Research Article - Neuropsychiatry (2016) Volume 6, Issue 5
Effects of very low birth weight on brain white matter measured by voxelwise diffusion tensor imaging in adolescents without neuromotor and cognitive deficits
- Corresponding Author:
- Pinchen Yang, MD
Department of Psychiatry, College of Medicine, Kaohsiung Medical University and Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
Tel: +886-9-36360220
Abstract
We applied voxelwise diffusion tensor magnetic imaging studying in a group of early adolescents born with very low birth weight (VLBW) who were currently without cognitive and neuromotor deficits. Our goal was to globally detect white matter alterations and explore their relationships with emotional-behavioral symptoms in this subgroup of VLBW adolescents. Subjects consisted of 24 adolescents: 12 VLBW adolescents (M:F=7:5, with average 13.1 years, birth weight of 998.3 grams and IQ of 86.8) and 12 healthy adolescents born full term (M:F=6:6, with average 13.7 years, birth weight of 3356.7 grams).
Keywords
Low birth weight, Brain white matter, Adolescents, DTI, Neuromotor, Cognitive deficits
Introduction
Weight-based classification of newborns recognizes Very Low Birth Weight (VLBW) which is birth weight less than 1500 grams. Almost all neonates of VLBW deliveries are preterm and are vulnerable to major organ injuries. The impact on the brain as a result of prematurity includes a spectrum of grey matter and white matter (WM) injuries and is associated with delayed and/or disrupted maturation of brain cells [1-3]. Clinically, longitudinal studies investigating the VLBW cohort revealed a spectrum of severity of functional outcomes, which may include neuromotor and cognitive deficits in later childhood and adult life [4,5]. Advances in perinatal care has led to improved survival rate and decreased prevalence of severe focal brain injuries but low intelligence quotient, poor executive function and academic outcome still are reported to be frequently associated with VLBW birth [6,7]. Nevertheless, a proportion of the babies born with VLBW grew up to be grossly healthy without overt neurological and cognitive disability. In this subgroup of children, follow-up study will be of interest to see whether there is any noticeable brain condition worthy of clinical attention.
Magnetic resonance imaging is an excellent assessment tool for brain injury related to prematurity. Non-invasive brain imaging assessments of preterm infants into later years have confirmed that a wide range of subtle to severe injuries at birth may persist into adolescence and adulthood [8-10]. Diffusion tensor imaging (DTI), a technique capable of assessing the orientational dependence of the diffusion process of water molecules [11], is powerful in visualizing and quantifying abnormalities in human brain WM connection in vivo. The information is obtained most frequently through the parameter of fractional anisotropy (FA), which describes the directional preference of diffusion process and is regarded as a measure of intra-voxel coherence in WM fiber direction, and therefore may reflect the integrity of axonal connections. Previously, deep grey matter abnormalities have been found in tandem with diffuse WM injuries among infants [12], toddlers [13] and school age children [14].
In this study, we applied voxelwise DTI in a group of early adolescents being born with VLBW but were without cognitive and neuromotor deficits currently. We hypothesized that the WM matter development would be different in VLBW adolescence from the control group. Possible relationship between the WM track of interest and emotional behavioral measures were investigated.
Materials and Methods
Subjects
This study reports on a subset of data from the VLBW cohort follow up at the Kaohsiung Medical University Hospital, Taiwan. This cohort is registered in the level III neonatal intensive care unit of this hospital and the follow-up results on the functional outcomes of total sixty-one adolescents born 1996-1999 with VLBW had already been reported [15]. For this current MRI study, we made written invitations to children who were without neuromotor disabilities and were tested to be above 70 in full scale intelligence quotient by Wechsler Intelligence Scale [16]. Of the total 41 invitation we made, 18 subjects gave informed consents to participate and completed the brain scan. For every subject who had no noticeable findings on conventional T1/T2 image, DTI scanning was performed. However, six MRI studies had to be excluded from the final DTI analysis (three were excluded due to large signal dropouts and distortions in the DTI images by dental braces, and three were excluded due to unsuccessful sequence acquisition or image artifacts due to motion, impartial scans or incorrect slice position).
Healthy control adolescents were recruited through advertisement in the community. They were of term and normal birth weight, and without major disease or physical handicap. Formal intelligence tests were not arranged but they were all reported to be without learning or daily living problems by their parents. Interview with a psychiatrist was arranged for each control adolescent to rule out any diagnosable psychiatric condition.
The subjects included for the final DTI analysis consisted of 24 adolescents: 12 VLBW adolescents without overt physical, neurological and cognitive disability (7 males and 5 females, with an average age of 13.1 years and average birth weight of 998.3±218.8 gm) and 12 control adolescents born full term (6 males and 6 females, with an average age of 13.7 years and average birth weight of 3356.7515.2 gm). The average full scale intelligence quotient of the case group was 86.8±8.3.
Instruments
▪ Child Behavior Checklist
Parents were asked to complete the Chineselanguage version of the Child Behavior Checklist (CBCL-Chinese) in the Achenbach system of Empirically Bases Assessment [17,18], based on the status of the adolescents in the 2 months preceding questionnaire completion. The CBCLChinese consists of 140 behavior/emotional items. Factor analysis can yield 8 syndromes, including aggressive behavior, anxiety/ depression, attention problems, delinquent rule-breaking behavior, social problems, somatic complaints, thought problems, and withdrawal. Three composite scales were computed, the internalizing scale (withdrawal, somatic complaints and anxiety/depression), externalizing scale (delinquent and aggressive behavior) and total problems (all syndrome scores).
▪ Chinese- version of the Swanson, Nolan and Pelham IV scales (SNAP-IV)
Parents were asked to complete the Chinese SNAP-IV, which is a reliable and valid instrument for rating attention deficit hyperactivity disorder (ADHD)-related symptoms in both clinical and community settings. The psychometrics properties of SNAP-IV-Chinese in a Taiwanese context have been well established [19].
MRI of the brain
▪ Data Acquisition
MRI scanning was performed with a 3.0T MRI scanner (Skyra, Siemens, Erlangen, Germany) using a 20-channel phase-array head-neck coil. After triplanar scans for slice localization, a high-resolution whole brain 3D T1-weighted magnetization-prepared rapid acquisition with gradient echo data (TR/TE/TI=2000/2.07/900 ms, matrix size = 256×256, number of sagittal slice = 160, and voxel size = 1×1×1 mm3) and fast spin-echo T2-weighted images (TR/ TE=6000/99 ms, echo train length = 18, matrix size = 320×320, number of slice = 25, slice thickness = 4 mm, and in-plane resolution = 0.34×0.34 mm2) were acquired. For all enrolled adolescents who had no noticeable findings on conventional T1 and T2 images, 3-repeated DTI data were further acquired using a spin-echo single-shot diffusion-weighted echo-planar pulse sequence with the following parameters: TR = 4200 sec, TE = 72 msec, matrix = 96×96, FOV = 240 mm × 240 mm, number of averages = 1, slice thickness = 3 mm, and 40 axial slices for wholebrain coverage, with the number of non-collinear diffusion direction = 30, number of b0 = 1, b-value = 1000 s/mm2, and total acquisition time for DTI was about 6 minutes and 30 seconds.
▪ Data Processing
After correction for eddy-current distortion, a diffusion tensor was fitted with a least square estimation to calculate FA map on a voxel-byvoxel basis. For voxel-based analysis, individual FA maps were spatially normalized to an International Consortium for Brain Mapping FA template by using linear affine and nonlinear demon registrations [20], respectively, to minimize global and local differences between the individual and template images. After normalization of all FA maps, the voxel-based analysis was performed by SPM8 (Wellcome Department of Imaging Neuroscience, London, UK) running on a MATLAB platform (MathWorks, Natick, Massachusatts). The 2-sample t-test was performed to reveal the differences in FA values between the VLBW subjects and normal controls. In addition, mean FA metrics from each of the identified ROI was calculated. The relationship between emotionalbehavioral measures and FA measures of ROIs were analyzed by Pearson correlational analysis. For VBA, a significant difference was reported if there was an uncorrected p<0.001 and cluster>100 voxels. For Pearson correlational analysis, a significant difference was reported if p<0.05.
Results
▪ Emotional-behavioral measures
The VLBW adolescents scored significantly higher on social problems (p=0.008), attention problems (p=0.019), and total problem scores (p=0.022) than the controls in the parental reported CBCL. In addition, the VLBW group scored higher in the Inattentive subscale (p=0.007) and Hyperactivityimpulsivity subscale (p=0.012) than the control subjects as reflected in the parental reported SNAPIV (Table 1).
| VLBW N=12 Mean(SD) |
Controls N=12 Mean(SD) |
|
|---|---|---|
| Sex(Male/Female) | 7/5 | 6/6 |
| Birth weight(g)* | 998.3(218.8) | 3418.0(551.0) |
| MRI assessment age(years) | 13.1(1.5) | 13.7(1.6) |
| CBCL scale | ||
| Anxious/depressed | 6.7(6.1) | 3.6(2.5) |
| Withdrawn | 4.8(2.4) | 2.9(2.4) |
| Somatic complaints | 4.8(4.9) | 1.3(1.4) |
| Delinquent problems | 5.3(3.7) | 2.8(3.5) |
| Aggressive behavior | 8.5(7.3) | 4.7(4.5) |
| Social problems* | 7.6(4.9) | 2.3(3.0) |
| Thought problems | 5.1(4.8) | 2.1(3.1) |
| Attention problems* | 12.5(5.2) | 6.3(5.3) |
| Internalizing | 16.4(11.4) | 7.8(5.7) |
| Externalizing | 13.7(10.8) | 7.4(7.7) |
| Total problem score* | 62.1(32.9) | 28.4(25.6) |
| SNAP-IV | ||
| Inattentive score* | 15.9(7.0) | 7.6(4.9) |
| Hyperactive-impulsivity score* | 8.3(5.6) | 2.6(2.7) |
| Oppositional-defiant score | 9.7(5.6) | 6.4(5.2) |
*p<0.05 VLBW versus controls
Table 1: Characteristics and emotional-behavioral conditions in the VLBW group compared with controls
▪ DTI finding
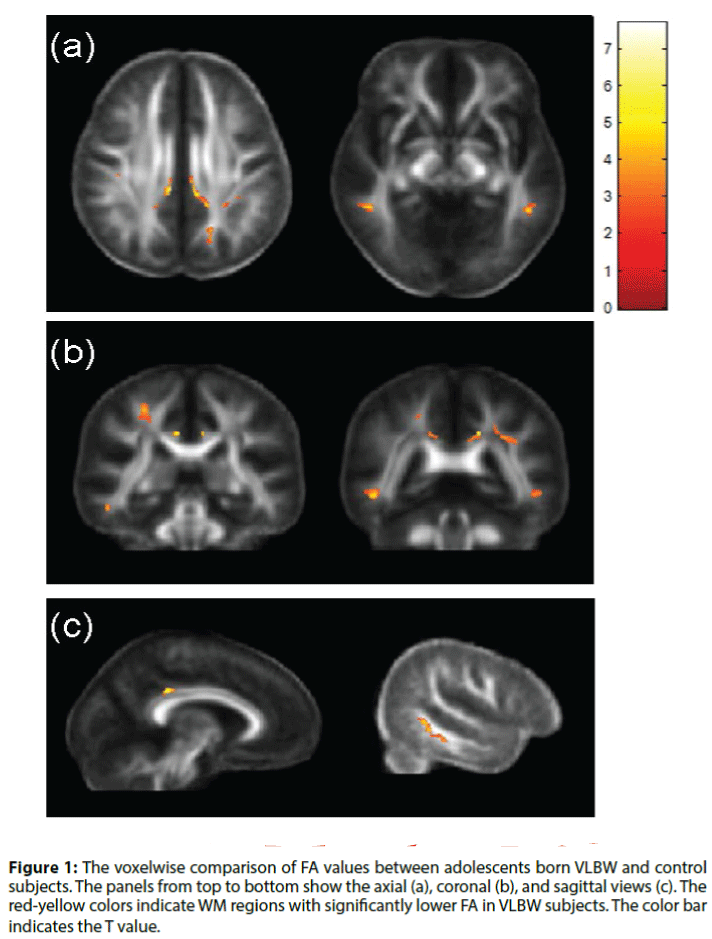
A significantly lower FA was observed in several areas of brain DTI (Table 2, Figure 1) in VLBW adolescents compared with the control group. These areas included the right superior corona radiata, bilateral posterior cingulum bundles, left superior longitudinal fasciculus (SLF) and bilateral inferior longitudinal fasciculus (ILF). No region indicated higher FA in the VLBW adolescents. Almost all the behavioral domains (i.e. social problem, attention problems, total problems, SNAP inattention, SNAP hyperactivity-impulsivity) showed a negative correlation with the FA values of the above identified region of interest (ROIs) (Table 3).
Figure 1: The voxelwise comparison of FA values between adolescents born VLBW and control subjects. The panels from top to bottom show the axial (a), coronal (b), and sagittal views (c). The red-yellow colors indicate WM regions with significantly lower FA in VLBW subjects. The color bar indicates the T value.
| Anatomical location | Hemisphere | MNI coordinates | Cluster size | FA VLBW | FA Control | ||
| x | y | Z | |||||
| Superior corona radiata | Right | 28 | -12 | 31 | 453 | 0.3536 | 0.5589 |
| Cingulum Cingulum |
Left | -7 | -17 | 16 | 312 | 0.2352 | 0.4331 |
| Right | 11 | -17 | 16 | 221 | 0.2334 | 0.4190 | |
| SLF | Left | -37 | -27 | 9 | 316 | 0.3469 | 0.5057 |
| ILF ILF |
Left | -45 | -27 | -22 | 298 | 0.3053 | 0.5073 |
| Right | 50 | -27 | -22 | 606 | 0.3306 | 0.5427 | |
The coordinates are in MNI space and correspond to the highest peak of the parametric map within each selected anatomical location
SLF: superior longitudinal fasciculus
ILF: inferior longitudinal fasciculus
Table 2: Location, cluster size (clusters of ≧100 voxels) and mean FA values of six white matter areas where the VLBW group had significantly lowers FA values than controls(p<0.01).
| Area | Social/CBCL | Attention/CBCL | CBCL | SNAP-I | SNAP-HI | |
|---|---|---|---|---|---|---|
| SCR | Right | -0.510* | -0.468* | -0.511* | -0.692** | -0.483* |
| Cingulum | Left | -0.454* | -0.499* | -0.439 | -0.631** | -0.605** |
| Cingulum | Right | -0.476* | -0.535* | -0.495* | -0.696** | -0.620** |
| SLF | Left | -0.536* | -0.537* | -0.568** | -0.750** | -0.627** |
| ILF | Left | -0.571** | -0.616** | -0.557* | -0.639** | -0.660** |
| ILF | Right | -0.630**- | -0.615** | -0.651** | -0.728** | -0.660** |
SCR: Superior corona radiate; SLF: superior longitudinal fasciculus;
ILF: inferior longitudinal fasciculus
CBCL: Child Behavioral Checklist Total Problem score;
SNAP-I: Swanson, Nolan and Pelham scales- Inattention subscale
SNAP-HI: Swanson, Nolan and Pelham scales – hyperactivity impulsivity subscale
*p<0.05; **p<0.01
Table 3: Pearson Correlation Coefficients of emotional-behavioral conditions and Fractional Anisotropy of the Six Identified White Matter Areas for the Whole Subjects.
Multiple regressions were conducted with behavioral scores (i.e social problem-CBCL, attention problems-BCL, SNAP inattention, SNAP hyperactivity-impulsivity) entered separately for four times as the outcome, and FA metrics from the six identified ROIs entered as predictors in a stepwise method. The results revealed that 1) the WM integrity of the right ILF is the predictor of social problems reported in CBCL: ILF was the only significant predictor for social problems in the model (F[1,18]=11.85, p=0.003), accounting for approximately 36.3% of the variance. 2) WM integrity of the left ILF and left SLF are the predictors of attention: FA of left ILF was the only significant predictor for attentional problems-CBCL in the model (F[1,18]=10.98, p=0.004), accounting for approximately 34.4% of the variance. FA of the left SLF was the only significant predictor for SNAP-Inattention subscale in the model (F[1,18]=23.20, p<0.001), accounting for approximately 53.9% of the variance. 3) WM integrity of the left ILF was the predictor of hyperactivity-impulsivity problems: FA of the left ILF was the only significant predictor for SNAP-Hyperactivity Impulsivity subscale in the model (F[1,18]=13.92, p=0.002), accounting for approximately 40.5% of the variance.
Discussion
Our cross-sectional study targeted on a subgroup, i.e. VLBW adolescents who were currently without cognitive and neuromotor deficits. The results showed lower FA in multiple areas of the brains of the subjects. The identified areas included the bilateral ILF (connecting the temporal lobe with the cerebellum), left SLF (a long bidirectional bundle connecting four lobes), right superior corona radiate (likely containing fibers from the SLF) and bilateral cingulum (a bundle of association fibers passing from the cingulate gyrus to the entorhinal cortex, encircling the corpus callosum). Our findings, albeit with very small sample size, corroborated the findings of European population-based cohort studies in demonstrating low FA values in brain areas of the VLBW subjects [21-23]. For example, one well-executed study found the VLBW adolescents had reduced FA values in the internal and external capsule, corpus callosum and superior, middle superior and inferior fasciculus than the age-matched controls [21]. However, not all studies on preterm and fullterm children find the significant difference by DTI scanning [24]. The difference in DTI finding may be explained by the subjects’ characteristics and DTI algorithm. The abovementioned findings of DTI with difference comes from the study on 34 VLBW adolescents (average age of 15 years) with average IQ of 77.9 [21]. The above-mentioned findings of DTI without difference comes from the study on 18 VLBW children (average age of 8 years) with average IQ of 97 [24]. It is possible that WM connectivity had unique developmental trajectory during post-natal development in subjects being born with VLBW and awaits future large-scale study to elucidate.
The identified WM tracts of lower FA in our study were previously implicated in imaging research on the pathophysiology of children with attention deficit hyperactivity disorder and were hypothesized to be of importance in subserving human attention [25-30]. We also noted that this group of adolescents was with more emotional-behavioral-attentional problems as compared with age and gender matched adolescents of normal birth weight. The structural characteristics of WM and clinical symptoms were demonstrated to have relationship. The increased emotional-behavioral symptoms of our study group also corroborated with previous European population-based researches investigating the VLBW cohort [21,22]. The Norwegian population-based follow-up study showed that VLBW adolescents had more psychiatric symptoms than controls, especially attention deficit, emotional, behavioral, social and academic problems [31]. Dahl et al investigated 99 families with VLBW adolescents aged 13 to 18 years and they found, from parents’ point of view using the parental reported CBCL questionnaire, significant proportions of VLBW adolescents experienced more emotional and behavioral problems and less competence than normative adolescents [5]. Even though the severity and complexity of our subjects’ symptoms did not meet full criteria of any psychiatric diagnose, the presence of these symptoms was issue of parental disciplinary concern, and hence these symptoms deserved clinical discussion and close follow-up.
The foremost limitation of our current study is a cross-sectional design targeting on a subgroup with very small sample size, and the subjects were recruited from the registry of a single institution. Conclusion draw from the results would be a preliminary one awaiting larger samples to replicate. Second, we used voxel base analysis (VBA) as an exploratory strategy because there were no hypotheses with regard to specific brain regions. VBA allows for whole-brain analysis, thus providing a complete overview of WM integrity in the brain. Generally, this analytic method renders researchers greater difficulty to detect differences between groups with small sample size. The fact that we still noted differences between groups was encouraging. Nevertheless, we applied uncorrected statistics in VBA to increase the sensitivity of WM abnormalities, thus a cautionary note was needed. In addition, recent studies of DTI on the myelination deficient model and demyelinated fibers indicate that FA might reflect predominantly axonal density rather than myelin content. Interpretation of FA may depend on factors other than directionality of water molecule movement, such as the presence of subvoxel fiber crossings and axonal density in the regions examined [32]. Future non-invasive assessment specifically targets against myelin content might be able to conciliate the current contradictory results from DTI researches.
Conclusion
In conclusion, being born with very low birth weight might has lasting effect on white matter in adolescents without neuromotor and cognitive deficits. FA index may be a biomarker of increased risk of emotional-behavioral problems in the development. It will be important to follow up the developmental course of children being born preterm with VLBW across physical, cognitive, emotional and behavioral domains beyond childhood so that proper intervention can be provided in time.
Acknowledgments
This work was supported by grants NSC100- 2314-B-037-017 and Ministry of Science and Technology, Taiwan & Kaohsiung Medical University Hospital.
References
- Skranes JS, Vik T, Nilsen G, et al.Cerebral magnetic resonance imaging and mental and motor function of very low birth weight children at six years of age. Neuropediatrics 28(3), 149-154 (1997).
- Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet. Neurol8(1), 110-124 (2009).
- Deng W. Neurobiology of injury to the developing brain. Nat. Rev. Neurol 6(6), 328-336 (2010).
- Volpe JJ. The encephalopathy of prematurity-brain injury and impaired brain development inextricably intertwined. Semin. Pediatr. Neurol16(4), 167-178 (2009)
- Dahl LB, Kaaresen PI, Tunby J, et al. Emotional, behavioral, social, and academic outcomes in adolescents born with very low birth weight. Pediatrics 118(2), e449-59 (2006).
- Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, et al.Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 124(2), 717-728 (2009).
- Lund LK, Vik T, Skranes J, et al.Low birth weight and psychiatric morbidity; stability and change between adolescence and young adulthood. Early. Hum. Dev88(8), 623-629 (2012).
- Stewart AL, Rifkin L, Amess PN, et al.Brain structure and neurocognitive and behavioural function in adolescents who were born very preterm. The Lancet353(9165), 1653-1657 (1999).
- Skranes JS, Martinussen M, Smevik O, et al.Cerebral MRI findings in very-low-birth-weight and small-for-gestational-age children at 15 years of age. Pediatric. Radiol35(8), 758-765 (2005).
- Cooke RW, Abernethy LJ. Cranial magnetic resonance imaging and school performance in very low birth weight infants in adolescence. Arch. Dis. Child. Fetal. Neonatal81(2), F116-F121 (1999).
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys.J66(1), 259-267 (1994).
- Boardman JP, Counsell SJ, Rueckert D, et al.Abnormal deep grey matter development following preterm birth detected using deformation-based morphometry. Neuroimage 32(1), 70-78 (2006).
- Lowe J, Duvall SW, MacLean PC, et al.Comparison of structural magnetic resonance imaging and development in toddlers born very low birth weight and full-term. J. Child. Neurol26(5), 586-592 (2011).
- Murray AL, Scratch SE, Thompson DK, et al.Neonatal brain pathology predicts adverse attention and processing speed outcomes in very preterm and/or very low birth weight children. Neuropsychology 28(4), 552-562 (2014).
- Yang P, Chen YH, Yen CF, et al.Psychiatric Diagnoses, Emotional-Behavioral Symptoms and Functional Outcomes in Adolescents Born Preterm with Very Low Birth Weights. Child. Psychiatry. Hum. Dev46(3), 358-366 (2015).
- Wechsler D. The Wechsler Intelligence Scale for Children, New York: Psychological Corp, USA, 2003.
- Achenbach H. Child Behavior Checklist in the Achenbach system of Empirically Bases Assessment -Chinese version. Taipei, Taiwan Psychological Publishing Company, 2009.
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families, 2001.
- Gau SS, Shang CY, Liu SK, et al.Psychometric properties of the Chinese version of the Swanson, Nolan, and Pelham, version IV scale - parent form. Int. J. Methods. Psychiatr. Res 17(1), 35-44 (2008).
- Vercauteren T, Pennec X, Perchant A, et al.Diffeomorphic demons: efficient non-parametric image registration. Neuroimage 45(1), S61-S72 (2009).
- Skranes J, Vangberg TR, Kulseng S, et al.Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain 130(3), 654-666 (2007).
- Vangberg TR, Skranes J, Dale AM, et al. Changes in white matter diffusion anisotropy in adolescents born prematurely. Neuroimage 32(4), 1538-1548 (2006).
- Pandit AS, Ball G, Edwards AD, et al.Diffusion magnetic resonance imaging in preterm brain injury. Neuroradiology 55(2), 65-95 (2013).
- Solsnes AE, Sripada K, Yendiki A, et al.Limited microstructural and connectivity deficits despite subcortical volume reductions in school-aged children born preterm with very low birth weight. Neuroimage 130(1), 24-34 (2016).
- van Ewijk H, Heslenfeld DJ, Zwiers MP, et al.Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci. Biobehav. Rev36(4), 1093-1106 (2012).
- Hamilton LS, Levitt JG, O'Neill J, et al.Reduced white matter integrity in attention-deficit hyperactivity disorder. Neuroreport 19(17), 1705-1708 (2008).
- Makris N, Buka SL, Biederman J, et al.Attention and executive systems abnormalities in adults with childhood ADHD: A DT-MRI study of connections. Cerebral.Cortex18(5), 1210-1220 (2008).
- Konrad A, Dielentheis TF, El Masri D, et al.Disturbed structural connectivity is related to inattention and impulsivity in adult attention deficit hyperactivity disorder. Euro.J.Neurosci31(5), 912-919 (2010).
- Qiu MG, Ye Z, Li QY, et al. Changes of brain structure and function in ADHD children.Brain. Topography24(3-4), 243-252 (2011).
- Nagel BJ, Bathula D, Herting M, et al.Altered white matter microstructure in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child. Adolesc. Psychiatry50(3), 283-2092 (2011).
- Indredavik MS, Vik T, Heyerdahl S, et al.Psychiatric symptoms in low birth weight adolescents, assessed by screening questionnaires. Eur. Child. Adolesc. Psychiatry14(4), 226-236 (2005).
- Lawrenz M, Brassen S, Finsterbusch J. Microscopic diffusion anisotropy in the human brain: reproducibility, normal values, and comparison with the fractional anisotropy. Neuroimage 109(1), 283-297 (2015).