Review Article - Neuropsychiatry (2018) Volume 8, Issue 1
Effect and Disease Indicative Role of Inflammation in Neurodegenerative Pathology: A Mechanistic Crosstalk of Promise and Dilemma
- *Corresponding Author:
- Amarendranath Choudhury, PhD
Jaipal Homes, Hightension Road, Kondapur
Hyderabad-500084, India & Alumnus
Department of Life Science and Bioinformatics
Assam University, Silchar-788011, India
Tel: +91-7003017920
Abstract
The pathobiology of neuronal cell loss due to various endogenous or exogenous influences is clinically termed as neurodegeneration. Neurodegeneration has been reported to be the major contributor to aging and central nervous system diseases. Apart from aging and endogenous involvement, neurodegeneration also has been reported from viral infection and prion diseases. Studies have shown that, chronic degeneration of neuronal cells initiate the pathology of Alzheimer’s disease-the most prevalent neurodegenerative disorder in the world. Similar neurodegenerative pathology is also evident in Parkinson’s disease, multiple sclerosis, and Amyotrophic Lateral Sclerosis.Neurodegeneration negatively affects the mental and physical functioning of the patient. Intriguingly, the involvement of inflammation has been linked as the most crucial entity in the mechanistic progress of neurodegeneration. Moreover, recent data also have shown that inflammatory biomarkers can prognosticate the silent progress of neurodegeneration through low-cost diagnostic approach. Mainly, Th17 and MDSCs are the particular immune cells, which have been reported to assist adequately to get a detailed insight into the underlying pathological process in neurodegeneration. Similarly, depression and dementia are also having a crucial association with pro-inflammatory cytokines, which in chronic spectrum indicates the degenerative pathology. Together, available literatures are depicting a direct association between neuroinflammation and neurodegeneration. In the present review, we have summed up all the neuropathologies in light of inflammation and emphasized the possible diagnostic measures by using inflammatory cells and mediators as biomarkers for neurodegenerative diseases.
Keywords
Neuroinflammation, Neurodegeneration, Inflammatory mediators, MDSC, Th17
Abbreviations
AD: Alzheimer’s Disease; PD: Parkinson’s Disease; MS: Multiple Sclerosis; ALS: Amyotrophic Lateral Sclerosis; HD: Huntington’s Disease; HTT: Huntingtin Gene; BBB: Blood Brain Barrier; CNS Central Nervous System; SVD: Cerebral Small Vessel Disease; CRP: C‑Reactive Protein; IL‑6: Interleukin-6; IL-4: Interleukin-4; IL-1β: Interleukin-1 Beta; DM: Diabetes Mellitus; CVD: Cardiovascular Disorders; MDD: Major Depressive Disorder; CCR5+: CC Chemokine Receptor 5; HIV: Human Immunodeficiency Virus; LPS: Lipopolysaccharide; Aβ: Amyloid-Beta Peptides; SPs: Senile Plaques; RAGE: Receptor For Advanced Glycoxidation End-Products; NLR: Nod Like Receptor; NALP3: NACHT, LRR And PYD Domains-Containing Protein 3; MHC: Major Histocompatibility Complex; Cox-2: Cyclooxygenase-2; MCP-1: Monocyte Chemoattractant Protein 1; TNF- α: Tumor Necrosis Factor-α; ApoE4: Apolipoprotein E4; TREM2: Triggering Receptors Expressed On Myeloid Cells 2; PET: Positron Emission Tomography; MPTP: 1-Methyl-4-Phenyl- 1,2,3,6-Tetrahydropyridine; SNpc: Substantia Nigra Par Compacta; ATP: Adenosine Triphosphate; PTEN: Phosphatase And Tensin Homolog; PARK 7: Parkinson Disease Protein 7; IFN- γ: Interferon- γ; IGF-1: Insulin Like Growth Factors 1; SOD1: Superoxide Dismutase 1; TDP43: TAR DNA-Binding Protein 43; GJ: Gap Japs; MBP: Myelin Basic Protein; HBV: Hepatitis B Virus; TGF-β: Transforming Growth Factor-β; RORγt: Retinoic Acid Receptors-Related Orphan Receptor γt; Th17: T Helper 17; IFN- γ: Interferon Gamma; MDSCs: Myeloid-Derived Suppressor Cells
Introduction
Neuronal cell loss has been reported to be the major cause underlying behavioural and psychological abnormalities [1-3]. It has been shown that, neurodegeneration is more frequent in aged persons but, the pathological incidences of neurodegeneration can occur regardless of age barrier [4]. Despite rigorous research and study, the actual cause and underlying molecular mechanisms of neurodegeneration seems to be equivocal. Globally, Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), Amyotrophic lateral sclerosis (ALS) are among most common pathological outcomes of neurodegeneration [5-8]. Besides age and endogenous influences, neurodegeneration also have been shown to be caused by viral or parasitic infections [9]. However, the scenario of neurodegeneration differs from disease to disease depending upon the mode of infection or brain tissue damage. Blood brain barrier (BBB) disruption and destruction of myelin sheath is a common feature of MS, where such anomalies results in axon terminal damage, gliosis, lymphocyte infiltration and chronic inflammation [10]. The pathological alteration in MS precipitates into physiological anomalies like neuropathic pain, paralysis, muscle spasms and optic neuritis [11]. The pathobiology of MS indicates a clear influence of inflammation; the same is also evident in any viral infection or immune‑mediated disorders that affect central nervous system (CNS) [10,12]. It is notable that, such inflammatory responses play a crucial role in health and disorders of human beings regardless of age and regulate the well-being of life support systems. The basic function of inflammatory cells like microglia is to supervise the ambient microenvironment containing glia and neurons [13]. Whenever there is any questionable particle or damage in CNS, inflammatory responses starts to communicate the supporting system to control the damage and initiate the tissue repair mechanisms [14,15]. Unfortunately, inflammatory mechanisms also promote the production of toxic entities, which not only amplify the disease tenure but also drag the fate of life support system into grave danger [16,17]. Similar mechanistic failure has been reported in neurodegenerative disorders, where inflammatory responses in chronic spectrum initiates abnormal production of inflammatory mediators like cytokines and interleukins [18,19]. AD, PD, MS, ALS and other psychological anomalies have been frequently reported with inflammatory dysregulation, which further has mechanistically been linked with proteopathy, mitochondrial dysfunction, aberrant cellular transport and cell death [20-23]. However, it is noteworthy that, chronic spectrum of inflammation with or without other disease risk factors (like oxidative stress, infection, cellular damage, necrosis) can drastically alter the fate of neuronal environment and even can cause neuronal cell loss [24,25]. Inflammation alone is less potent for neurodegeneration in AD, MS, PD and ALS. Moreover, peripheral expressions of inflammatory cells and mediators have been found to be a potential diagnostic tool for the neurodegeneration [26]. Together, role of inflammation in neurodegenerative pathology is truly dual-faced [26-28]. In one hand, it is influencing the degenerative pathology; on the other hand, it promises to be a biomarker to indicate the status of neurodegeneration and assists to prognosticate the therapeutic measures. Such cross-talk could enrich the therapeutic knowledge for neurodegenerative diseases. Research and study on the particular role of inflammation in neurodegenerative disease pathology is the urgent need of time. Keeping such rationale in mind, present review intended to present all the putative roles of inflammation in CNS disorders and discussed the possible disease indicative potency for future therapeutics.
Neuroinflammation and Neurodegenerative Disease Pathologies
▪ Atherosclerosis, dementia and lifestyle disorders
Atherosclerosis is a major factor in several CNS and stroke characterised diseases where vascular inflammation is witnessed as a result of rise in IL-6 levels accompanied by the movement of monocytes into damaged vascular wall following subsequent intracranial large artery stenosis progression [29]. The C-reactive protein (CRP) serves as an inflammation marker in cardiovascular diseases (CVD) predicting hemorrhage progression and atherosclerosis in the event of a stroke. In case of cardiovascular diseases, diabetes mellitus, and stroke, lowgrade inflammation causes severe pathological complications [30]. In such diseases, the inflammation caused, is attributed to adipose tissue dysfunction as seen in case of hypertension and obesity [29-32]. Micro-vascular, macrovascular, and sensory neuropathic complications at times lead to fatal consequences. Warning signs like foot ulcers are observed during a heart attack. Unhealthy food habits often lead to disorders like CVD, hypertension, stroke, metabolic syndromes, and a series of related diseases characterised by mataflammation [33].
Hypertension, depression, diabetes, dementia and cerebral strokes are notable among common lifestyle disorders, which are found to be associated with inflammation at cellular and molecular levels [34,35]. Rather than exhibiting symptoms, neuroinflammation has been reported to act silently through a distinct mechanism, believed to be associated with the development of dementia along with functional impairment in older people [36]. Risk of stroke escalates on account of oligodendrocyte death accompanied by constant myelinated fiber degeneration; a phenomenon caused due to inflammation in the CNS which induces cerebral small vessel disorder (SVD) or better known as vascular dementia [37].
▪ Depression
The aging process in living beings is associated with increased expression levels of inflammatory factors similar to pro-inflammatory cytokines [38]. It is observed initially as a chronic activation of both perivascular and parenchymal microglia that produce these pro-inflammatory cytokines alongside an increased astrocytes number in brain. Such pro-inflammatory signals produced by these cytokines increases vulnerability towards quite a few neuropsychiatric diseases [15]. Experimental studies revealed elevated levels of pro-inflammatory markers like adipokines, IL-6, and CRP in overweight females which correlated to symptoms of depression and anxiety [39]. Following surgical removal of adipose tissue from the body, anxiety is observed accompanied by reduced inflammation. Patients suffering from major depressive behaviour show greater susceptibility towards diseases such as depression, cognitive dysfunction and dementia; moreover, immune, cardiovascular, cerebrovascular, and neuroendocrine systems are affected alongside the occurrence of metabolic diseases like hypertension, obesity, ageing, etc. [40,41]. Conventional antidepressant therapy not only fails to show therapeutic effects in depression but also results in treatment resistance. Reports in this regard, consider elevated IL-6 and IL-8 levels, endothelial nitric oxide synthase uncoupling, hyperglutamatergia, and oxidative stress as the primary mechanism behind inflammation. Major depressive disorders (MDD), is a severe neurological disorder associated with elevated levels of inflammatory markers like chemokines, cytokines and acute phase proteins alongside neurovascular dysfunction [14,34,36].
▪ Infections
On several occasions dynamic inflammatory responses are observed in CNS, viral and prion infections are crucial among such kinds. Soon after the CNS is invaded by virus, two hypothetical mechanisms are believed to activate which are, neuronal retrograde dissemination and hematogenous dissemination, through which pathogen reach the brain by traversing the BBB [42,43]. Virus can replicate in macrophage and CC chemokine receptor 5 (CCR5+) T cells in the CNS region during developmental stages and assists in the progression of dementia [44]. As evident in case of human immunodeficiency virus (HIV), the protein gp120 and Tat has been reported to induce neuronal death by enhancing CXCR4-PKC, thereby causing neuronal dysfunction through interruption of miRNA expression [45]. HIV infection and other viral disorders are associated with rise in cytokines, cholesterol, and lipopolysaccharide (LPS) levels. The same infection is also found to be cooccurring in insulin resistance and testosterone deficiency related pathobiology [46], which are also having influential role in inflammation in the CNS [47]. Inflammatory responses trigger tissue damage and are believed to be associated with different age related diseases [38].
▪ Alzheimer’s disease
AD is characterised by symptoms like impaired cognition, behavioral and neuropsychiatric disturbances and memory loss. For over two decades, structural changes in AD have attracted attention throughout the globe [19,48]. Amyloid β (Aβ) and Tau proteins found to exist as clusters in AD neurons, which is regarded as the hallmark pathological features for AD [15]. An important hypothesis in this regard is the ‘amyloid hypothesis’ which was framed after observing the aggregates of Aβ fragments in ‘senile plaques’ (SPs) of AD brain [49]. The hypothesis postulates that accumulation of Aβ in brain initiates a neurochemical cascade; thereby, adversely affecting the neuronal and synaptic functions and resulting into cognitive impairment [49]. Aβ activates microglia, which in turn provokes the production of inflammatory mediators, involved in neuronal death [50]. Microglial activation triggers the receptor for advanced glycoxidation end-products (RAGE), which is responsible for sensing Aβ [51]. In patients with type II diabetes, increased RAGE activation is associated with greater risk of AD via activation of glial cells, which mainly mediates through Nod like receptor (NLR) family. These are expressed when Aβ oligomers and fibrils induces lysosomal damage via NACHT, LRR and PYD domainscontaining protein 3 (NALP3). The NLR proteins are also a key component of signaling platforms, which detects inflammation together with participation in caspase1 dependent activation of the pro-inflammatory cytokines (IL-1β and IL-18) [52]. Microglial cells act on the region around senile plaques, causes increased in size, rapid multiplication and motility [19]. Activation of pro-inflammatory markers like major histocompatibility complex (MHC) class II, IL-1β, IL-6, Cyclooxygenase-2 (Cox-2), Monocyte chemoattractant protein 1 (MCP- 1), and tumor necrosis factor-α (TNF-α) [53]. Many a times, onset of AD is random or late due to innate immunity caused by Apolipoprotein E4 (ApoE4). Additionally, ApoE4 is also a risk factor for AD as it triggers triggering receptors expressed on myeloid cells 2 (TREM2) genes. Phytochemicals with activities similar to ApoE have shown therapeutic effects on experimental models of neurodegenerative diseases [54-59].
▪ Parkinson’s disease
Among neurodegenerative disorders, PD is one of the most predominant motor-neurodegeneration with a prevalence rate of 0.13% (globally) above 65 years of age [60]. PD causes a number of movement abnormalities and shows visible symptoms which accounts both behavioural and psychiatric. Besides, PD is also associated with autonomic function impairment due to degradation of nigrostriatal connections, which is an important component of PD pathology [61,62]. PD development is characterised on the extent of damage caused to the dopamergic neurons of the substantia nigra, which occur through the intricate interactions between external factors and genetic susceptibility [63]. Risk of PD is linked to the increase of systemic inflammatory molecules in the midbrain region activating microglia and killing dopaminergic neurons [64]. It is now known through positron emission tomography (PET) and neuropathology studies that neurovascular dysfunction and increase in permeability of BBB are the main causes for PD risk. Inflammatory response for this disease can be seen through activation of peripheral lymphocytes or rise in serum cytokine (TNF-α, IL-2, and IL-6) levels [65,66]. In a murine PD model, adaptive immune response was observed, as increase in MHC-II in ventral mid brain microglia and astrocytes along with inflammatory response to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [67]. Experimental evidence has shown that, Aquaporin-4 (AQP4) deficiency alters the expression of nuclear factor kB (NF-kB) level and related gliosis. Basically, AQP4 controls the trend of neuroinflammation through release of pro-inflammatory cytokines and Adenosine triphosphate (ATP) through astrocytes instead of microglia activation [68]. Reports also depict that, inflammatory mediators are responsible for localised damage particularly in substantia nigra par compacta (SNpc). Neurons in this region operate under high oxygen conditions resulting in reduced antioxidant glutathione and therefore are more prone to oxidative damage [69]. What follows reactive oxygen species (ROS) generation by microglia, instantly activates upon oxidative stress [6]. Hereditary PD and Phosphatase and tensin homolog (PTEN) is associated with mutation in DJ-1 or Parkinson disease protein 7 (PARK 7), loss of function of which is evident in PD patients. Mutation in DJ-1 impairs antioxidant response, which further reported to be associated with microglia activation in rodent model of PD [70]. Microglia activation can be triggered by a number of factors which include damage signals arising from toxins, endogenous protein products, by-products from neuronal death, and pathogens. Genomic studies report several pro-inflammatory factors such as IL-1β, TNF-α, IL-2, IL-6 and interferon-γ (IFN-γ) that are expressed in PD patients due to such mechanism of action [65,69]. It is also reported that, pathologically altered form of α-synuclein activates microglia and hampers the clearance of α-synuclein, which ultimately results into inflammation-mediated neurodegeneration, neuronal dysfunction, and phagocytosis [71,72]. This process is influenced by pro-inflammatory cytokines produced by microglia triggering oxidative stress [6].
▪ Amyotrophic lateral sclerosis
ALS is a collective degeneration of motor neurons in the brain cortex, motor cortex, and lower motor neurons in the spine [73]. Other symptoms of ALS include dysphagia, speech difficulties, and weakness in hands and/or legs [74]. Studies conducted on both animal and human subjects confirm that ALS pathogenesis is attributed to microglia [75]. Inflammation basically, serves as a key factor in course of the disease with effective involvement at two different stages i.e., initially in a protective mode, later causing neurotoxicity [76]. Experimental data from lumbar spinal cord SOD1G93A mice was compared with end stage SOD1G93A mice microglia and the result showed loss of motor neurons in end group, which revealed the direct influence of inflammation in ALS pathology [77,78]. Similar experiment also proved the involvement of interleukin-4 (IL-4) along with insulin like growth factors 1 (IGF- 1), which considerably increases microglia expression [77,78]. In advanced stages of ALS, pro-inflammatory properties are observed, as rise in interleukin-1 beta (IL-1β) and tumor necrosis factor α (TNF-α) in spinal cord [76]. ALS autopsy reports have shown the extent of microgliosis, which mainly predominant in spinal cord. PET imaging study also established the similar result of microglia activation [79]. In vivo studies found microgliosis in different motor regions of the brain and dorsolateral perfrontal cortex and thalamus [80]. Recent finding in transgenic mice model showed aggregation and mislocalisation of toxic protein aggregates in motor neurons, which triggers microglia and astrocytes mediated inflammatory processes [76]. Together, inflammatory process through LPS and TNF-α and other inflammatory mediators provide a direct link between microglia activation, neuroinflammation and ALS pathology [76]. Gap japs (GJ) play crucial role in the event of neuroinflammation. GJs are providing intercellular channels connecting cytoplasmic compartment of nearby cells letting passage of small molecules and ions upto ~1000Da [81]. GJ contains six subunits protein complex called connexins, which after mutation or during inflammation abnormally regulates hemi-channel function [82]. Moreover, chronic damage signals result in microglial activation, after which constant release of cytotoxic factors promoting neurodegeneration in motor neurons of hypothalamus subsequently leading to development of AD, ALS and PD [28,83].
▪ Huntington’s disease
Huntington’s disease (HD) is an inherited autosomal dominant neurodegenerative disorder characterised by onset of motor dysfunctions, psychiatric issues along with intellectual weakening in late adulthood [84]. Post-mortem brain tissues have revealed that, microglial activation is evident in HD brain, which profoundly accounts striatum, globus pallidus, and cortex region [84]. It is also reported that, quantitative increase in activated microglia is correlated with the neurodegeneration in HD [85]. Interestingly, similar pathobiology of HD due to microglial activation is also reproducible in rodent model of HD [86]. Besides microglial activation, HD pathology is also reported with increased activity of astrocytes, which is crucial for the generation of inflammatory mediators like IL-6, IL-8, TNF-α, MCP-1, CCL2, and IL-10 [87,88]. However, whether the actual role of inflammation covers the HD pathology actively or reactively is a matter of further experimentation and discussion.
▪Multiple sclerosis
MS is an autoimmune disorder characterized by demyelination, inflammation, and axon degeneration in the CNS. Most prevalent form of MS is the relapsing-remitting MS wherein the disease alternates between phases of inflammation, demyelination, and remission [10,12,89]. The inflammatory response in MS is caused, once T and B lymphocytes recognise the myelin basic protein (MBP) present in the myelin sheath in neurons as an auto-antigen. The perivascular region of CNS is prone to movement by antibodies and lymphocytes. These are also observed in demyelinated lesions suggesting inflammatory response [14,90]. In relapsing-remitting MS, despite remyelination take place during remission; the process remains compromised and causing severe neurodegeneration [91,92]. Among exogenous factors, viruses and bacteria are believed to be one of the most possible initiators of MS. These initiators function through molecular mimicry otherwise as self-antigens [93,94]. This is evident in case of hepatitis B virus (HBV) which encodes a protein resembling MBP in terms of epitope structure. In response to HBV, T-cells are immediately activated and cross react with the MBP present in major histocompatibility complex available on the surface of antigen presenting cells (APCs). It is indeed the cytokines secreted out of APC that triggers differentiation of T cells into effector cells [95]. Together with astrocytes, APC express toll-like receptors (TLRs) that are involved in MS. Even though the participation of TLRs in MS has long been established, the nature of TLR signaling is still unclear, whether it is disease promoting or protective [10]. However, reports indicates that, owing to transforming growth factor-β (TGF-β) and IL-6 secretion, naïve T-cells differentiate into retinoic acid receptors-related orphan receptor γt (RORγt) expressing T helper 17 (Th17) cells, which are having immense importance in MS pathogenesis [96].
Neuroinflammation and Neurodegeneration: A Tale of Promise and Dilemma
CNS remains under high surveillance and protection through innate and acquired immune responses. Both of these remain closely regulated by endogenous and exogenous influences [97]. In neuroinflammation perspective, involvement of leukocyte movement from surrounding regions into the CNS has been reported to be the crucial in the pathobiology of neurodegeneration [98,99]. Infiltration of leukocytes results in a strong inflammatory response in the peripheral organs during pathogenic invasion or systemic LPS exposure [100,101]. As a result, microglial activation has been reported. Furthermore, proinflammatory mediators have been reported to release following such activation of microglia making BBB permeable [102,103]. This results in transport of peripheral leukocytes like T-cells and macrophages into the CNS. Invaded lymphocytes and macrophages share quite a few functional similarities with microglia like TLR expression, activation through aggregated proteins or pathogens, MHC class II expression, controlling T-cell phenotypes, and polarising functional phenotypes headed for an inflammatory M1 or anti-inflammatory M2 state. Thereby, influencing both inflammatory as well as lymphocyte regulatory T-cells to spread the range of inflammation throughout systemic and CNS regions [104-106]. Alteration of CD4+ and CD8+ T-cells are detected in subjects suffering from neurodegenerative disorders suggesting involvement of T-cells in such diseases apart from posing a persistent antigenic contest [10,107-109]. Presence of antibodies alongside neuronal antigen confirms role of immunity in neurodegenerative disorders [110]. An acute neuroinflammatory response is therefore considered helpful in reducing risk of damage as it activates our innate immunity. Conversely, chronic inflammation involves standby activation of microglia preventing release of inflammatory mediators causing increase oxidative stress together with nitrosative stress endless extending the inflammation cycle, which adversely affects neurodegenerative diseases [111- 113]. It has been reported that, T-cells infiltrate CNS and regulate the functions of neurons and glial cell population. By doing so, T-cell marks its association in innate immune response during an inflammation or infection [114,115]. Similar mechanism with microglia activation along with existing cellular stress and chronic inflammation has significant role in the pathophysiology of neurodegenerative diseases [8,114]. In AD and ALS patients, abnormal phosphorylation of proteins has been observed and intriguingly, IL-1 positive microglia also found in such pathologies, which confers the putative role of inflammatory mediators in neurodegenerative pathology [116,117]. Moreover, it’s been always consistent that, neuroinflammation accompanied with oxidative stress exerts detrimental consequence in chronic neurodegenerative disease profile. Neurotoxic species such as tumor necrosis-α (TNF-α), glutamate, ROS and NOS play crucial role in the manifestation of such action [25].
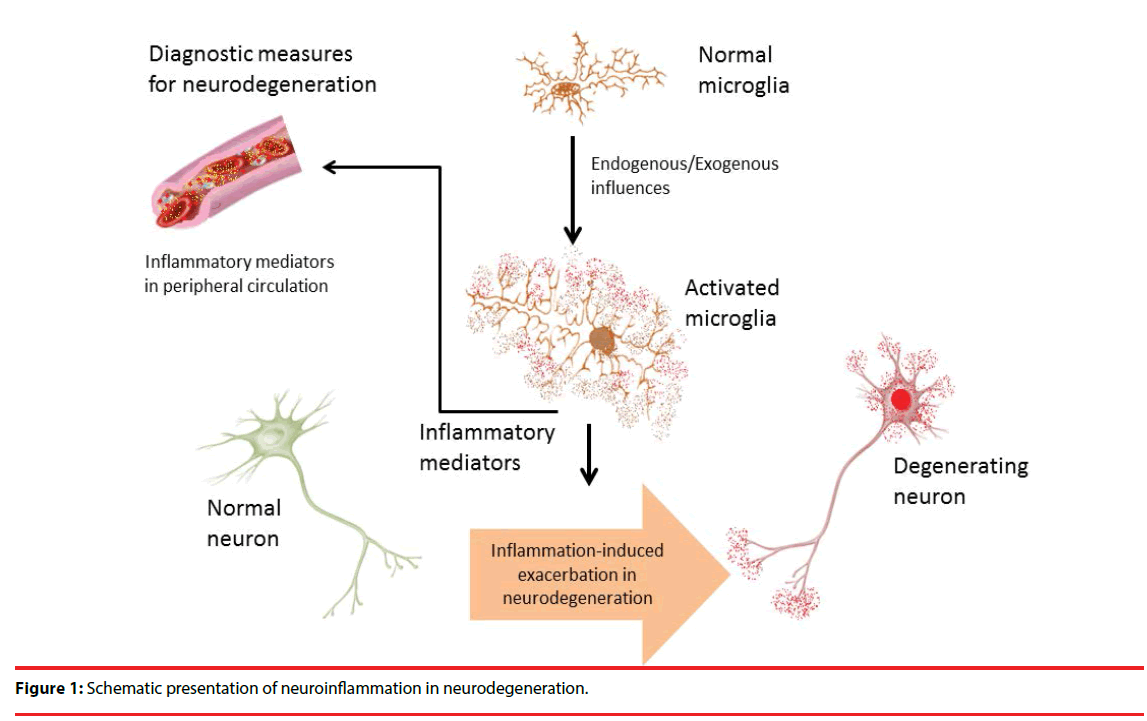
With respect to the promising attributes of inflammation as disease indicator; several studies have been conducted which opens up a new avenue of therapeutics for neurodegenerative disorders [118]. In PD, it has been reported that, inflammation plays a crucial role in neurodegenerative pathologies by releasing interferon gamma (IFN-γ), interleukin family molecules, and cytokines in the dopaminergic center, which are also found in higher levels during disease condition [64,119]. Among various inflammatory mediators and cells Th17 and myeloid-derived suppressor cells (MDSCs) are notable in this regard [26]. Studies have shown that, Th17 cells infiltrate the region causing uncontrolled microglial activation thereby resulting in neurodegeneration [120,121]. Available reports have shown that, BBB dysfunction is the main reason behind such increased levels of inflammatory mediators in peripheral circulation [103]. Moreover, Th17 also capable of influencing the secretion of IL-17, which in turn assists increased secretion of other detrimental inflammatory factors like tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1) [122-124]. In this way Th17 spreads the inflammatory responses promptly throughout the brain by activating microglial population [116]. On the other hand, expression of MDSCs is known to inhibit inflammation, which is quite opposite to the function of Th17 [125- 127]. Though, maturation and transformation of Th17 cells are highly regulated by MDSCs, the overall increase in there peripheral levels are the determining criteria for the diagnosis of chronic inflammation [128]. Moreover, recent evidences are indicating that, MDSCs are also associated with increased inflammation by transforming CD4+ T lymphocytes into Th17 cells, which in turn increase the inflammation and promotes progressive neurodegeneration [128-130]. The development and occurrence of PD have shown a positive correlation with Th17 and MDSCs; however, the actual mechanism of action remains unknown [21]. Literature sources nevertheless confirm its involvement in early disease developmental stages; therefore, these could be considered as useful biomarkers for neurodegeneration [31,131]. More research are need to be done to get a clear insight into the mechanisms of neurodegeneration and such inflammatory mediator quantification from cerebrospinal fluid could provide better understanding on the neurodegenerative progress in various neurological diseases (Figure 1).
Conclusion
Neuroinflammation and neurodegeneration are mechanistically inseparable and exhibiting profound effects in the health and well-being of subjects. Particularly in neurodegeneration, abnormalities of the immune response components have been reported frequently. As a result inflammatory mediator molecules and cells are found in elevated levels. Due to disruption of BBB, such mediators traverse the limiting barriers and reaching the peripheral circulation. It is also reported that, both the innate and acquired immune components along with inflammatory mediators from glial cells serve as sensors for brain tissue. Therefore, peripheral quantification of such immune components could provide an insight into the actual disease scenario in case of neurodegeneration. However, specific inflammatory mediator identification, correlative analysis and region specific quantification strategies could promise for a novel diagnostic tool for neurodegenerative pathologies. Together, neuroinflammation is having a potential to enrich the diagnostic manifestation for neurodegeneration, besides having its putative role in neurological anomalies.
Acknowledgement
We sincerely acknowledge the continuous support and co-operation provided by Amit Kumar Banerjee.
References
- Zhang Y, Zhang C, Cheng Z, et al. Serum levels of brain-derived neurotrophic factor and clinical efficacy of mirtazapine in geriatric patients with major depression. Biomed. Res 26(2), 338-342 (2015).
- Svensson M, Lexell J, Deierborg T. Effects of Physical Exercise on Neuroinflammation, Neuroplasticity, Neurodegeneration, and Behavior. Neurorehabil. Neural. Repair 29(6), 577-589 (2015).
- Postuma RB, Gagnon J-F, Montplaisir JY. REM sleep behavior disorder: From dreams to neurodegeneration. Neurobiol. Dis 46(3), 553-558 (2012).
- McCray BA, Taylor JP. The role of autophagy in age-related neurodegeneration. NeuroSignals 16(1), 75-84 (2007).
- Nikam S, Nikam P AS. Role of free radical and antioxidant imbalance in pathogenesis of Parkinson’s disease. Biomed. Res 20(1), 55-58 (2009).
- VD R. The molecular crosstalk: oxidative stress, genomic instability and neurodegenerative disorders. Biomed. Res. Ind 28(14), 6446-6451 (2017).
- Li X-L, Hu N, Tan M-S, et al. Behavioral and Psychological Symptoms in Alzheimer’s Disease. Biomed Res. Int 2014, 1-9 (2014).
- Cai D. Neuroinflammation and neurodegeneration in overnutrition- induced diseases. Trends. Endocrinol. Metab 24(1), 40-47 (2013).
- De Chiara G, Marcocci ME, Sgarbanti R, et al. Infectious agents and neurodegeneration. Mol. Neurobiol 46(3), 614-638 (2012).
- Frischer JM, Bramow S, Dal-Bianco A, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 132(5), 1175-1189 (2009).
- Compston A, Coles A. Multiple sclerosis.Lancet 1221-1231 (2002).
- Lassmann H. Multiple sclerosis: Is there neurodegeneration independent from inflammation? J. Neurol. Sci 259(1-2), 3-6 (2007).
- Aloisi F. Immune function of microglia. Glia36(2), 165–179 (2001).
- Meinl E, Krumbholz M, Derfuss T, et al. Compartmentalization of inflammation in the CNS: A major mechanism driving progressive multiple sclerosis. J. Neurol. Sci 274(1–2), 42–44 (2008).
- Graeber MB, Li W, Rodriguez ML. Role of microglia in CNS inflammation. FEBS. Lett 585(23), 3798-3805 (2011).
- Ek M, Engblom D, Saha S, et al. Inflammatory response: pathway across the blood-brain barrier. Nature 410(1), 430–431 (2001).
- Skaper SD, Facci L, Leon A. Inflammatory Mediator Stimulation of Astrocytes and Meningeal Fibroblasts Induces Neuronal Degeneration via the Nitridergic Pathway. J. Neurochem 64(1), 266-276 (1995).
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci 2(10), 734–744 (2001).
- Weisman D, Hakimian E, Ho GJ. Interleukins, Inflammation, and Mechanisms of Alzheimer’s Disease. Vitam. Horm 74, 505–530 (2006).
- IJ M. Autoimmune disease and mitochondrial dysfunction in chronic diseases. Res. Chron. Dis 1(11–12) (2017).
- Urrutia PJ, Mena NP, Núñez MT. The interplay between iron accumulation, mitochondrial dysfunction, and inflammation during the execution step of neurodegenerative disorders. Front.Pharmacol (2014).
- Cho D-H, Nakamura T, Lipton SA. Mitochondrial dynamics in cell death and neurodegeneration. Cell. Mol. Life Sci 67(20), 3435-3447 (2010).
- Cassarino DS, Bennett JP. An evaluation of the role of mitochondria in neurodegenerative diseases: Mitochondrial mutations and oxidative pathology, protective nuclear responses, and cell death in neurodegeneration. Brain Res. Rev 29(1), 1-25 (1999).
- Gandhi S, Abramov AY. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longev (2012).
- Fischer R, Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: Role of TNF. Oxid. Med. Cell. Longev (2015).
- Yang L, Guo C, Zhu J, et al. Increased Levels of Pro-Inflammatory and Anti-Inflammatory Cellular Responses in Parkinson’s Disease Patients: Search for a Disease Indicator. Med. Sci. Monit 23(6), 2972-2978 (2017).
- Martins IJ. Biomarker Tests and Ageing Science. Ageing Sci Ment Heal. Stud 1(1), 1-2 (2017).
- Cunningham C. Microglia and neurodegeneration: The role of systemic inflammation. Glia 61(1), 71-90 (2013).
- Libby P. Inflammation in atherosclerosis.Nature 420(6917), 868–874 (2002).
- Matas AJ, Gillingham KJ, Payne WD, et al. Dissociation of pentameric to monomeric C-reactive protein on activated platelets localizes inflammation to atherosclerotic plaques. Circulation 366(8), 857-859 (2014).
- Martins IJ. Defective Interplay between Adipose Tissue and Immune System Induces Non Alcoholic Fatty Liver Disease.Updat. Nutr. Disord. Ther 1(1), 1-4 (2017).
- Tsai S, Clemente-Casares X, Revelo XS, Winer S, Winer DA. Are obesity-related insulin resistance and type 2 diabetes autoimmune diseases? Diabetes 64(6), 1886-1897 (2015).
- Nishide K, Nagase T, Oba M, et al. Ultrasonographic and thermographic screening for latent inflammation in diabetic foot callus. Diabetes Res. Clin. Pract 85(3), 304- 309 (2009).
- Lopresti AL, Hood SD, Drummond PD. A review of lifestyle factors that contribute to important pathways associated with major depression: Diet, sleep and exercise. J. Affect. Disord 148(1), 12-27 (2013).
- Kolb H, Mandrup-Poulsen T. The global diabetes epidemic as a consequence of lifestyle-induced low-grade inflammation. Diabetologia 53(1), 10-20 (2010).
- Leonard BE. Inflammation, depression and dementia: Are they connected? Neurochem. Res 32(10), 1749-1756 (2007).
- O’Brien JT, Thomas A. Vascular dementia.Lancet 386(10004), 1698-1706 (2015).
- Woods JA, Wilund KR, Martin SA, Kistler BM. Exercise, inflammation and aging. Aging Dis 3(1), 130-140 (2012).
- Toker S, Shirom A, Shapira I, et al. The Association Between Burnout, Depression, Anxiety, and Inflammation Biomarkers: C-Reactive Protein and Fibrinogen in Men and Women. J. Occup. Health Psychol 10(4), 344- 362 (2005).
- Vogelzangs N, Beekman ATF, De Jonge P, et al. Anxiety disorders and inflammation in a large adult cohort. Transl. Psychiatry 3(1), (2013).
- Salim S, Chugh G, Asghar M. Inflammation in anxiety. Adv. Protein Chem. Struct. Biol 88(1), 1-25 (2012).
- Wickersham IR, Finke S, Conzelmann KK, et al. Retrograde neuronal tracing with a deletion- mutant rabies virus. Nat. Methods 4(1), 47-49 (2007).
- Aktas O, Ullrich O, Infante-Duarte C, et al. Neuronal damage in brain inflammation. Arch. Neurol 64(2), 185-189 (2007).
- Griffin DE. Cytokines in the brain during viral infection: Clues to HIV-associated dementia. J. Clin. Invest 100(12), 2948-2951 (1997).
- Sullivan CS, Ganem D. MicroRNAs and viral infection. Mol. Cell 20(1), 3-7 (2005).
- Samaras K, Gan SK, Peake PW, et al. Proinflammatory markers, insulin sensitivity, and cardiometabolic risk factors in treated HIV infection. Obesity 17(1), 53-59 (2009).
- de la Monte SM, Longato L, Tong M, et al. Insulin resistance and neurodegeneration: roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr. Opin.Investig. Drugs 10(10), 1049-1060 (2009).
- Ballard C, Gauthier S, Corbett A, et al. Alzheimer’s disease. Lancet 377(9770), 1019- 1031 (2011).
- Hardy J, Higgins G. Alzheimer’s disease: the amyloid cascade hypothesis. Science 256(5054), 184–185 (1992).
- Karran E, Mercken M, Strooper B De. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov 10(9), 698- 712 (2011).
- Srikanth V, Maczurek A, Phan T, et al. Advanced glycation endproducts and their receptor RAGE in Alzheimer’s disease. Neurobiol. Aging 32(5), 763-777 (2011).
- Fitzgerald KA. NLR-containing inflammasomes: Central mediators of host defense and inflammation. Eur. J. Immunol 40(3), 595-598 (2010).
- Bauer ME, De la Fuente M. Oxidative Stress, Inflammaging, and Immunosenescence. Inflammation, Advancing Age and Nutrition: Research and Clinical Interventions 39–47 (2013).
- Lee CH, Huang GC. Bioactive compounds from natural product extracts in Taiwan cosmeceuticals-Mini review. Biomed. Res 28(15), 6561-6566 (2017).
- Hui ZG, Zhou XW, Li RJ, et al. Studies on the extraction process of total flavonoids in Radix puerariae and their hypoglycemic effect in mice. Biomed. Res 26(1), 51-54 (2015).
- Zhang W LS. Study on the determination of lactone contents in Ginkgo biloba leaves and their effects in schizophrenia. Biomed. Res 26(1), 31-36 (2015).
- Sase T, Arito M, Onodera H, et al. Effects of edaravone on hypoxic human astrocytes revealed by a proteomic approach. Biomed. Res 27(4), 1064-1070 (2016).
- Lee CH, Huang GC, Chen CY. Bioactive compounds from natural product extracts in Taiwan cosmeceuticals-mini review. Biomed. Res 28(15), 6561-6566 (2017).
- Chang MY, Liu CM, Shieh DE CC. Evaluation and analysis of phytochemical antioxidant capacity. Biomed. Res 28(14), 6431-6434 (2017).
- Hirsch L, Jette N, Frolkis A, et al. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology. 46(4), 292-300 (2016).
- Kalia LV, Lang AE. Parkinson’s disease. Lancet386(9996), 896-912 (2015).
- Park A, Stacy M. Non-motor symptoms in Parkinson’s disease. J. Neurol (2009).
- Lotharius J, Brundin P. Pathogenesis of parkinson’s disease: Dopamine, vesicles and α-synuclein. Nat. Rev. Neurosci 3(12), 932-942(2002).
- Tufekci KU, Meuwissen R, Genc S, Genc K. Inflammation in parkinson’s disease. Adv. Protein Chem. Struct. Biol 88, 69-132 (2012).
- Lee JK, Tran T, Tansey MG. Neuroinflammation in Parkinson’s disease. J. Neuroimmune Pharmacol 4(4), 419-429 (2009).
- Przedborski S. Inflammation and Parkinson’s disease pathogenesis. Mov. Disord 25 Suppl 1 (2010).
- Imamura K, Hishikawa N, Sawada M, et al. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol 106(6), 518-526 (2003).
- Sun H, Liang R, Yang B, et al. Aquaporin-4 mediates communication between astrocyte and microglia: Implications of neuroinflammation in experimental Parkinson’s disease. Neuroscience 317(3), 65-
75 (2016). - Jenner P. Oxidative stress in Parkinson’s disease. Ann. Neurol 53(S3), S26-S38 (2003).
- Yanagida T, Kitamura Y, Yamane K, et al. Protection against oxidative stress-induced neurodegeneration by a modulator for DJ-1, the wild-type of familial Parkinson’s disease- linked PARK7. J. Pharmacol. Sci 109(3), 463- 468 (2009).
- Devos D, Lebouvier T, Lardeux B, et al. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis. 50(1), 42–48 (2013).
- McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism. Relat. Disord S3-S7 (2004).
- Okado-Matsumoto A, Fridovich I. Amyotrophic lateral sclerosis: a proposed mechanism. Proc. Natl. Acad. Sci. U. S. A 99(13), 9010–9014 (2002).
- Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet 369(9578), 2031–41 (2007).
- McGeer PL, McGeer EG. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve 26(4), 459–470 (2002).
- Evans MC, Couch Y, Sibson N, et al. Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Mol. Cell. Neurosci. 53, 34–41 (2013).
- Gasco S, Zaragoza P, García-Redondo A, et al. Inflammatory and non-inflammatory monocytes as novel prognostic biomarkers of survival in SOD1G93A mouse model of Amyotrophic Lateral Sclerosis. PLoS. One 12(9), e0184626 (2017).
- Dibaj P, Zschüntzsch J, Steffens H, et al. Influence of methylene blue on microglia- induced inflammation and motor neuron degeneration in the SOD1G93A model for ALS. PLoS. One 7(8), e43963 (2012).
- Renard D, Collombier L, Castelnovo G, et al. Brain FDG-PET changes in ALS and ALS-FTD. Acta Neurol. Belg 111(4), 306-309 (2011).
- pathogenesis in ALS: insights in cell interconnectivity. Front. Cell. Neurosci 8(1), 117 (2014).
- Boillee S, Yamanaka K, Lobsiger CS, et al. Onset and Progression in Inherited ALS Determined by Motor Neurons and Microglia. Science 312(5778), 1389-1392 (2006).
- Chiu IM, Morimoto ETA, Goodarzi H, et al. A neurodegeneration-specific gene- expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell. Rep 4(2), 385- 401 (2013).
- Holtman IR, Raj DD, Miller JA, et al. Induction of a common microglia gene expression signature by aging and neurodegenerative conditions: a co-expression meta-analysis. Acta.Neuropathol. Commun 3(1), 31 (2015).
- Walker FO. Huntington’s disease. Lancet 369(9557), 218-228 (2007).
- Staal RGW, Möller T. Neuroinflammation in Huntington’s disease. Neuroinflammation and Neurodegeneration 179–197 (2014).
- Petersén Å, Hult S, Kirik D. Huntington’s disease - new perspectives based on neuroendocrine changes in rodent models. Neurodegener. Dis 6(4), 154–164 (2009).
- Hsiao HY, Chen YC, Chen HM, et al. A critical role of astrocyte-mediated nuclear factor-κB-dependent inflammation in huntington’s disease. Hum. Mol. Genet 22(9), 1826–1842 (2013).
- Sofroniew M V. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci 16(5), 249-263 (2015).
- Compston A, Coles A. Multiple sclerosis.Lancet 372(9648), 1502-1517 (2008).
- Bitsch A. Acute axonal injury in multiple sclerosis: Correlation with demyelination and inflammation. Brain 123(6), 1174-1183 (2000).
- Amato MP, Portaccio E, Goretti B, et al. Relevance of cognitive deterioration in early relapsing-remitting MS: A 3-year follow-up study. Mult. Scler 16(12), 1474- 1482 (2010).
- Rosti E, Hämäläinen P, Koivisto K, Hokkanen L. PASAT in Detecting Cognitive Impairment in Relapsing-Remitting MS. Appl. Neuropsychol 14(2), 101-112 (2007).
- Sospedra M, Martin R. Immunology of Multiple Sclerosis. Semin. Neurol 36(2), 115- 127 (2016).
- Ascherio A, Munger KL. Epstein-barr virus infection and multiple sclerosis: A review. J. Neuroimmune Pharmacol. 5(3), 271-277 (2010).
- Mormile R. Hepatitis B virus (HBV) infection and multiple sclerosis: One more reason to undergo vaccination? Immunol. Lett 165(1), 60-61 (2015).
- Mohammadzadeh Honarvar N, Harirchian MH, Koohdani F, et al. The effect of vitamin A supplementation on retinoic acid- related orphan receptor γt (RORγt) and interleukin-17 (IL-17) gene expression in avonex-treated multiple sclerotic patients. J. Mol. Neurosci 51(3), 749-753 (2013).
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science 327(5963), 291-295 (2010).
- Guthrie GJK, Charles KA, Roxburgh CSD, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol 88(1), 218–230 (2013).
- Sakai Y, Kobayashi M. Lymphocyte “homing” and chronic inflammation. Pathol. Int 65(7), 344–354 (2015).
- Chen HC, Lai SY, Sung JM, et al. Lymphocyte activation and hepatic cellular infiltration in immunocompetent mice infected by dengue virus. J. Med. Virol 73(3), 419-431 (2004).
- Kintscher U, Hartge M, Hess K, et al.T-lymphocyte infiltration in visceral adipose tissue: A primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance.
Arterioscler. Thromb. Vasc. Biol 28(7), 1304- 1310 (2008). - Nimmerjahn A, Kirchhoff F, Helmchen F. Neuroscience: Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308(5726), 1314-1318 (2005).
- da Fonseca ACC, Matias D, Garcia C, et al. The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell. Neurosci 8(11), 362 (2014).
- Boche D, Perry VH, Nicoll JAR. Review: Activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol 39(1), 3-18 (2013).
- Marques CP, Cheeran MC-J, Palmquist JM, et al. Lokensgard JR. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. J. Immunol 181(9), 6417–
6426 (2008). - Venneti S, Lopresti BJ, Wiley CA. Molecular imaging of microglia/macrophages in the brain. Glia 61(1), 10-23 (2013).
- Lidman O, Swanberg M, Horvath L, et al. Discrete gene loci regulate neurodegeneration, lymphocyte infiltration, and major histocompatibility complex class II expression in the CNS. J. Neurosci 23(30), 9817-9823 (2003).
- Andersen JK. Oxidative stress in neurodegeneration: Cause or consequence? Nat. Rev. Neurosci 10(7), S18 (2004).
- Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8 + T lymphocyte mobilization to virus- infected tissue requires CD4 + T-cell help. Nature 462(7272), 510-513 (2009).
- Ransohoff RM, Schafer D, Vincent A, et al. Neuroinflammation: Ways in Which the Immune System Affects the Brain. Neurotherapeutics 12(4), 896-909 (2015).
- Kim YK, Na KS, Myint AM, et al. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 64, 277-284 (2016).
- Rivat C, Becker C, Blugeot A, et al. Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety- induced hyperalgesia. Pain 150(2), 358–368 (2010).
- Faden AI, Loane DJ. Chronic Neurodegeneration After Traumatic Brain Injury: Alzheimer Disease, Chronic Traumatic Encephalopathy, or Persistent Neuroinflammation? Neurotherapeutics 12(1), 143-150 (2015).
- Suzumura A. Neuron-microglia interaction in neuroinflammation. Curr. Protein. Pept. Sci 14(1), 16-20 (2013).
- Carson MJ, Cameron Thrash J, Walter B. The cellular response in neuroinflammation: The role of leukocytes, microglia and astrocytes in neuronal death and survival. Clin. Neurosci. Res 6(5), 237–245 (2006).
- Lloret a, Fuchsberger T, Giraldo E, et al. Molecular mechanisms linking amyloid β toxicity and Tau hyperphosphorylation in Alzheimers disease. Free Radic. Biol. Med 83, 186–191 (2015).
- Sawmiller D, Habib A, Li S, et al. Diosmin reduces cerebral Aβ levels, tau hyperphosphorylation, neuroinflammation, and cognitive impairment in the 3xTg-AD mice. J. Neuroimmunol 299, 98–106 (2016).
- Glass CK, Saijo K, Winner B, et al. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 140(6), 918–934 (2010).
- Neurology TL. Biomarker promise for Parkinson’s disease. Lancet Neurol 9(12), 1139 (2010).
- Segal BM. Th17 cells in autoimmune demyelinating disease. Semin. Immunopathol 32(1), 71-77 (2010).
- Tesmer LA, Lundy SK, Sarkar S, et al. Th17 cells in human disease. Immunol. Rev 223(1), 87-113 (2008).
- Kimura A, Kishimoto T. Th17 cells in inflammation. Int. Immunopharmacol 11(3), 319-322 (2011).
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL- 17 and Th17 Cells. Annu. Rev. Immunol 27(1), 485-517 (2009).
- Peters A, Lee Y, Kuchroo VK. The many faces of Th17 cells. Curr. Opin. Immunol 23(6), 702- 706 (2011).
- Ray A, Chakraborty K, Ray P. Immunosuppressive MDSCs induced by TLR signaling during infection and role in resolution of inflammation. Front. Cell. Infect. Microbiol 3(9), 52 (2013).
- Goedegebuure P, Mitchem JB, Porembka MR, et al. Myeloid-derived suppressor cells: general characteristics and relevance to clinical management of pancreatic cancer. Curr. Cancer Drug Targets 11(6), 734-51 (2011).
- Schmid MC, Varner J a. Myeloid cells in tumor inflammation. Vasc. Cell 4(1), 14 (2012).
- Chatterjee S, Das S, Chakraborty P, et al.Myeloid derived suppressor cells (MDSCs) can induce the generation of Th17 response from na??ve CD4+ T cells. Immunobiology 218(5), 718-724 (2013).
- Kebir H, Kreymborg K, Ifergan I, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med 13(10), 1173- 1175 (2007).
- Zhu J, Yamane H, Paul WE. Differentiation of Effector CD4 T Cell Populations. Annu. Rev. Immunol 28(1), 445-489 (2010).
- Martins IJ. The Future of Biomarkers Tests and Genomic Medicine in Global Organ Disease. Arch. Infect. Dis. Ther 1(1), 1-6 (2017).