) according to the depression severity.
̢̻̉ Patients with and without LLD according to atherosclerosis severity
According to atherosclerosis severity, 154 patients had not atherosclerosis (Normal), 262 patients had mild atherosclerosis (Mild), and 40 patients had moderate-severe atherosclerosis (Moderate-severe).
Demographic, affective, cognitive and clinical characteristics of LLD and noLLD patients according to atherosclerosis severity are summarized in Table 5. The three groups of patients did not differ in following parameters: age (p = 0.303), educational level (p = 0.463), MMSE (p = 0.525), FAB ((p = 0.864) and CDT ((p = 0.937). The patients with atherosclerosis were mainly and progressively more man (43.5% vs. 52.7% vs. 77.5%, p = 0.001), and more depressed (12.88 vs. 13.39 vs. 18.60, p < 0.0001) than patient without atherosclerosis. The three groups of patients did not differ in all CGA domains, as shown below: 1) ADL (p = 0.911), 2) IADL (p = 0.938), 3) SPMSQ (p = 0.754), 4) MNA (p = 0.833), 5) ESS (p = 0.818), CIRS-CI (p = 0.942), number of medications (p = 0.463), and social support network distribution (p = 0.682).
| Normal n=154 |
Mild n=262 |
Moderate-severe n=40 |
P-value | |
|---|---|---|---|---|
| Sex | 0.001 | |||
| Males/Females | 67/87 | 138/124 | 31/9 | |
| Males (%) | 43.50 | 52.70 | 77.50 | |
| Age (years) | 0.303 | |||
| Mean ± SD | 77.40 ± 7.64 | 78.10 ± 7.35 | 79.38 ± 7.73 | |
| Range | 55 – 92 | 55 – 93 | 59 – 91 | |
| Educational level (years) | 0.463 | |||
| Mean ± SD | 5.49 ± 3.90 | 5.84 ± 4.41 | 6.38 ± 4.07 | |
| Range | 0 – 18 | 0 – 18 | 0 - 18 | |
| HRSD-21* | <0.0001 | |||
| Mean ± SD | 12.88 ± 7.74 | 13.39 ± 7.96 | 18.60 ± 5.68 | |
| Range | 0 – 30 | 0 – 35 | 8 – 30 | |
| MMSE† | 0.525 | |||
| Mean ± SD | 21.33 ± 5.74 | 21.91 ± 4.75 | 21.89 ± 5.09 | |
| Range | 0 - 30 | 4 - 30 | 9 - 30 | |
| FAB‡ | 0.864 | |||
| Mean ± SD | 11.26 ± 5.55 | 10.93 ± 5.10 | 11.56 ± 6.27 | |
| Range | 0 – 18 | 0 – 18 | 2 – 18 | |
| CDT§ | 0.937 | |||
| Mean ± SD | 3.48 ± 2.15 | 3.58 ± 1.97 | 3.62 ± 1.96 | |
| Range | 1 – 6 | 0 – 6 | 1 – 6 | |
| ADLâÃâ¬Ãâ | 0.911 | |||
| Mean ± SD | 4.66 ± 1.69 | 4.65 ± 1.63 | 4.77 ± 1.47 | |
| Range | 0 – 6 | 0 – 6 | 1 - 6 | |
| IADL# | 0.938 | |||
| Mean ± SD | 4.20 ± 3.28 | 4.10 ± 3.09 | 4.05 ± 3.09 | |
| Range | 0 – 8 | 0 – 8 | 0 - 8 | |
| SPMSQ** | 0.754 | |||
| Mean ± SD | 2.59 ± 2.36 | 2.94 ± 2.44 | 2.83 ± 2.17 | |
| Range | 0 – 8 | 0 – 10 | 0 - 6 | |
| MNA†† | 0.833 | |||
| Mean ± SD | 23.39 ± 4.77 | 23.61 ± 3.73 | 23.81 ± 3.88 | |
| Range | 7 – 30 | 10 – 30 | 15 – 29 | |
| EES‡‡ | 0.818 | |||
| Mean ± SD | 17.56 ± 2.39 | 17.41 ± 2.38 | 17.53 ± 2.25 | |
| Range | 10 – 20 | 10 – 20 | 12 - 20 | |
| CIRS-CI§§ | 0.942 | |||
| Mean ± SD | 2.35 ± 1.59 | 2.35 ± 1.58 | 2.46 ± 1.27 | |
| Range | 0 – 10 | 0 – 9 | 0 – 5 | |
| N of medications | 0.463 | |||
| Mean ± SD | 4.75 ± 2.83 | 4.43 ± 2.55 | 5.67 ± 3.35 | |
| Range | 1 – 12 | 0 – 10 | 1 – 11 | |
| Social support network | ||||
| Living with family N (%) | 109 (70.8) | 178 (67.9) | 31 (77.5) | 0.682 |
| Institutionalized N (%) | 7 (4.5) | 18 (6.9) | 2 (5.0) | |
| Living alone N (%) | 38 (24.7) | 66 (25.2) | 7 (17.5) |
Abbreviations *HRSD-21: Hamilton Rating Scale for Depression with 21 items †MMSE: Mini Mental State Examination ‡FAB: Frontal Assessment Battery §CDT: Clock Drawing Test âÃâ¬ÃâADL: Activities of Daily Living #IADL: Instrumental Activities of Daily Living **SPMSQ: Short Portable Mental Status Questionnaire ††MNA: Mini Nutritional Assessment ‡‡EES: Exton-Smith Scale §§CIRS-CI: Cumulative Illness Rating Scale-Comorbidity Index
Table 5: Demographic, affective, cognitive and clinical characteristics of older patients according to the atherosclerosis severity.
Vascular risk assessment is summarized in Table 6. The three groups of patients did not differ in the following parameters, as shown below: hypertension (p = 0.099), dyslipidemia (p = 0.302), diabetes (p = 0.981), and BMI (p = 0.699). The patients with atherosclerosis were mainly and progressively more smokers (15.6% vs. 21.4% vs. 30.0%, p = 0.002) and showed progressively more WMLs (47.4% vs. 60.3% vs. 77.5%, p = 0.001) than patient without atherosclerosis.
| Normal n=154 |
Mild n=262 |
Moderate-severe n=40 |
P-value | |
|---|---|---|---|---|
| Tobacco use | 0.002 | |||
| Smoker – N (%) | 24 (15.6) | 56 (21.4) | 12 (30.0) | |
| Ex smoker - N (%) | 29 (18.8) | 45 (17.2) | 15 (37.5) | |
| No smoker – N (%) | 101 (65.6) | 161 (61.5) | 13 (32.5) | |
| WML* | 0.001 | |||
| Yes – N (%) | 73 (47.4) | 158 (60.3) | 31 (77.5) | |
| No - N (%) | 81 (52.6) | 104 (39.7) | 9 (22.5) | |
| Hypertension | 0.099 | |||
| Yes – N (%) | 53 (34.4) | 95 (36.3) | 21 (52.5) | |
| No – N (%) | 101 (65.6) | 167 (63.7) | 19 (47.5) | |
| Dyslipidemia | 0.302 | |||
| Yes – N (%) | 23 (14.9) | 49 (18.7) | 10 (25.0) | |
| No – N (%) | 131 (85.1) | 213 (81.3) | 30 (75.0) | |
| Diabetes | 0.981 | |||
| Yes – N (%) | 26 (16.9) | 43 (16.4) | 7 (17.5) | |
| No – N (%) | 128 (83.1) | 219 (83.6) | 33 (82.5) | |
| BMI† | 0.699 | |||
| Mean ± SD | 26.59 ± 3.89 | 26.82 ± 4.22 | 27.66 ± 5.12 | |
| Range | 15 – 34 | 16 – 35 | 19 - 35 |
Table 6. Vascular risk assessment of older patients according to the atherosclerosis severity.
̢̻̉ Distribution of atherosclerosis severity in patients with and without LLD
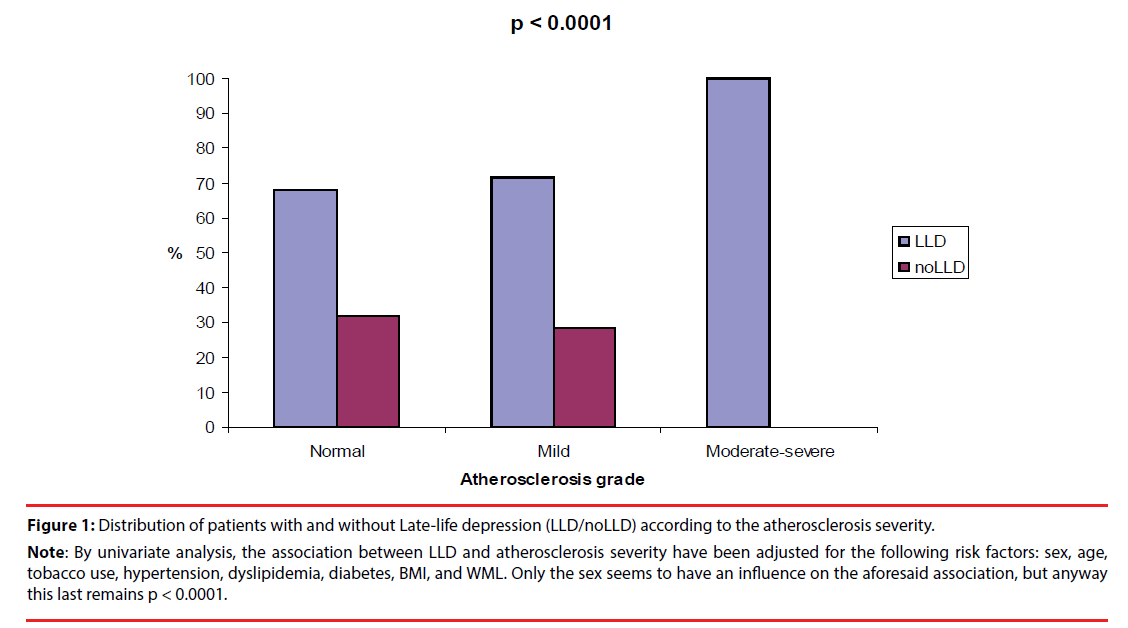
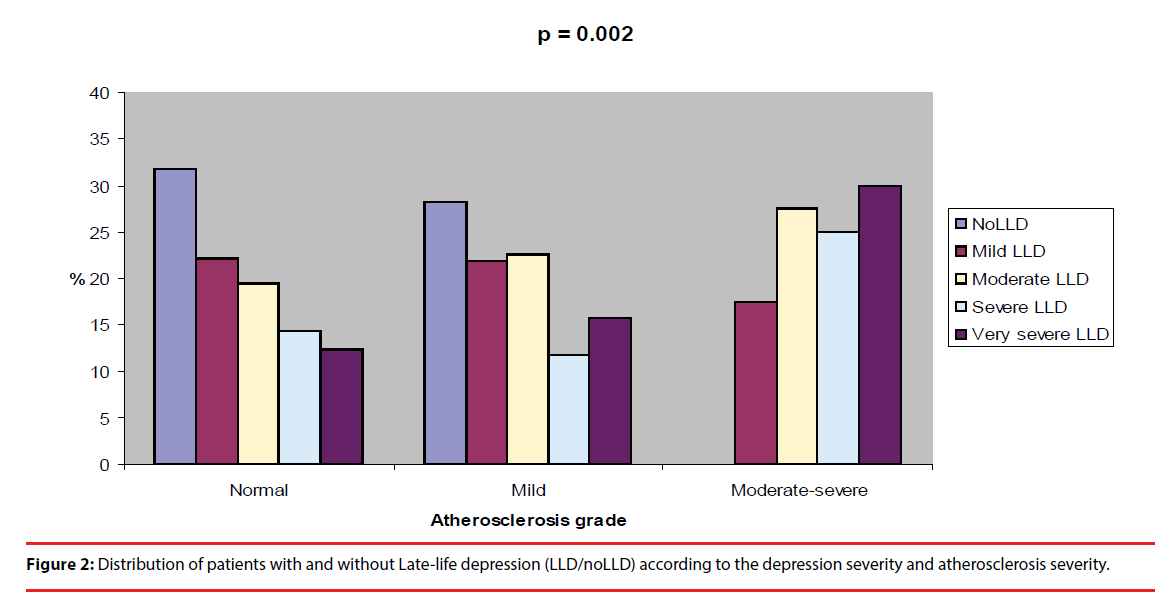
Figure 1 shows a visual analogic picture of the patients with and without LLD by atherosclerosis severity. Patients with LLD demonstrated significantly a higher frequency in Moderatesevere atherosclerosis (p < 0.0001). Figure 2 shows a visual analogic picture of the patients with and without LLD according to the depression severity and atherosclerosis severity. The severity of LLD seems increasing progressively in patients with Mild and Moderate-severe atherosclerosis, showing that the patients with Very severe LLD were significantly more frequent in Moderate-severe atherosclerosis (p = 0.002).
Figure 1: Results of significant positive correlations at baseline for depressed patients 1) between the total score of the KSAS and the score on autonomic symptoms of the TMI (ρ=0.402, p=0.028, N=30), 2) between the total score of the KSAS and score of the BDI-II (ρ=0.471, p=0.009, N=30), and 3) between the total score of the KSAS and the score on trait-anxiety scale of the STAI (ρ=0.392, p=0.032, N=30).
Note: By univariate analysis, the association between LLD and atherosclerosis severity have been adjusted for the following risk factors: sex, age, tobacco use, hypertension, dyslipidemia, diabetes, BMI, and WML. Only the sex seems to have an influence on the aforesaid association, but anyway this last remains p < 0.0001.
Discussion
In the present study, using a relatively large sample of patients with and without LLD, it was found that subjects with atherosclerosis were more likely to be depressed. Moreover the severity of LLD seems increasing progressively in patients with Mild and Moderate-severe atherosclerosis, showing that the patients with very severe LLD were significantly more frequent in Moderate-severe atherosclerosis. A significantly relationship was observed between moderate-severe atherosclerosis and LLD. According to these results, clinical evidences suggest that LLD may operate with several pathophysiological mechanism in promoting and accelerating atherosclerosis and vice versa [43]. In addition to an unhealthy lifestyle, several biological and immunopathological pathways may mediate the relationship between depression symptoms and atherosclerosis: these alterations include increased glucocorticoids, catecholamines and inflammation which contribute to platelet activation and aggregation, and endothelial dysfunction which may result in an increased risk of progression to atherosclerosis and thrombus formations [44].
Further results had shown that LLD diagnosis, LLD severity and carotid atherosclerosis severity were positively associated with WMLs. In light of these outcomes, it may be considered that atherosclerosis in the carotids is more strongly associated with WMLs than atherosclerosis in other vascular territories in the body [45]. This would suggest that WMLs is not merely a consequence of a generalized artheriosclerotic disease process but that the hemodynamic effects of carotid atherosclerosis predispose to WMLs [45]. This assumption imply that factors other than carotid atherosclerosis contribute to the increased WML load in LLD, e.g., dysregulation of immune mechanisms, vascular endothelial dysfunction, dysregulation of the hypothalamicpituitary- adrenal axis, and autonomic dysfunction, which have all been linked to depression [20].
Two opposing previous studies have investigated the relationship between WMLs and carotid atherosclerosis in depressed patients. Chen et al. found that WML severity was positively correlated with carotid atherosclerosis in 14 LLD patients, but not in 11 noLLD patients [46]. On the contrary, Paranthaman et al. found no association between WMLs and carotid atherosclerosis in either 25 LLD patients or 21 noLLD patients [47].
In our study, the role of sex seems to impact the development of LLD: indeed LLD patients were mainly more females. It was widely demonstrated that depression symptoms are twice as common in women than in men [43]. Interestingly, compared with females, we found that males were generally more likely to have the moderate-severe carotid atherosclerosis. This is in line with a of the largest comparable studies analyzing the effect of sex on plaque morphology. In the aforementioned study of 450 carotid specimens, Hellings et al [48] showed that atheromatous plaques were also more frequent in men than in women.
Moreover, in this study, it was emerged that LLD impacts the cognitive functions (as shown through MMSE, FAB, and CDT scores), functional (as shown through ADL and IADL scores) and clinical (as shown through MNA and EES scores) aspects in older patients. Coexisting cognitive impairment is common in persons with LLD and can involve multiple cognitive domains, including executive function, attention, and memory [49].Cognitive deficits may thus be signs of accelerated brain aging that confers a predisposition to and perpetuates depression [50].
Some limitations of the present study must be acknowledged. In fact, nonetheless the large sample of LLD/noLLD patients with an epiaortic trunk evaluation were investigated, the main limitation was the very loss number of patients with moderate-severe carotid atherosclerosis. Furthermore, the study population comprising only Caucasian patients recruited in a single centre, so it could be possible that our results may not be applicable in other populations. Larger prospective multicenter studies are therefore needed to confirm the present findings.
Conflict of Interests
The authors declare that there are no conflicts of interests regarding the publication of this article.
Acknowledgment
GD, MGL and MP conceived of the study. GD, MGL, MP and DSa created the search protocol. GD examined the literature. MGL and MP had performed the Duplex ultrasound scans in Vascular disease Evaluation Unit. GD, DSa, MLa, LC and AM had recruited the patients in Ageing Evaluation Unit. GD contributed to manuscript preparation. MPD, DSe, MS, DSG, VI, MLe, AR and AG contributed to review the paper. The authors confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. The authors further confirm that the order of authors listed in the manuscript has been approved by all.
Funding
This work was fully supported by “Ministero della Salute”, I.R.C.C.S. Research Program, Ricerca Corrente 2015-2017, Linea n. 2 “Malattie complesse e terapie innovative” and by the “5 x 1000” voluntary contribution.
References
- Centers for Disease Control and Prevention (2016).
- Naismith SL, Norrie LM, Mowszowski L, et al. The neurobiology of depression in later-life: Clinical, neuropsychological, neuroimaging and pathophysiological features. Prog. Neurobiol 98(1), 99–143 (2012).
- Alexopoulos GS. Depression in the elderly. Lancet 365(1), 1961– 1970 (2005).
- World Health Organization. Mental health and older adults (2016).
- Dombrovski AY, Mulsant BH, Houck PR, et al. Residual symptoms and recurrence during maintenance treatment of late-life depression. J. Affect. Disord 103(1), 77-82 (2007).
- Lenze EJ, Schulz R, Martire LM, et al. The course of functional decline in older people with persistently elevated depressive symptoms: longitudinal findings from the Cardiovascular Health Study. J. Am. Geriatr. Soc 53(1), 569-575 (2005).
- Katon WJ, Lin E, Russo J, et al. Increased medical costs of a population-based sample of depressed elderly patients. Arch. Gen. Psychiatry 60(1), 897-903 (2003).
- Panza F, Frisardi V, Capurso C, et al. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am. J. Geriatr. Psychiatry 18(1), 98-116 (2010).
- Ganguli M, Dodge HH, Mulsant BH. Rates and predictors of mortality in an aging, rural, community-based cohort: the role of depression. Arch. Gen. Psychiatry 59(1), 1046-1052 (2002).
- Devantier TA, Nørgaard BL, Poulsen MK, et al. White Matter Lesions, Carotid and Coronary Atherosclerosis in Late-Onset Depression and Healthy Controls. Psychosomatics 57(4), 369-377 (2016).
- Videbech P. MRI findings in patients with affective disorder: a meta-analysis. Acta. Psychiatr. Scand 96(3), 157–168 (1997).
- Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J. Neurol. Neurosurg. Psychiatr 79(6), 619–624 (2008).
- Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J. Appl. Physiol 105(5), 1652–1660 (2008).
- Tarumi T, Shah F, Tanaka H. Association between central elastic artery stiffness and cerebral perfusion in deep subcortical gray and white matter. Am. J. Hypertens 24(10), 1108–1113 (2011).
- Paranthaman R, Burns AS, Cruickshank JK, et al. Age at onset and vascular pathology in late-life depression. Am. J. Geriatr. Psychiatry 20(6), 524–532 (2012).
- Tiemeier H, Breteler MMB, van Popele NM, et al. Late-life depression is associated with arterial stiffness: a population-based study. J. Am. Geriatr. Soc 51(8), 1105–1110 (2003).
- Firbank MJ, Teodorczuk A, van der Flier WM, et al. LADIS group Relationship between progression of brain white matter changes and late-life depression: 3-year results from the LADIS study. Br. J. Psychiatry 201(1), 40–45 (2012).
- Taylor WD, Steffens DC, MacFall JR, et al. White matter hyperintensity progression and late-life depression outcomes. Arch. Gen. Psychiatry 60(11),1090–1096 (2003).
- Dalby RB, Frandsen J, Chakravarty MM, et al. Depression severity is correlated to the integrity of white matter fiber tracts in late-onset major depression. Psychiatry. Res 184(1), 38–48 (2010).
- Stapelberg NJC, Neumann DL, Shum DHK, et al. A topographical map of the causal network of mechanisms underlying the relationship between major depressive disorder and coronary heart disease. Aust. N Z J. Psychiatry 45(5), 351–369 (2011).
- Bots ML, van Swieten JC, Breteler MM, et al. Cerebral white matter lesions and atherosclerosis in the Rotterdam Study. Lancet 341(8855), 1232–1237 (1993).
- de Leeuw FE, de Groot JC, Bots ML, et al. Carotid atherosclerosis and cerebral white matter lesions in a population based magnetic resonance imaging study. J. Neurol 247(4), 291–296 (2000).
- Shu M, Zhang JJ, Dong Y, et al. Significance of increased CIMT with coexisting carotid plaques in cerebral white matter lesions in elders. J. Huazhong. Univ. Sci. Technolog. Med Sci 33(1), 69–74 (2013).
- Sneed JR, Culang-Reinlieb ME. The vascular depression hypothesis: An update. Am. J. Geriatr. Psychiatry 19(2), 99-103 (2011).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), American Psychiatric Association, Arlington (2013).
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23(1), 56-62 (1960).
- Katz S, Downs TD, Cash HR, et al. Progress in the development of an index of ADL. Gerontologist 10(1), 20–30 (1970).
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9(1), 179-186 (1969).
- Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc 23(1), 433-441 (1975).
- Parmelee PA, Thuras PD, Katz IR, et al. Validation of the Cumulative illness rating scale in a ge6riatric residential population. J. Am. Geriatr. Soc 43(1), 130-137 (1995).
- Vellas B, Guigoz Y, Garry PJ, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 15(1), 116-122 (1999).
- Bliss MR, McLaren R, Exton-Smith AN. Mattresses for preventing pressure sores in geriatric patients. Mon. Bull. Minist. Health. Public. Health. Lab. Serv 25(1), 238-268 1966.
- Folstein M, Folstein S, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res 12(1), 189–198 (1975).
- Rouleau I, Salmon DP, Butters N, et al. Quantitative and qualitative analyses of clock drawings in Alzheimer's and Huntington's disease. Brain. Cogn 18(1), 70-87 (1992).
- Dubois B, Litvan I. The FAB: A frontal assessment battery at bedside. Neurology55(1), 1621-1626 (2000).
- Gabb GM, Mangoni AA, Arnolda L. Guideline for the diagnosis and management of hypertension in adults - 2016. Med. J. Aust 206(3), 141 (2017).
- Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists And American College of Endocrinology Guidelines For Management of Dyslipidemia And Prevention of Cardiovascular Disease. Endocr Pract 23(2), 1-87 (2017).
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm - 2017 Executive Summary. Endocr. Pract 23(2), 207-238 (2017).
- van Popele NM, Grobbee DE, Bots ML, et al. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke 32(1), 454-460 (2001).
- Touboul P, Hennerici M, Meairs S, et al. Mannheim Carotid Intima-Media Thickness and Plaque Consensus (2004–2006–2011): An Update on Behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovascular. Diseases (Basel, Switzerland) 34(4), 290-296 (2012).
- Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler. Thromb. Vasc. Biol 20(1), 1177–1178 (2000).
- Pizzi C, Manzoli L, Mancini S, et al. Autonomic nervous system, inflammation and preclinical carotid atherosclerosis in depressed subjects with coronary risk factors. Atherosclerosis 212(1), 292-298 (2010).
- Pizzi C, Santarella L, Costa MG, et al. Pathophysiological mechanisms linking depression and atherosclerosis: an overview. J. Biol. Regul. Homeost. Agents 26(4), 775-782 (2012).
- Devantier TA, Nørgaard BL, Poulsen MK, et al. White Matter Lesions, Carotid and Coronary Atherosclerosis in Late-Onset Depression and Healthy Controls. Psychosomatics 57(4), 369-377 (2016).
- Chen CS, Chen CC, Kuo YT, et al. Carotid intima-media thickness in late-onset major depressive disorder. Int. J .Geriatr. Psychiatry 21(1), 36-42 (2006).
- Paranthaman R, Greenstein AS, Burns AS, et al. Vascular function in older adults with depressive disorder. Biol. Psychiatry 68(2), 133-139 (2010).
- Hellings WE, Pasterkamp G, Verhoeven BA, et al. Gender-associated differences in plaque phenotype of patients undergoing carotid endarterectomy. J. Vasc. Surg 45(1), 289–296 (2007).
- Saczynski JS, Beiser A, Seshadri S, et al. Depressive symptoms and risk of dementia: the Framingham Heart Study. Neurology 75(1), 35-41 (2010).
- Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol. Psychiatry18(1), 963-974 (2013).