Research Article - (2018) Volume 8, Issue 4
Effect of Physical Activity on Auditory Sensory Gating in Chronic Schizophrenia
- Corresponding Author:
- Hsin-Yung Chen
Department of Occupational Therapy, Chang Gung University, No.259, Wenhua 1st Rd., Guishan Dist., Taoyuan City-33302, Taiwan
Tel: +886-3-2118800 ext. 3633
Fax: +886-3-2118700
Abstract
Schizophrenia (SCZ) is associated with diminished gating components in auditory processing. The hippocampus is one of the brain regions functionally altered in SCZ and plays a crucial role in the modulation of auditory processing. Although physical activity (PA) is beneficial for hippocampal neurogenesis and cognitive function in SCZ, its effect on auditory processing has been rarely studied. The auditory-paired stimulus paradigm with a binaural pure tone was presented on each side, and time-voltage domain responses were qualified with evoked potential under conditions before, during, and after PA as well as at follow-up. Gating ratios were compared between 10 patients with four phases of chronic SCZ (SCZ, n=10) and a group of age- and sex-matched healthy controls (Ctrl, n=10) to determine whether moderate PA influenced sensory gating in this crossover study. An inherent deficit in the P50 gating ratio was found in a baseline comparison between SCZ and Ctrl groups. However, the P50 gating ratio improved during and after PA in the SCZ group. In an intragroup comparison of SCZ, PA application significantly improved N100 gating, both during and after PA. The results of this study suggest the beneficial effect of PA in improving sensory gating in SCZ.
Keywords
Schizophrenia, Sensory gating, Physical activity, Paired-Click paradigm
Introduction
Cognitive functions may be protected from potentially interfering information by brain mechanisms that filter relevant information from irrelevant information across multiple sensory domains. These mechanisms operate during the earliest levels of sensory information processing and within progressively higher-order faculties (such as attention, memory, language, and social functioning) while an individual is interacting with the physical environment [1-3]. Sensory gating is the first sensory processing stage that occurs as a part of the behavioral organization processes of central nervous system functions, and it is considered that P50, N100, and P200 sensory gating reflect distinct biological substrates that preserve the integrity of cognitive functioning by preventing irrelevant information from reaching higher-order functions [1,4]. The paired-stimulus sensory gating paradigm is the most important method used for determining impaired sensory gating. This method involve`s presenting the subject with a brief initial stimulus, Stimulus 1 (S1), which activates an inhibitory mechanism to minimize the disruptive effects of an identical second stimulus, Stimulus 2 (S2), which occur 500 ms later [4-6]. The magnitude of inhibition is defined as the ratio of the evoked response amplitude to the first stimulus, S1, to the evoked response amplitude of the second stimulus, S2, (i.e., S2/S1). Lower numbers reflect stronger attenuation of irrelevant input and thus a higher gating capability [1,3,7]. Deficits in sensory processing in the auditory system of individuals with schizophrenia (SCZ) have been documented, and the integrity of sensory function has been assessed using wellcharacterized event-related potentials, such as P50, N100, and P200 [2,6]. In this respect, the amplitude attenuation of auditory evoked potentials P50, N 100, and P200 can be used to examine this process in a paired-stimulus paradigm.
These abovementioned sensory gating measures, which indicate an individual’s sensory processing ability, have been shown to be decreased in people with SCZ compared with the healthy population, reflecting a sensory processing deficit connected with impairment in cognitive functioning [3,5,8,9]. Although evidence indicates that sensory gating is a core deficit in individuals with SCZ, cognitive correlates for sensory gating have not yet been clearly established. Thus, identifying novel approaches to develop treatments that target neurocognitive deficits in such individuals is particularly necessary.
Physical activity (PA) is a lifestyle factor that can lead to a life-long increase in physical and mental health. Routine participation in PA is associated with a reduction in the number of physical and mental disorders across an adult’s lifespan. Studies have also examined the effect of PA on both healthy and schizophrenic individuals. In healthy individuals, participating in PA results in increased volume and synaptic plasticity of the hippocampus [10-12] and a core structure of cognitive functioning in the brain, which correlates with an improvement in cognitive performance compared with sedentary individuals, particularly in relation to attention, processing speed, visuospatial learning, and memory processing [12-14]. The benefits of PA include not only increased cardiovascular fitness (reflected by physical fitness, physical endurance, and physical tolerance) but also improved mental health, cognitive functioning, and social functioning [11,15]. For patients with SCZ, PA is a crucial treatment modality that has a positive effect on cardiopulmonary fitness and metabolic health, and it may reduce physical health problems associated with SCZ, such as obesity and diabetes [16]. The effect of PA interventions also results in increased hippocampal volume and synaptic plasticity functioning [10,11], which is associated with significant enhancement in cognitive performance domains, such as executive function, working memory, visual learning, and processing speed, all of which could favorably influence disease progression [15,17-20]. Therefore, PA is considered a necessary and vital component of the comprehensive management of cognitive impairment in individuals with SCZ.
Engaging in PA might provide even larger cognitive benefits that can be transferred to the everyday functioning of individuals with SCZ. However, such individuals are generally highly sedentary and experience a range of barriers to PA, such as negative symptoms, pain, and the side effects of medication. Therefore, understanding the timing and intensity of PA required has crucial implications for an individual to successfully participate in PA. Previous findings have indicated that commitment to target training intensity is significantly correlated with an improvement in cognitive performance in this population [21]. Recently, several metaanalyses of exercise interventions conducted in SCZ trials (involving mostly 90–120 min per week of moderate-to-vigorous exercise) have reported significant improvements not only in fitness but also in psychiatric symptoms [22,23]. However, an individualized clinical intervention program may not be sufficient to ensure that training intensity is adhered to. Thus, supervised group PA is the recommended program of intervention that promotes a high attendance rate at a moderate intensity, and this program may have a positive impact on the daily life of people with SCZ [17].
Although evidence has repeatedly demonstrated that PA is a promising strategy for improving cognitive function in people with SCZ, the basic mechanisms involved in modulating multilevel cognitive correlates and brain activity are not well-defined. It is believed that sensory gating contributes to cognitive function by enabling elementary neuronal processing that involves an inhibitory neuron forming a synaptic loop between a sensory neuron and a major output neuron of the organism to pre-attentively filter repetitious incoming irrelevant stimuli [24]. However, research on the effect of PA on the performance of sensory gating in patients with SCZ is scant, despite the strong association between cognitive function and clinical outcomes. Therefore, this study examined the relationship between PA and sensory gating abnormalities in patients with SCZ. Patients with SCZ were hypothesized to show a change in their sensory gating ratio after undergoing PA.
Materials and Methods
▪ Patients
Patients with SCZ aged between 20 and 50 years were recruited from the chronic ward of Taoyuan Chang Gung Memorial Hospital, Taoyuan, Taiwan. The psychiatric diagnosis was assessed by a senior psychiatrist at the recruiting institution. Patients with SCZ were included in the study if they met the following criteria: (1) met the criteria for the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) for SCZ, (2) had no hearing problems, (3) were able to tolerate earphones in the ear without evoking an emotional response for at least 15 min, and (4) agreed to participate in the study and complete the informed consent form. However, patients with any one of the following criteria were not included in the study: (1) a history of cardiovascular disorders, neurological disorders, or head trauma with loss of consciousness; (2) current comorbid DSM-IV axis I condition with emotional disorders, anxiety disorders, or substance dependence; (3) current comorbid DSM-IV axis II condition with mental retardation; or (4) active symptoms that warranted antipsychotic dose adjustment 4 weeks prior to or during participation. All the experimental procedures were approved by the Institutional Review Board of Chang Gung Medical Foundation (101-4435A3).
▪ Moderate PA
Activity was conducted in a therapeutic room of the chronic psychiatric ward. The program involved three 1-h PA sessions every week for a period of 8 weeks. These sessions began with a 10-min warm-up period, after which participants exercised individually for 45 min, and ended with a 5-min cool-down period that was supervised by certified occupational therapists. Patients were required to participate in a minimum of 90% of sessions. The program consisted of sensory-motor activities at a moderate energy consumption level between 3 and 6 MET. The patients were permitted to stop any activity if they felt uncomfortable.
▪ Stimuli and experimental procedure
The patients sat upright in a relaxed upright position on a high-backed chair in a quiet room that was not soundproofed. No patients reported having a hearing deficit, and all were instructed to relax and sit quietly with their eyes closed during passive listening to the paired-click stimuli.
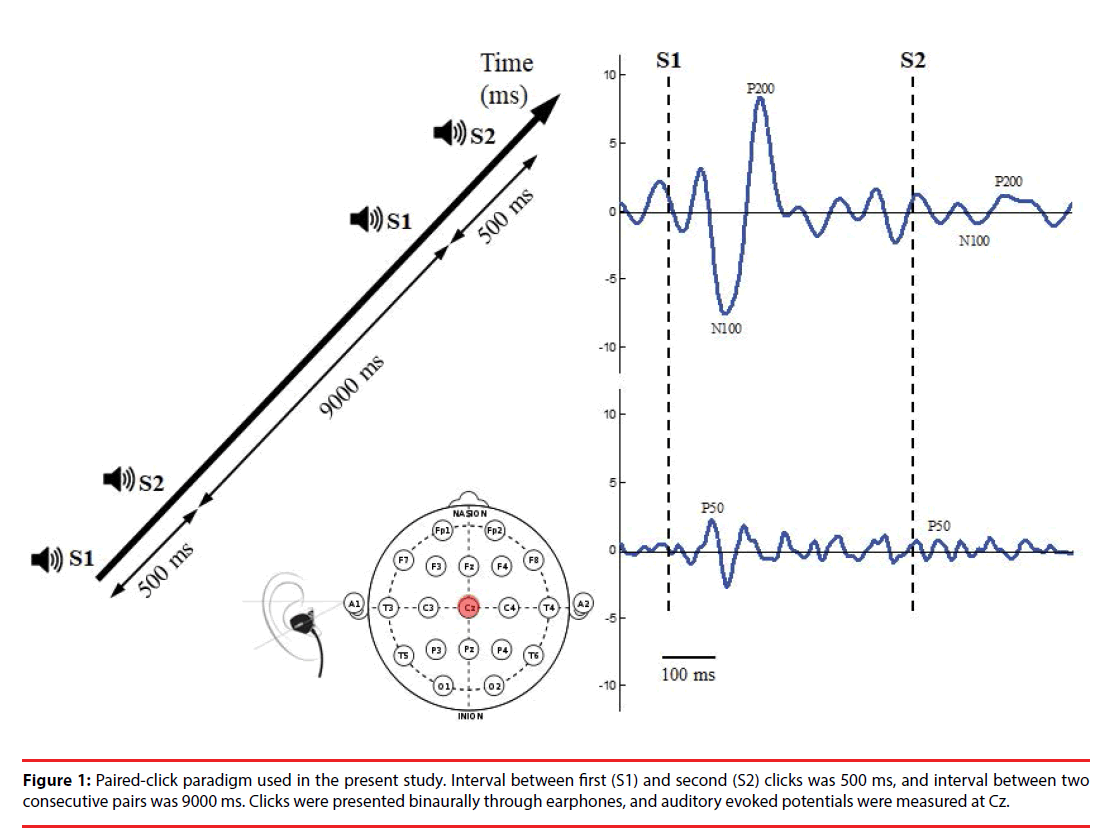
The paired-click paradigm (Figure 1) consisted of 76 pairs of stimuli with two identical clicks (each click with 10 ms in latency, 90 dB in amplitude, and 1500 Hz in frequency); these were presented to participants binaurally through inserted earphones. The stimulus interval between the first (S1) and second (S2) click of each pair of stimuli was 500 ms, and the trial interval between two consecutive stimulus pairs was 9000 ms, which allowed full recovery of the evoked amplitude prior to the subsequent stimulus click. The total recording time was approximately 12 min.
▪ Electrophysiological data acquisition
During pair-click stimuli, brain activity was recorded using an electroencephalography (EEG) with two Ag/AgCl electrodes attached by means of a plastic adhesive tape and positioned to the vertex point (Cz) according to the international 10–20 system. Linked earlobes served as reference. The electrode impedance was maintained below 5 kΩ. EEG was recorded at a sampling rate of 2048 Hz and filtered between 0.1 and 100 Hz and a gain factor of 19.5 through a Bluetooth-based telemetry wireless acquisition system, the Nexus-10 system (Mind Media BV, The Netherlands).
▪ Data processing
Raw signals were digitally filtered off-line between 0.1 and 50 Hz (48 dB/oct roll off) by using Matlab software. Data were epoched from 100 ms preceding (baseline) to 300 ms following S1 and S2 and then corrected for baseline activity for further analyses. Any epoch with artifact was detected manually and rejected from further analysis. Epochs were rejected as a pair; that is, if S1 was rejected by the detection procedure, the accompanying S2 epoch was then rejected in analysis. Both S1 and S2 signals were averaged, respectively, per trial block. Signals were filtered with a 10-Hz high-pass filter to optimize P50 scoring or with a 20-Hz low-pass filter optimize scoring of N100 and P200 before epoch averaging [25].
In the present study, P50 components associated with S1 and S2 were defined as the most positive peaks in electrode Cz occurring between 35 and 85 ms post stimulus. If two equal-amplitude peaks were present, the later peak was selected.
Amplitude was measured relative to the immediately preceding negativity, through this trough was not permitted to have a latency of <30 ms post stimulus. The S1 amplitude was then computed as the difference between the P50 maxima and minima associated with the preceding trough. If no P50 was detected in that time window, the P50 was identified as the most positive peak preceding the N100. The N100 and P200 components associated with S1 and S2 were identified as the largest negative deflection in the time window between 80 and 150 ms and the largest positive deflection between 150 and 250 ms post stimulus [26], respectively. The peak amplitudes of the N100 component were computed as the sum of the absolute value of the amplitude from the preceding P50 peak to the minima of the N100 component. If no N100 peak was identified between 80 and 150 ms, the time window between 0 and 300 ms was used, in which the most negative peak was considered to be the N100 component. The peak amplitudes of the P200 component were computed as the sum of the absolute value of the preceding N100 minima and the P200 maxima. If no P200 peak was found in that window, P200 was identified as the most positive peak after the N100 component, but only if it peaked before 300 ms. The sensory gating ratio was calculated as the amplitude of the second click (S2) divided by the amplitude of the first click (S1) (S2/S1 ratio).
▪ Statistical analyses
The mean and standard deviation of demographic variable data (age and sex) were analyzed for all participants, and results were presented as descriptive statistics. Dependent variables were the peak amplitudes and latencies of P50, N100, and P200 evoked by S1 and S2 scored at Cz; P50, N100, and P200 gating ratio (S2 amplitude/S1 amplitude); and scores of amplitude differences (S1 amplitude − S2 amplitude, ΔS1S2amplitude). The independent t test was used to determine any significant difference in the gating variables and difference scores between the control and the four-phase conditions of the SCZ group. One-way repeated measure ANOVA was used to investigate the distinct phases, the main effects of gating variables, and the difference scores in the SCZ group, where a lower ratio and higher difference score indicated superior gating. All statistical tests were two tailed, with a significance level (α) set at 0.05, and were performed using SPSS version 19.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
▪ Participants
Eighteen patients with SCZ who were determined through a chart review were assessed for eligibility in this study. Of the 18 patients, eight were excluded because of the following reasons: four did not meet the inclusion criteria, two did not sign the informed consent for study participation, one was discharged during the intervention, and one was excluded from analyses because data were destroyed. Finally, 10 patients were included in the SCZ group. Another 10 healthy controls, who were age- and sex-matched, were recruited to enable comparison with the SCZ group. Demographic characteristics did not significantly differ between the two groups (p=1.00; Table 1).
| Control Group | Schizophrenia Group | |
|---|---|---|
| N | 10 | 10 |
| Gender (Male/Female) | 4/6 | 4/6 |
| Age (years) | 34.9 (7.84) | 34.9 (7.84) |
Table 1: Demographic characteristics of groups.
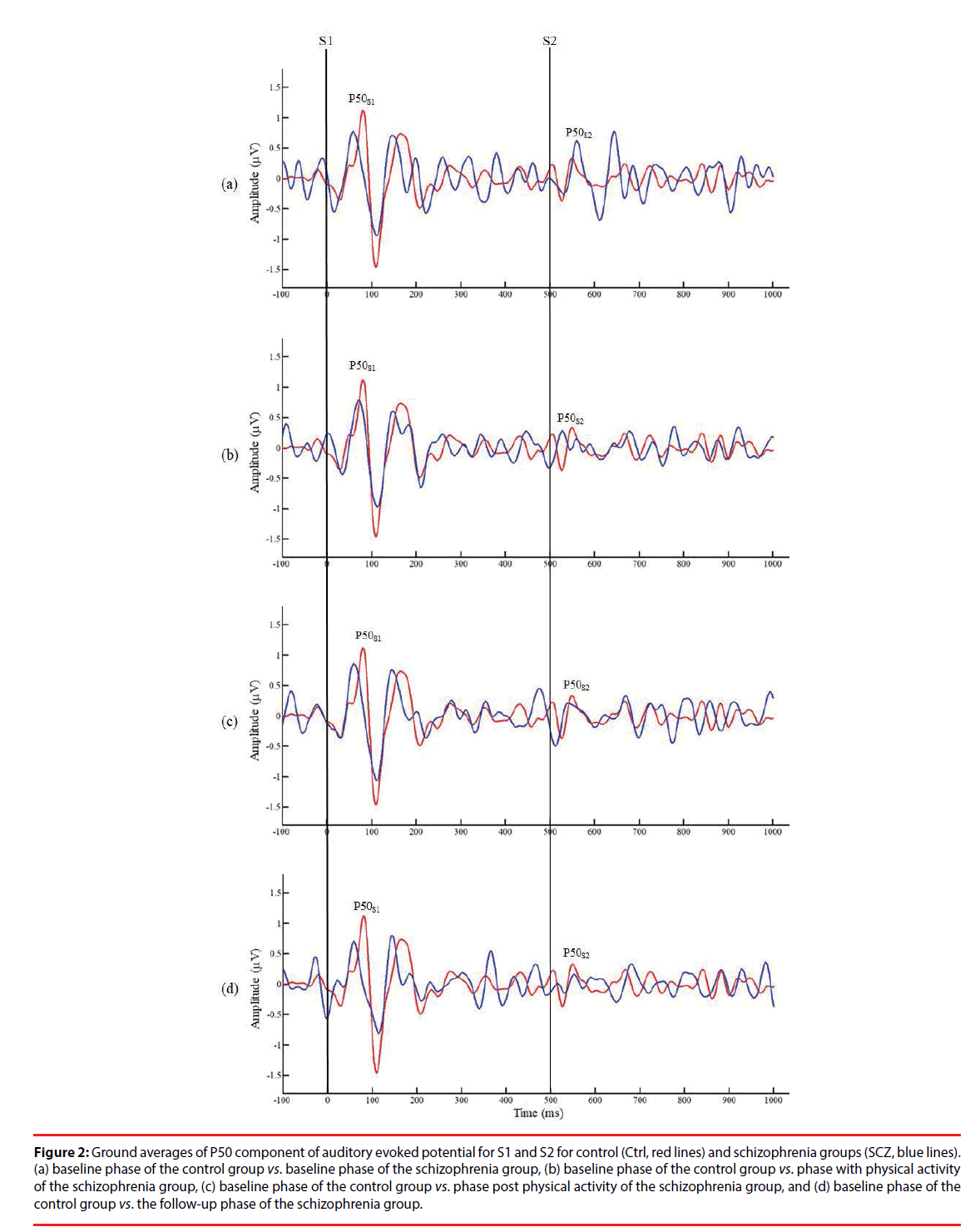
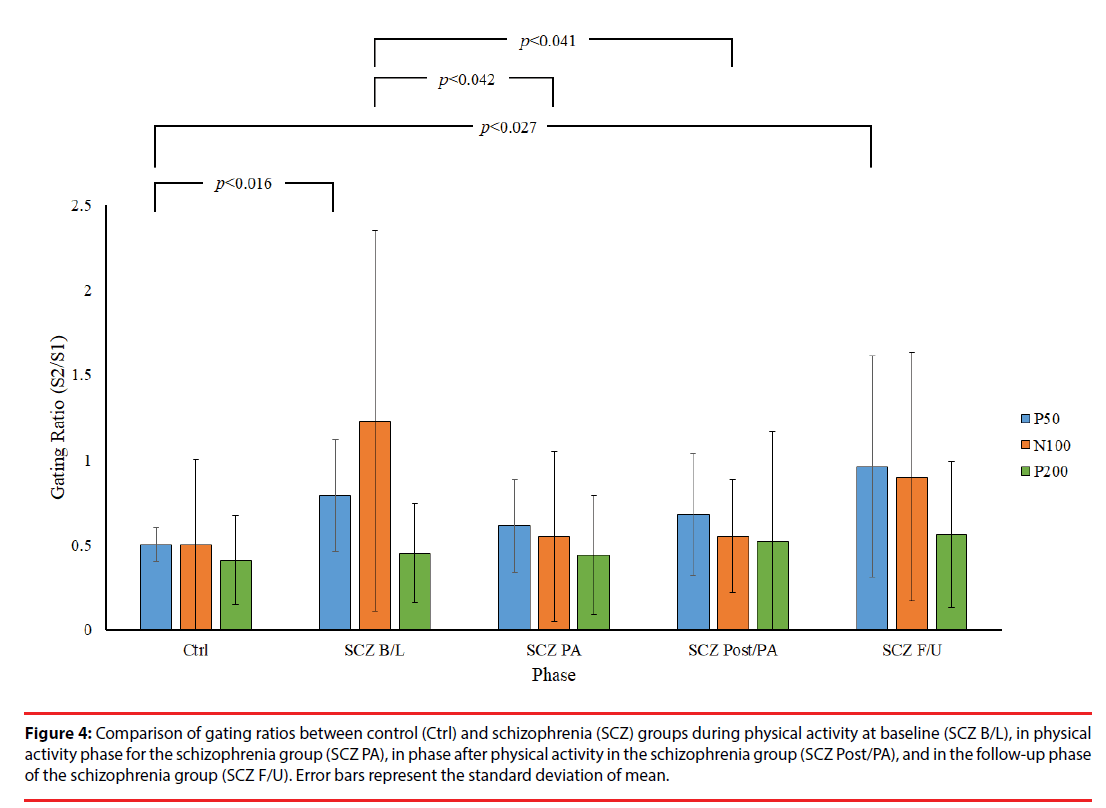
Figure 2 presents a comparison of P50 grand averages for control and SCZ groups. Although a higher amplitude of P50 was recorded in response to S1 and S2 in the control group than in the SCZ group, no other significant differences in this respect were observed between the control and SCZ groups or between relative differences in the phases of the SCZ group (p>0.05). However, independent t test results showed significantly lower P50 gating ratios in the control group than in the B/L and F/U phases of the SCZ group (Ctrl: 0.50 ± 0.10 vs. SCZ_B/L: 0.79 ± 0.33, p=0.016; Ctrl: 0.50 ± 0.10 vs. SCZ_F/U: 0.99 ± 0.63, p=0.027), indicating an inherent intergroup difference between the two groups for the P50 gating ratio (Table 2). By contrast, there was no significant difference in the gating ratio between the PA and Post/PA phases of the SCZ and control groups (SCZ_ PA: 0.61 ± 0.27 vs. Ctrl: 0.50 ± 0.10, p=0.240; SCZ_Post/PA: 0.68 ± 0.36 vs. Ctrl: 0.50 ± 0.10, p=0.130), which may reveal an improvement in the P50 gating effect with moderate PA in the SCZ group. Furthermore, a lower P50 gating ratio was observed in PA and Post/PA than in the B/L phase in intragroup data, but there was no significant difference between the phases in the SCZ group (p>0.05).
| Control | Schizophrenia | ||||
|---|---|---|---|---|---|
| Condition | B/L | B/L | PA | Post-PA | F/U |
| Gating ratio | |||||
| P50 | 0.50 (0.10) | 0.79 (0.33)* | 0.61 (0.27) | 0.68 (0.36) | 0.98 (0.67)* |
| N100 | 0.50 (0.50) | 1.23 (1.12) | 0.57 (0.52)† | 0.55 (0.33) † | 0.76 (0.63) |
| P200 | 0.41 (0.26) | 0.45 (0.29) | 0.44 (0.36) | 0.42 (0.18) | 0.56 (0.46) |
| ΔS1S2 amplitude (µV) | |||||
| P50 | 1.76 (0.71) | 0.83 (1.24) | 1.08 (0.75) | 1.52 (2.08) | 0.83 (1.80) |
| N100 | 4.37 (4.50) | 0.81 (3.03) | 1.14 (1.30)* | 3.67 (4.54) | 1.94 (4.61) |
| P200 | 9.17 (5.50) | 6.03 (4.16) | 8.63 (7.00) | 9.63 (6.93) | 5.80 (5.13) |
| ΔS1S2 latency (ms) | |||||
| P50 | 5.68 (27.72) | -6.97 (19.06) | 13.57 (15.74) | -3.20 (25.33) | -0.23 (21.74) |
| N100 | -5.87 (21.66) | -13.08 (27.65) | 13.10 (34.74) | 18.63 (47.19) | -25.89 (28.50) |
| P200 | -20.33 (34.54) | -21.76 (51.58) | 19.1 (79.47) | 2.22 (57.27) | -38.26 (31.24) |
Table 2: Gating variables of participants.
Figure 2: Ground averages of P50 component of auditory evoked potential for S1 and S2 for control (Ctrl, red lines) and schizophrenia groups (SCZ, blue lines). (a) baseline phase of the control group vs. baseline phase of the schizophrenia group, (b) baseline phase of the control group vs. phase with physical activity of the schizophrenia group, (c) baseline phase of the control group vs. phase post physical activity of the schizophrenia group, and (d) baseline phase of the control group vs. the follow-up phase of the schizophrenia group.
▪ N100
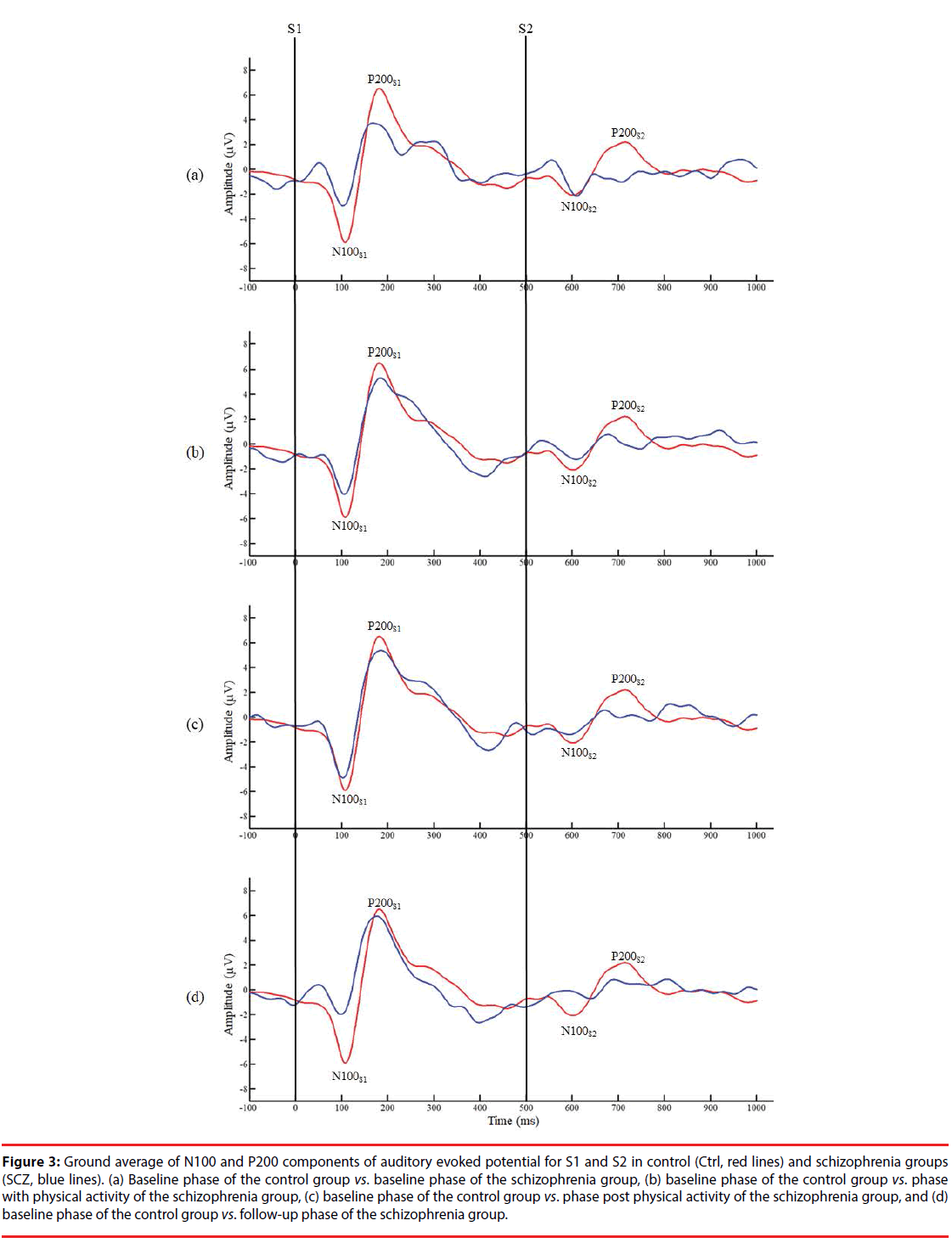
Figure 3 presents the grand averages of N100 and P200 waveforms evoked by S1 and S2 for both groups. Intergroup data reveal a significantly lower amplitude of N100 to S1 in the B/L phase of the SCZ group than in the control group (SCZ_B/L: 4.04 ± 2.64 μv vs. Ctrl: 7.37 ± 4.08 μv, p=0.044). In addition, a significantly lower amplitude difference was observed in the PA phase of the SCZ group than for the control group (SCZ_PA: 1.14 ± 1.30 vs. Ctrl: 4.37 ± 4.50, p=0.043), as shown in Figure 4. However, there were no significant differences in the gating ratios between the control and in each phase of the SCZ group, respectively (p>0.05), indicating that different amplitudes of N100 to S1 do not affect the gating ratios between the groups. In the intragroup data, a significantly higher N100 gating ratio was observed in the B/L phase in the SCZ group than for PA (SCZ_B/L: 1.23 ± 1.12 vs. SCZ_PA: 0.57 ± 0.52, p=0.042) and the Post/PA phase (SCZ_B/L: 1.23 ± 1.12 vs. SCZ_ Post/PA: 0.55 ± 0.33, p=0.041). Furthermore, there were no significant differences in P200 parameters. The intragroup comparison shows that moderate PA can attenuate the deficit of sensory gating induced by auditory stimuli in the SCZ group.
Figure 3: Ground average of N100 and P200 components of auditory evoked potential for S1 and S2 in control (Ctrl, red lines) and schizophrenia groups (SCZ, blue lines). (a) Baseline phase of the control group vs. baseline phase of the schizophrenia group, (b) baseline phase of the control group vs. phase with physical activity of the schizophrenia group, (c) baseline phase of the control group vs. phase post physical activity of the schizophrenia group, and (d) baseline phase of the control group vs. follow-up phase of the schizophrenia group.
Figure 4: Comparison of gating ratios between control (Ctrl) and schizophrenia (SCZ) groups during physical activity at baseline (SCZ B/L), in physical activity phase for the schizophrenia group (SCZ PA), in phase after physical activity in the schizophrenia group (SCZ Post/PA), and in the follow-up phase of the schizophrenia group (SCZ F/U). Error bars represent the standard deviation of mean.
In summary, our data reveal that moderate PA alters the sensory gating response by decreasing the P50 and N100 gating ratio in the SCZ group.
Discussion
The current study investigated sensory gating in patients with SCZ in response to the application of moderate PA, with the aim of further understanding the associated effect on auditory processing. Results revealed three major findings. First, auditory sensory gating, which was evaluated using the P50 and N100 gating ratio, was found to be impaired in patients with SCZ. Second, there was no significant sensory gating impairment in the deficit forms of SCZ under a baseline phase than in phases under and after treatment. Third, the application of moderate PA may have provided an opportunity to patients with SCZ to improve their sensory processing performance when subjected to auditory stimuli. These findings may indicate that the application of PA increases the sensory gating ability and attention allocation during auditory stimuli in patients with SCZ.
Auditory sensory gating is a rudimentary physiological assay of the brain’s ability to process novel or salient information (amplitude response to first stimulus) and to filter out extraneous information (amplitude suppression of second stimulus), in order to protect higherorder cognitive functioning. This goal-directed cortical process reflects the identification of context-specific irrelevance at the encoding (input) stage and/or subsequent to selectively attending to relevant stimuli based on the previous identification [27], which involves sensory gating activity as early as P50 and as late as N100. Neurophysiological evidence points to the hippocampus and rhinal regions as the main brain locations mediating an early phase [28] and a later phase [7] of auditory sensory gating. Understanding this pre-attentive mechanism is an important step toward revealing neuronal mechanisms, because it plays a role in underlying the automatic detection of stimulus change in the auditory modality. The inhibitory interneuron originating from the hippocampus has been assumed to mediate such sensory habituation and gating processes in both animal and human studies [24,29-31]. Inhibition is an important form of cognitive control that functions to limit the processing of sensory information in our environment [27]. Sensory gating abnormalities have been hypothesized to be associated with various cognitive impairments in SCZ [31,32]. In the inhibitory filter process, P50 suppression (which has no requirement of voluntary attention and occurs automatically to an initial stimulus) provides an index of sensory gating, whereby S1 automatically activates an inhibitory process and then suppresses responsiveness to S2. The role of P50 sensory gating reflects the pre-attentive mechanism that can protect the integrity of higher-order functioning under stimulus detection [1,27]. N100 is not merely a low order of the auditory response, and the hippocampus has a modulatory effect on N100 amplitudes ranging from pre-attentive to attentive conditions [30]. The present study confirms a reduced gating performance in patients with SCZ, which agrees with previous results of a significant higher P50 gating S2/S1 ratio in patients with SCZ compared with a healthy control group [1,8,33]. Our findings therefore corroborate previous results and show weaker gating of P50 in patients with SCZ. Attention is the principal cognitive dimension correlated with sensory gating [27,34], and attention functions represent the foundations for other neurocognitive domains. Briefly, if a participant has deteriorated attentional functions, then tests for other neurocognitive domains cannot be performed to the person’s full ability [20].
In the present study, we specifically tested whether SCZ and control groups differ in gating of auditory evoked N100. The gating response of N100 reflects a trigger to allocate attention and enables improved discrimination between signal and noise [1]. The results of this present study show a significantly higher N100 gating S2/S1 ratio in the SCZ group than in the controls, indicating weaker gating in SCZ beyond the pre-attentive to attentive level, which is reflected by P50 and N100 gating. These findings are consistent with those of previous studies in healthy controls, which have shown stronger P50 and N100 sensory gating with better inhibition of interfering stimuli than in the SCZ group.
Moderate PA (i.e., aerobic exercise) is a simple, cheap, effective, and potentially widely available nonpharmacological treatment for cognitive impairment. It has been shown to contribute to improvements in cognitive functioning, including (but not limited to) modest effects on attention, processing speed, memory, and executive function, both in healthy participants and patients with SCZ [12,23]. Cognitive findings have shown a parallel regional increase in brain volume and functioning, primarily with respect to the hippocampus [13]. Because the hippocampus is a key structure for spatial learning and a certain type of memory, it is possible that PA improves cognitive and daily functioning associated with enhancements in short-term memory, along with increases in hippocampal volume in patients with SCZ [10,12,13]. However, the benefits of PA in patients with SCZ cannot be attributed to hippocampal volume alone. The upregulation of the brain-derived neurotrophic factor in the hippocampus is associated with changes in the hippocampal volume and has extensive involvement in the inhibitory response to synaptic plasticity and neurogenesis to enhance the effect of PA on improvements in cognitive control [18,23,35,36]. The results of our present study show no significant differences in P50 and the N100 gating ratio between patients with SCZ undertaking moderate PA and those in the control group. PA is a promising approach for use in remediating hippocampal harm and alleviating cognitive deficits [11,13,18]. In the present study, changes were elicited in regions related to sensory gating in patients with SCZ. Therefore, the application of PA may provide the opportunity for such patients to perform better in sensory gating tasks.
The hippocampus is involved in memory and perceptual processing [30]. With respect to auditory perception, the hippocampus receives afferent inputs from the auditory association cortices, and it projects back to the primary auditory cortex and auditory association areas in turn [4]. The hippocampus is also believed to play a critical role in relaying auditory information to later perceptual and cognitive processes [4,30]. PA has been shown to stimulate hippocampal neuroplasticity and successfully counteract deteriorating hippocampal function [20,37,38]. The findings of the present study suggest that PA intervention can provide benefits in alleviating certain aspects of sensory gating deficits in patients with SCZ.
The major limitation of present study is the small number of subjects, and thus not being able to use more elaborate study design and analysis to find differences between groups. In future studies, we hope to overcome this problem by increasing the number of subjects to provide the more relevant information for further treatment approaches in SCZ rehabilitation.
Conclusion
This study investigated changes in sensory gating induced by a paired-click paradigm before and after the application of moderate PA in patients with SCZ. We observed a substantial correlation between the effect of moderate PA and sensory gating performance in both the P50 and N100 gating ratios. Undertaking physiological measurements with a paired-click paradigm is a feasible strategy for objectively assessing sensory gating responses. Our findings provide empirical evidence that a moderate amount of PA can influence sensory gating by reducing the gating ratio during paired-click stimuli. Our results further suggest that moderate PA can be an appropriate therapeutic strategy for people with SCZ who have sensory gating deficit, both during or in preparation for a paired-click paradigm. However, to enable application in clinical practice, additional studies are needed to better investigate the underlying mechanism involved and its correlation with sensory gating modulation.
Acknowledgments
This project was partially supported by grants from the Chang Gung Memorial Hospital (BMRPA70). We thank Nan-Wen Yu, M. D. and I-Ting Hsieh, O. T. R. for their support and guidance as this project progressed to its fruition. The authors express their gratitude to the participants of this study.
References
- Lijffijt M, Lane SD, Meier SL, et al. P50, N100, and P200 sensory gating: relationships with behavioral inhibition, attention, and working memory. Psychophysiology 46(5), 1059-1068 (2009).
- Javitt DC. Sensory processing in schizophrenia: neither simple nor intact. Schizophr. Bull 35 (6), 1059-1064 (2009).
- Smucny J, Olincy A, Eichman LC, et al. Early sensory processing deficits predict sensitivity to distraction in schizophrenia. Schizophr. Res 147(1), 196-200 (2013).
- Javitt DC, Freedman R. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am. J. Psychiatry 172(1), 17-31 (2015).
- Sánchez-Morla EM, Santos JL, Aparicio A, et al. Neuropsychological correlates of P50 sensory gating in patients with schizophrenia. Schizophr. Res 143(1), 102-106 (2013).
- Williams TJ, Nuechterlein KH, Subotnik KL, et al. Distinct neural generators of sensory gating in schizophrenia. Psychophysiology 48(4), 470-478 (2011).
- Cromwell HC, Mears RP, Wan L, et al. Sensory gating: a translational effort from basic to clinical science. Clin. EEG Neurosci 39(2), 69-72 (2008).
- Boutros NN, Brockhaus-Dumke A, Gjini K, et al. Sensory-gating deficit of the N100 mid-latency auditory evoked potential in medicated schizophrenia patients. Schizophr. Res 113(2-3), 339-346 (2009).
- Hall MH, Taylor G, Salisbury DF, et al. Sensory gating event-related potentials and oscillations in schizophrenia patients and their unaffected relatives. Schizophr. Bull 37(6), 1187-1199 (2011).
- Pajonk FG, Wobrock T, Gruber O, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch. Gen. Psychiatry 67(2), 133-143 (2010).
- Kandola A, Hendrikse J, Lucassen PJ, et al. Aerobic Exercise as a Tool to Improve Hippocampal Plasticity and Function in Humans: Practical Implications for Mental Health Treatment. Front. Hum. Neurosci 10(1), 373 (2016).
- Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 108(7), 3017-3022 (2011).
- Nuechterlein KH, Ventura J, McEwen SC, et al. Enhancing cognitive training through aerobic exercise after a first schizophrenia episode: theoretical conception and pilot study. Schizophr. Bull Suppl 1(1), S44-S52 (2016).
- Voelcker-Rehage C, Niemann C. Structural and functional brain changes related to different types of physical activity across the life span. Neurosci. Biobehav. Rev 37(9 PtB), 2268-2295 (2013).
- Kimhy D, Vakhrusheva J, Bartels MN, et al. Aerobic fitness and body mass index in individuals with schizophrenia: Implications for neurocognition and daily functioning. Psychiatry. Res 220(3), 784-791 (2014).
- Boyer L, Richieri R, Dassa D, et al. Association of metabolic syndrome and inflammation with neurocognition in patients with schizophrenia. Psychiatry. Res 210(2), 381-386 (2013).
- Gomes E, Bastos T, Probst M, et al. Effects of a group physical activity program on physical fitness and quality of life in individual with schizophrenia. Ment. Health. Phys. Act 7(3), 155-162 (2014).
- Kimhy D, Vakhrusheva J, Bartels MN, et al. The impact of aerobic exercise on brain-derived neurotrophic factor and neurocognition in individuals with schizophrenia: a single-blind, randomized clinical trial. Schizophr. Bull 41(4), 859-868 (2015).
- Oertel-Knöchel V, Mehler P, Thiel C, et al. Effects of aerobic exercise on cognitive performance and individual psychopathology in depressive and schizophrenia patients. Eur. Arch. Psychiatry. Clin. Neurosci 264(7), 589-604 (2014).
- Kurebayashi Y, Otaki J. Correlations between physical activity and neurocognitive domain functions in patients with schizophrenia: a cross-sectional study. BMC. Psychiatry 17(1), 4 (2017).
- Kimhy D, Lauriola V, Bartels MN, et al. Aerobic exercise for cognitive deficits in schizophrenia - The impact of frequency, duration, and fidelity with target training intensity. Schizophr. Res 172(1-3), 213-215 (2016).
- Firth J, Cotter J, Elliott R, et al. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol. Med 45(7) 1343-1361 (2015).
- Dauwan M, Begemann MJ, Heringa SM, et al. Exercise improves clinical symptoms, quality of life, global functioning, and depression in schizophrenia: a systematic review and meta-analysis. Schizophr. Bull 42(3), 588-599 (2016).
- Freedman R, Adler LE, Myles-Worsley M, et al. Inhibitory gating of an evoked response to repeated auditory stimuli in schizophrenic and normal subjects. Human recordings, computer simulation, and an animal model. Arch. Gen. Psychiatry 53(12), 1114-1121 (1996).
- Jerger K, Biggins C, Fein G. P50 suppression is not affected by attentional manipulations. Biol. Psychiatry 31(4), 365-377 (1992).
- Boutros NN, Korzyukov O, Jansen B, et al. Sensory gating deficits during the mid-latency phase of information processing in medicated schizophrenia patients. Psychiatry. Res 126(3), 203-215 (2004).
- Jones LA, Hills PJ, Dick KM, et al. Cognitive mechanisms associated with auditory sensory gating. Brain. Cogn 102, 33-45 (2016).
- Braff DL, Light GA. Preattentional and attentional cognitive deficits as targets for treating schizophrenia. Psychopharmacology 174(1), 75-85 (2004).
- Grunwald T, Boutros NN, Pezer N, et al. Neuronal substrates of sensory gating within the human brain. Biol. Psychiatry 53(6), 511-519 (2003).
- Chatani H, Hagiwara K, Hironaga N, et al. Neuromagnetic evidence for hippocampal modulation of auditory processing. Neuroimage 124(), 256-266 (2016).
- Tregellas JR, Davalos DB, Rojas DC, et al. Increased hemodynamic response in the hippocampus, thalamus and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophr. Res 92(1-3), 262-272 (2007).
- Potter D, Summerfelt A, Gold J, et al. Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophr. Bull 32(4), 692-700 (2006).
- Brockhaus-Dumke A, Schultze-Lutter F, Mueller R, et al. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol. Psychiatry 64(5), 376-384 (2008).
- Yadon CA, Bugg JM, Kisley MA, et al. P50 sensory gating is related to performance on select tasks of cognitive inhibition. Cogn. Affect. Behav. Neurosci 9(4), 448-458 (2009).
- Firth J, Stubbs B, Rosenbaum S, et al. Aerobic Exercise Improves Cognitive Functioning in People With Schizophrenia: A Systematic Review and Meta-Analysis. Schizophr. Bull 43(3), 546-556 (2017).
- Ploughman M. Exercise is brain food: the effects of physical activity on cognitive function. Dev. Neurorehabil 11(3), 236-240 (2008).
- Malchow B, Reich-Erkelenz D, Oertel-Knöchel V, et al. The effects of physical exercise in schizophrenia and affective disorders. Eur. Arch. Psychiatry. Clin. Neurosci 263(6), 451-467 (2013).
- Nithianantharajah J, Hannan AJ. The neurobiology of brain and cognitive reserve: mental and physical activity as modulators of brain disorders. Prog. Neurobiol 89(4), 369-382 (2009).