Review Article - Neuropsychiatry (2017) Volume 7, Issue 4
Effects of selective serotonin reuptake inhibitors on the pharmacokinetics of proton pump inhibitors
- Corresponding Author:
- Tsukasa Uno, PhD
Department of Hospital Pharmacy, Zikeikai-Aoimori Hospital, 16-3 Ohtani-Yamanouti, Aomori 030-0155, Japan
Tel: +81-17-729-3330
Fax: +81-17-729-3147
Abstract
By now, the differential effects of several selective serotonin reuptake inhibitors (SSRIs) on the cytochrome P450 (CYP) enzymes are well defined and that the drug-drug interactions (DDIs) are a major issue in the management of depression. In many cases of DDIs in relation to SSRIs, SSRIs plays as a potent CYP inhibitor. Fluvoxamine has inhibited various CYPs-mediated pathways (especially CYP1A2, 2C9/19 and 3A4) and P-gp-mediated transport of substrate drugs. While, the metabolism of proton pump inhibitors (PPIs) is related to cytochrome P450 (CYP) 3A4 and polymorphic CYP2C19, and PPIs such as omeprazole and lansoprazole have also shown to be substrates of P-glycoprotein (P-gp) in in vitro study. Therefore, this review summarized the DDIs of Fluvoxamine-PPIs in Japanese healthy volunteers and the findings indicated that the DDIs of fluvoxamine-PPIs may be associated with the sum of polymorphic CYP2C19, CYP3A4 and P-gp.
Keywords
Selective serotonin reuptake inhibitors, Proton pump inhibitors, Drug-drug interactions, Fluvoxamine, Rabeprazole
Fluvoxamine Overview
Over the course of more than 20 years, selective serotonin reuptake inhibitors (SSRIs) have been widely prescribed in the treatment of depression [1,2]. By now, the differential effects of several selective serotonin reuptake inhibitors (SSRIs) on the cytochrome P450 (CYP) enzymes are well defined and that the drug-drug interactions (DDIs) are a major issue in the management of depression [3,4]. In many cases of DDIs in relation to SSRIs, SSRIs plays as a potent CYP inhibitor. For example, when fluvoxamine was co-administered with tizanidine (a centrally acting skeletal muscle relaxant), the area under the concentration-time curve (AUC) of tizanidine increased 33-fold compared with when tizanidine was administered alone, and caused side effects such as the decrease in systolic blood pressure and Digit Symbol Substitution Test (DSST) [5]. Inhibition of tizanidine-metabolizing enzyme(s), mainly CYP1A2, by fluvoxamine seems to explain the observed interaction. Furthermore, in a case of fluvoxamine-ramelteon (a melatonin receptor agonist used as a treatment for insomnia) DDIs, the AUC of ramelteon increased 128-fold by fluvoxamine. As, ramelteon is metabolized by CYP1A2, CYP2C19, and CYP3A4; fluvoxamine simultaneously may be inhibited by multiple CYPs-mediated pathways of ramelteon [6]. As, fluvoxamine sometimes yields an amazing DDIs, due to the quite low bioavailability of coadministered drugs that metabolized by multiple CYPs, these findings show the magnitude of DDIs estimated from in vitro data may be limited (11.4- fold estimated versus 128-fold actual) [6].
While, multidrug resistance P-glycoprotein (P-gp; also known as MDR1 and ABCB1) regulates the pharmacokinetics of a wide range of compound structures [7-9]. P-gp-mediated transport is saturable and is subject to modulation by inhibition and induction, which can affect the pharmacokinetics and several in vitro and in vivo studies have demonstrated that SSRIs also have inhibitory effects on P-gp-mediated transport [8-10]. A previous in vitro study revealed that the concentrations of L-MDR1 cells required inhibiting P-gp activity by 50% (IC50) for paroxetine (29.8 μM) and sertraline (31.8 μM) were similar to that of quinidine (33.8 μM), a known P-gp inhibitor, and fluoxetine (IC50 115.5 μM) showed a weak P-gp inhibition [8]. In addition, an in vivo study indicated that SSRIs increase fexofenadine (as a P-gp substrate) exposure in healthy volunteers, and that these effects are greatest for fluvoxamine (fluvoxamine by 1.8-fold, paroxetine by 1.4-fold, sertraline by 0.8-fold) [11]. This finding therefore suggests that SSRIs act as P-gp inhibitors in the clinical situation, and may cause P-gp-mediated drug interactions in patients receiving P-gp substrates.
While, fluvoxamine has been known to interact with many psychotropic drugs [12]. As shown in fluvoxamine-ramelteon DDIs, fluvoxamine has inhibited various CYPs-mediated pathways (especially CYP1A2, 2C9/19 and 3A4) [13-15]. In a case of fluvoxamine-imipramine DDIs [16], the imipramine AUC with fluvoxamine increased 3.0-fold compared with imipramine when administered alone. This DDIs system show the following results: first, fluvoxamine strongly inhibits the CYPs-mediated metabolism of imipramine (CYP2D6, 1A2, 2C19 and 3A4); next, it inhibits the P-gp-mediated efflux transport systems; accordingly, the blood concentrations of imipramine increased through the inhibition of both systems. However, comparing the DDIs of tizanidine- or ramelteon-fluvoxamine, illustrates that a moderate effect of imipramine disappear to be quite low bioavailability thus exhibiting a great first-pass effect. Similar to fluvoxamine-DDIs cases, since other SSRIs have inhibitory effects on CYPs [17,18], they may also cause clinical significances as shown in fluvoxamine-DDIs.
Rabeprazole Overview
Rabeprazole is one of proton pump inhibitors (PPIs) and possesses suppressive activity on gastric acid secretion by inhibiting (H+/K+)- ATPase in gastric parietal cells [19,20]. Like other PPIs (omeprazole, lansoprazole, and pantoprazole), rabeprazole is effective for treating various peptic diseases, including gastric and duodenal ulcer, gastroesophageal reflux disease, and Zollinger-Ellison syndrome [19,20]. The metabolism of proton pump inhibitors (PPIs) is mainly related to CYP3A4 and polymorphic CYP2C19 [21,22]. In contrast, as for rabeprazole, rabeprazole thioether formulated by the nonenzymatic reduction is a major metabolite, but, some is oxidized to demethylated rabeprazole and rabeprazole sulfone by CYP2C19 and CYP3A4, respectively [22-25]. Therefore, the pharmacokinetics and pharmacodynamics of rabeprazole by the polymorphic CYP2C19 activity appear to be a lesser effect compared with other PPIs. However, there have been reported that plasma concentrations of rabeprazole are significantly different among CYP2C19 genotype groups [26-30] and then that gastric acid inhibition by rabeprazole are different among CYP2C19 genotype groups [29-30]. However, no published data suggest an in vivo contribution of CYP3A4 to the pharmacokinetics of rabeprazole, even though in vitro studies have shown that rabeprazole is metabolized to rabeprazole sulfone by CYP3A4 [24,25]. Furthermore, to date, although other PPIs (omeprazole, lansoprazole and pantoprazole) were shown to be substrates of P-gp in in vitro study [31], it is unknown whether rabeprazole is the substrate of P-gp. In Japanese, the frequency of the defective CYP2C19 alleles is 19% (35 of 186) [32] and this frequency is very higher than that (2-3%) of Caucasians [33]. In terms of this genetic data, PPIs pharmacokinetics of Japanese may be quite different from PPIs pharmacokinetics of Caucasian and then DDIs in relation to PPIs and CYP2C19 in Japanese may be different from that in Caucasians. On the basis of these observations, the sum of polymorphic CYP2C19, CYP3A4 and P-gp should be noted in DDIs of Fluvoxamine-PPIs.
Fluvoxamine-PPIs DDIs
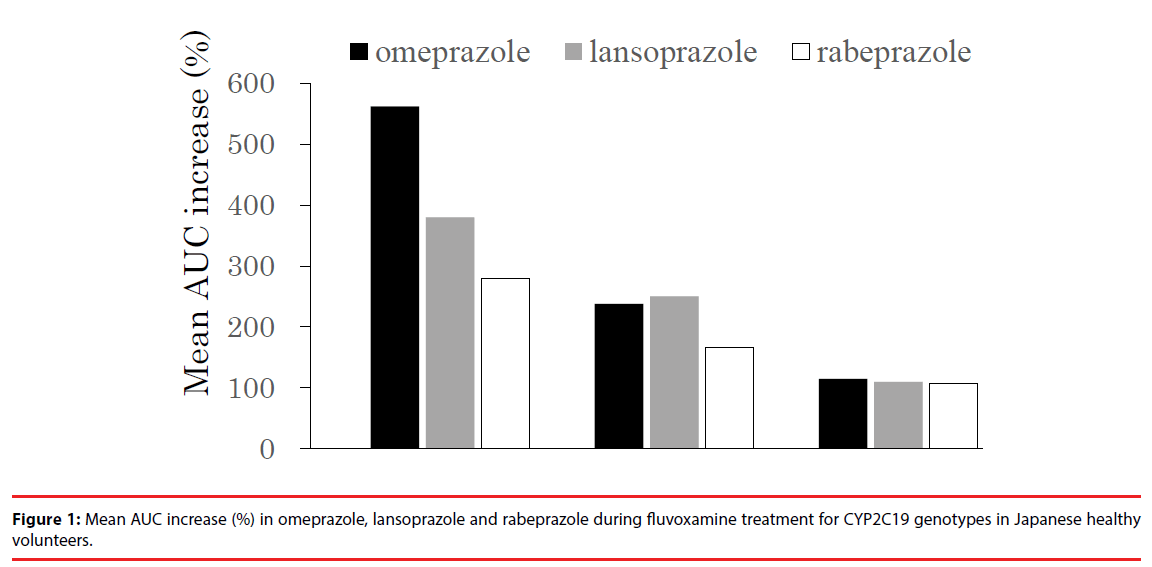
In the DDIs of Fluvoxamine-PPIs that the authors’ laboratory conducted, Japanese healthy volunteers were divided into three CYP2C19 genotype groups, homozygous EMs (CYP2C19*1/*1), heterozygous EMs (CYP2C19*1/*2 and *1/*3), and PMs (CYP2C19*2/*2 and *2/*3). Previous studies showed that fluvoxamine treatment increased the AUC of PPIs (e.g. omeprazole and lansoprazole) and prolonged elimination half-life of PPIs in homozygous EMs and heterozygous EMs, but not in PMs [34,35], indicating a potent inhibitory effect of fluvoxamine on CYP2C19 activity and no effect on fluvoxamine on CYP3A4 activity. While, previous papers revealed that clarithromycin, as a potent CYP3A4 inhibitor, increased the AUC of omeprazole and lansoprazole in all CYP2C19 genotypes through the inhibition of CYP3A4 pathways [36,37]. In only one report as a role of CYP3A4 inhibitor, fluvoxamine (100 mg per day) increased plasma concentrations of alprazolam, a substrate of CYP3A4, suggesting that fluvoxamine has a moderate inhibitory effect (2.0-fold) on CYP3A4 to some degree [38]. Since all fluvoxamine-PPIs DDIs were carried out in low daily-dose (50 mg per day), these results therefore imply that the concentrations of fluvoxamine in low dailydose is not enough to inhibit CYP3A4-mediated metabolism of PPIs and the high-dose ( âÃâ°Ã§ 100 mg per day) of fluvoxamine may induce a greater change of PPIs pharmacokinetics.
In the rabeprazole-fluvoxamine DDIs [39], similar to results of other PPIs-fluvoxamine DDIs, the inhibitory effect of fluvoxamine on rabeprazole pharmacokinetics showed a same tendency [34,35]: the inhibitory effect was greatest in homozygous EMs, less in heterozygous EMs and least in PMs. In addition, when considering the inhibitory effect of fluvoxamine on the three PPIs pharmacokinetics in homozygous EMs, the order is as follows: omeprazole > lansoprazole > rabeprazole (Figure 1), which is acceptable because the relative effect of CYP2C19 polymorphism on the three PPIs pharmacokinetics is also similar [22]. Furthermore, fluvoxamine simultaneously did not inhibit the CYP3A4 metabolic pathway of rabeprazole. On the other hand, our separate report revealed that potent CYP3A4 inhibitors such as clarithromycin and verapamil had little effect on rabeprazole pharmacokinetics, so unexpected DDIs between rabeprazole and CYP3A4 inhibitors is unlikely to occur in the clinical situation. [40]. Therefore, this negative finding may be attributed to less contribution of CYP3A4 to rabeprazole disposition in an in vivo study. In addition, clarithromycin and verapamil are P-gp inhibitors, as well as CYP3A4 inhibitors [41-44]. Our previous study showed that the increased AUC of lansoprazole by clarithromycin might be due to the combination of the inhibition of CYP3A4 and P-gp [37]. However, although rabeprazole has an inhibitory effect on MDR1- mediated transport at 100 μM of higher therapeutic ranges [45], it is unclear whether rabeprazole is a substrate of P-gp. Therefore, these findings suggest that the effect of P-gp inhibitors involving fluvoxamine on rabeprazole pharmacokinetics would be a minimal effect in comparison with the effects on other PPIs pharmacokinetics.
Conclusion and Future Perspective
In view of the pointed information described above, the potential for DDIs between fluvoxamine and PPIs should be noted and the degree of DDIs by fluvoxamine may be different according to co-administered various PPIs. Concurrently, these CYP2C19-inhbitted effects are predicted to be different among CYP2C19 genotype groups. Therefore, the typical CYP2C19 substrates such as clopidogrel [46], escitalopram [47] and voriconazole [48] showing inter-individual differences among CYP2C19 genotypes should be cautioned for the pharmacodynamics (PD) with changes of pharmacokinetics when fluvoxamine was coadministered. Simultaneously, the following information should be also noted that there may be a limit on the magnitude of fluvoxamine-DDIs that can be estimated from in vitro data because fluvoxamine can inhibit multiple CYPs-mediated pathways and P-gp-mediated transport of substrate drugs. While, the pharmacokinetic changes of rabeprazole should be noted when CYP2C19 inhibitors such as fluvoxamine and voriconazole [49] were co-administered, however, the potential of DDIs between rabeprazole and the inhibitors of CYP3A4 and P-gp may be limited to date.
Conflict of Interest
The authors have no conflicts of interest in relation to this paper.
References
- Hansen RA, Gartlehner G, Lohr KN, et al. Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder. Ann. Intern. Med 143(6), 415-426 (2005).
- Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet 373(9665), 746-758 (2009).
- Spina E, Santoro V, D'Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin. Ther 30(7), 1206-1227 (2008).
- Preskorn SH, Flockhart D. Guide to Psychiatric Drug Interaction. Primary Pscyhiatry 16(12), 45-74 (2009).
- Granfors MT, Backman JT, Neuvonen M, et al. Fluvoxamine drastically increases concentrations and effects of tizanidine: a potentially hazardous interaction. Clin. Pharmacol. Ther 75(4), 331-341 (2004).
- Obach RS, Ryder TF. Metabolism of ramelteon in human liver microsomes and correlation with the effect of fluvoxamine on ramelteon pharmacokinetics. Drug Metab. Dispos 38(8), 1381-1391 (2010).
- Lee CA, Cook JA, Reyner EL, et al. P-glycoprotein related drug interactions: clinical importance and a consideration of disease states. Expert. Opin. Drug. Metab. Toxicol 6(5), 603-619 (2010).
- Weiss J, Dormann SM, Martin-Facklam M, et al. Inhibition of P-glycoprotein by newer antidepressants. J. Pharmacol. Exp. Ther 305(1), 197-204 (2003).
- El Ela AA, Hartter S, Schmitt U, et al. Identification of P-glycoprotein substrates and inhibitors among psychoactive compounds-implications for pharmacokinetics of selected substrates. J. Pharm. Pharmacol 56(8), 967-975 (2004).
- Fenner KS, Troutman MD, Kempshall S, et al. Drug-drug interactions mediated through P-glycoprotein: clinical relevance and in vitro-in vivo correlation using digoxin as a probe drug. Clin. Pharmacol. Ther 85(2), 173-81 (2009).
- Saruwatari J, Yasui-Furukori N, Niioka T, et al. Different effects of the selective serotonin reuptake inhibitors fluvoxamine, paroxetine, and sertraline on the pharmacokinetics of fexofenadine in healthy volunteers. J. Clin. Psychopharmacol 32(2), 195-199 (2012).
- Akamine Y, Yasui-Furukori N, Ieiri I, et al. Psychotropic drug-drug interactions involving P-glycoprotein. CNS Drugs 26(11), 959-973 (2012).
- Sandson NB. Drug-Drug Interaction Primer: A Compendium of Case Vignettes for the Practicing Clinician. American Psychiatric Publishing, Inc., Washington, D.C. and London, UK (2007).
- von Moltke LL, Greenblatt DJ, Court MH, et al. Inhibition of alprazolam and desipramine hydroxylation in vitro by paroxetine and fluvoxamine: comparison with other selective serotonin reuptake inhibitor antidepressants. J. Clin. Psychopharmacol 15(2), 125-131 (1995).
- Christensen M, Tybring G, Mihara K, et al. Low daily 10-mg and 20-mg doses of fluvoxamine inhibit the metabolism of both caffeine (cytochrome P4501A2) and omeprazole (cytochrome P4502C19). Clin. Pharmacol. Ther 71(3), 141-152 (2002).
- Spina E, Pollicino AM, Avenoso A, et al. Effect of fluvoxamine on the pharmacokinetics of imipramine and desipramine in healthy subjects. Ther. Drug. Monit 15(3), 243-246 (1993).
- Ereshefsky L. Drug-drug interactions with the use of psychotropic medications. CNS Spectr 14(8), 01-08 (2009).
- Keltner NL, Moore RL. Biological perspectives psychiatric drug-drug interactions. Perspect. Psychiatr. Care 46(3), 244-251 (2010).
- Prakash A, Faulds D. Rabeprazole. Drugs 55(2), 261-267 (1998).
- Williams MP, Pounder RE. Review article: the pharma-cology of rabeprazole. Aliment. Pharmacol. Ther 13(3), 3-10 (1999).
- Andersson T. Pharmacokinetics, metabolism and inter-actions of acid pump inhibitors. Focus on omeprazole, lansoprazole and pantoprazole. Clin. Pharmacokinet 31(1), 09-28 (1996).
- Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors-emphasis on rabeprazole. Aliment. Pharmacol. Ther 13(3), 27-36 (1999).
- Yasuda S, Horai Y, Tomono Y, et al. Comparison of the kinetic disposition and metabolism of E3810, a new proton pump inhibitor, and omeprazole in relation to S-mephenytoin 4′-hydroxylation status. Clin. Pharmacol. Ther58(2), 143-154 (1995).
- VandenBranden M, Ring BJ, Binkley SN, et al. Interaction of human liver cytochromes P450 in vitro with LY307640, a gastric proton inhibitor. Pharmacogenetics 6(1), 81-91 (1996).
- Kita T, Sakaeda T, Baba T, Aoyama N, et al. Different contribution of CYP2C19 in the in vitro metabolism of three proton pump inhibitors. Biol. Pharm. Bull 26(3), 386-390 (2003).
- Horai Y, Kimura M, Furuie H, et al. Pharmacodynamic effects and kinetic disposition of rabeprazole in relation to CYP2C19 genotypes. Aliment Pharmacol. Ther 15(6), 793-803 (2001).
- Shirai N, Furuta T, Moriyama Y, et al. Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment. Pharmacol. Ther 15(12), 1929-1937 (2001).
- Ieiri I, Kishimoto Y, Okochi H, et al. Comparison of the kinetic disposition of and serum gastrin change by lansoprazole versus rabeprazole during an 8-day dosing scheme in relation to CYP2C19 polymorphism. Eur. J. Clin. Pharmacol 57(6-7), 485-492 (2001).
- Sugimoto M, Furuta T, Shirai N, et al. Different dosage regimens of rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotype status. Clin. Pharmacol. Ther 76(4), 290-301 (2004).
- Shimatani T, Inoue M, Kuroiwa T, et al. Rabeprazole 10 mg twice daily is superior to 20 mg once daily for night-time gastric acid suppression. Aliment. Pharmacol. Ther 19(1), 113-122 (2004).
- Pauli-Magnus C, Rekersbrink S, Klotz U, et al. Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein. Naunyn. Schmiedebergs. Arch. Pharmacol 364(6), 551-557 (2001).
- Kubota T, Chiba K, Ishizaki T. Genotyping of S-mephenytoin 4ïÿà-hydroxylation in an extended Japanese population. Clin. Pharmacol. Ther 60(6), 661-666 (1996).
- Scordo MG, Pengo V, Spina E, et al. Influence of CYP2C9 and CYP2C19 genetic polymorphisms on warfarin maintenance dose and metabolic clearance. Clin. Pharmacol. Ther 72(6), 702-710 (2002).
- Yasui-Furukori N, Takahata T, Nakagami T, et al. Different inhibitory effect of fluvoxamine on omeprazole metabolism between CYP2C19 genotypes. Br. J. Clin. Pharmacol 57(4), 487-494 (2004).
- Yasui-Furukori N, Saito M, Uno T, et al. Effects of fluvoxamine on lansoprazole pharmacokinetics in rela-tion to CYP2C19 genotypes. J. Clin. Pharmacol 44(11), 1223-1229 (2004).
- Furuta T, Ohashi K, Kobayashi K, et al. Effects of clarithromycin on the metabolism of omeprazole in relation to CYP2C19 genotype status in humans. Clin. Pharmacol. Ther 66(3), 265-274 (1999).
- Saito M, Yasui-Furukori N, Uno T, et al. Effects of clarithromycin on lansoprazole pharmacokinetics between CYP2C19 genotypes. Br. J. Clin. Pharmacol 59(3), 302-309 (2005).
- Fleishaker JC, Hulst LK. A pharmacokinetic and pharmacodynamic evaluation of the combined administration of alprazolam and fluvoxamine. Eur. J. Clin. Pharmacol 46(1), 35-39 (1994).
- Uno T, Shimizu M, Yasui-Frukori N, et al. Different effects of fluvoxamine on rabeprazole pharmacokinetics in relation to CYP2C19 genotype status. Br. J. Clin. Pharmacol 61(3), 309-314 (2006).
- Shimizu M, Uno T, Yasui-Furukori N, Sugawara K, Tateishi T. Effects of clarithromycin and verapamil on rabeprazole pharmacokinetics between CYP2C19 genotypes. Eur. J. Clin. Pharmacol 62(8), 597-603 (2006).
- Wakasugi H, Yano I, Ito T, et al. Effect of clarithromycin on renal excretion of digoxin: interaction with P-glycoprotein. Clin. Pharmacol. Ther 64(1), 123– 128 (1998).
- Kurata Y, Ieiri I, Kimura M, et al. Role of human MDR1 gene polymorphism in bioavail- ability and interaction of digoxin, a substrate of P-glycoprotein. Clin. Pharmacol. Ther 72(2), 209-219 (2002).
- Yasui-Furukori N, Uno T, Sugawara K, et al. Different effects of three transporting inhibitors, verapamil, cimetidine, and probenecid, on fexofenadine pharmacokinetics. Clin. Pharmacol. Ther 77(1), 17-23 (2005).
- Sakugawa T, Miura M, Hokama N, et al. Enantioselective disposition of fexofenadine with the P-glycoprotein inhibitor verapamil. Br. J. Clin. Pharmacol 67(5), 535-540 (2009).
- Itagaki F, Homma M, Takara K, et al. Effect of rabeplazole on MDR1- mediated transport of rhodamine 123 in Caco-2 and Hvr 100-6 cells. Biol. Pharm. Bull 27(10), 1694-1696 (2004).
- Hulot JS, Bura A, Villard E, et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood 108(7), 2244-2247 (2006).
- von Moltke LL, Greenblatt DJ, Giancarlo GM, et al. Escitalopram (S-citalopram) and its metabolites in vitro: cytochromes mediating biotransformation, inhibitory effects, and comparison to R-citalopram. Drug. Metab. Dispos 29(8), 1102-1109 (2001).
- Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug. Metab. Dispos 31(5), 540-547 (2003).
- FDA Antiviral Drugs Advisory Committtee. Briefing Document for Voriconazole (Oral and Intravenous Formulations) (2001).