Research Article - Neuropsychiatry (2017) Volume 7, Issue 6
Early CT Signs of Pituitary Dysfunction Following Acute Traumatic Brain Injury
- Corresponding Author:
- Ping Zheng
Department of Neurosurgery
Shanghai Pudong New area People’s Hospital
490 South Chuanhuan Road, Shanghai, 201299, China
Phone: +86-21-5898 1990
Abstract
Objective:
In this study, we aimed to identify the characteristics of early head CT scan in patients with traumatic brain injury (TBI) and to evaluate the association of such changes with hypopituitarism (HPT) in TBI patients.
Methods:
A cohort of 230 TBI patients was enrolled and further grouped with or without HPT based on the secretion status of pituitary hormones at six months post injury. Data were analyzed and compared including different signs of the early head CT scan. Logistic regression analysis was used to show the risk factors related to HPT.
Results:
Our study included 106 (46.1%) TBI patients suffered from pituitary dysfunction and 124 TBI patients with normal pituitary function. The differences between HPT and non-HPT were statistically significant in the initial CT scans showing basal fracture, subarachnoid hemorrhage (SAH), brain contusion, epidural hematoma (EDH), subdural hematoma (SDH), and multiple hematoma as well as the midline shift (P<0.01). Logistic regression analysis showed that early CT scans with basal fracture, SAH and midline shift were strong predictors of HPT (P<0.01).
Conclusion:
For patients with the initial CT scan shows basal fracture, SAH, and midline shift, dynamic laboratory tests for pituitary hormones should be performed for detection of HPT as early as possible and the medical intervention would be enforced in time.
Keywords
Traumatic brain injury (TBI), Hypopituitarism (HPT), Early CT scan, CT signs
Introduction
Traumatic brain injury is known as the primary cause of death and disability in adults in developed countries [1], and its incidence rate is rising worldwide with the increased use of motor vehicles. Clinically, TBI is associated with lasting symptoms of impaired cognitive function, memory disturbance, and decreased activity, failures of emotional control, carelessness, excessive tenacity and planning failure for execution [2]. These symptoms often cause social problems in families, schools, work places, and communities, which are related to the hypopituitarism following TBI [3].
More and more researchers and clinicians are becoming aware of the high incidence of hypopituitarism following TBI from 21% to 54% [4]. With the increased care about the hormone-secreting status in patients with TBI, some of these patients develop hypopituitarism in the chronic stage even after one year of TBI and associated with the long-term neurological outcomes. The delayed diagnosis and multiple tests of pituitary’s hormones would hinder the early targeting intervention and the relationship between initial CT signs in patients with traumatic brain injury (TBI) and hypopituitarism remains unknown. Nevertheless, almost no study has correlated these characteristics in early head CT scan with the hypopituitarism in patients, especially TBI patients.
In this study, we prospectively collected the clinical data of acute traumatic brain injury patients, focusing on their initial head CT signs, to examined the role of the these characteristics in predicting the prognosis of pituitary function in TBI patients.
▪ Clinical materials and methods
We prospectively enrolled TBI patients between Jan 2014 and April 2015 at Shanghai Pudong New Area People’s Hospital. Our study population consisted of 230 patients (127 males and 103 females) whose mean age was 43 years (range 19–59 years).
Our study protocol was approved by our institutional review board; written patient consent was obtained from family relatives before the study registration.
▪ Head CT data
The first CT scan of 230 TBI patients was obtained within 24 h of the injury. Data recorded included patient age, gender, time from injury to the first CT scan, and early head CT signs including common types of intracranial hematoma, basal and linear fracture, contusion, intracranial air, midline shift, and brain swelling. Inclusion criteria included (1) history of head injuries; (2) no severe thoracic or abdomen injury; (3) no cardiac pulmonary resuscitation or shocks; and (4) patients without previous or current hormonal replacement treatment during the study.
HPT was diagnosed if a patient showed abnormality in one or more hormone axis at six months post onset according to our previous studies [5-7]. Our patients were divided into two groups: HPT group and non-HPT group. All patients were treated by the Clinical Guidelines for the Management of Head Injury [8].
Statistical analysis
The data of the patients were analyzed using SPSS (SPSS, Chicago) software. Chi-square (χ2) statistics and Wilcoxon rank sum test were calculated for categorical comparisons and continuous variables, respectively. Values for the continuous parameters are given as the mean±standard deviation. After determining the risk factors related to HPT by single factor analysis, a further stepwise regression analysis was performed to find out the predictors of HPT. We performed stepwise regression using variables stored in a dataset array and specified the starting model using Wilkinson notation, and identified the response and predictor variables according to the statistical values. One variable was added at each time and if the p value from the equation was larger than 0.05, the new variable was removed, until the top three variables were identified. Predictors were defined as being significant if probability values were less than 0.05.
Results
Demographic data were available for all patients including age, gender and initial CT signs, as summarized in Table 1. The age of TBI patients with HPT (mean 22.7±4.8) did not differ from the subjects without HPT (mean 50.1±6.7, p=0.2163), and the proportion of men in the HPT group (n=60, 57.69%) did not differ from that in the non-HPT group (n=70, 55.56%, P=1.0000).
| Characteristic | HPT 106 | Non-HPT 124 | P Value |
|---|---|---|---|
| Age (yrs)a | 52.7 ± 14.8 | 50.1 ± 16.7 | 0.2163 |
| Genderb | 60 (57.69%) | 70 (55.56%) | 0.9998 |
| Early CT signsb | |||
| Basal fracture | 58 (55.76%) | 41 (32.54%) | 0.0013* |
| SAH | 68 (65.38%) | 60 (47.62%) | 0.0173* |
| Contusion | 35 (33.65%) | 26 (20.63%) | 0.0511 |
| Intracranial Air | 11 (10.58%) | 15 (11.90%) | 0.4081 |
| EDH | 23 (22.12%) | 14 (11.11%) | 0.0467* |
| SDH | 21 (20.19%) | 16 (12.70%) | 0.2072 |
| ICH | 6 (5.76%) | 4 (3.17%)c | 0.5193 |
| Multiple hematoma | 13 (12.5%) | 3 (2.38%) | 0.0039* |
| Midline shift | 11 (10.58%) | 3 (2.38%) | 0.0136* |
| Brain swelling | 3 (2.88%) | 2 (1.59%) | 0.6638 |
aStudent’s t test, bChi-square test, cFisher’s exact test when one or more than one cell in the Chi-square less than 5, *=P<0.05
Table 1: Comparison of the related clinical factors between HPTs and non-HPTs.
▪ Hormone profiles
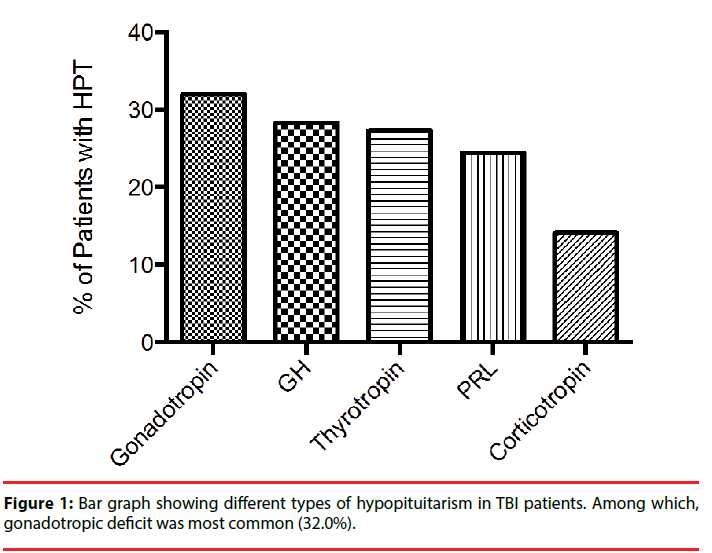
The total incidence of hypopituitarism was about 46.1 % at six months post TBI, and each hormone profile was listed in Figure 1. The most frequent deficiency (n=35, 32.0%) was Gonadotropin deficiency. Growth hormone deficiency was relatively common (n=30, 28.3%). Thyroid deficiency was found in 29 cases (27.4%), followed by 26 cases with abnormal prolactin (PRL) (24.5%). Corticotropin deficiency was quite infrequent (n=15, 14.2%).
The results of this study demonstrated that early CT signs showing basal fracture, SAH, primary hematoma type including EDH and multiple hematomas as well as the midline shift were statistically different between two groups. Table 1 shows a comparison of the statistical data of each group.
Of these potential factors, a logistic regression model of five predictors including basal fracture, SAH, EDH, multiple hematoma and midline shift based on the single factor analysis were identified. In Table 2, the CT signs showed that basal fracture (P<0.001), SAH (P=0.001), and midline shift (P=0.003) during the early injury phase were the strongest predictors of HPT.
| Risk factor | Odds ratio | 95% CI | P Valuea |
|---|---|---|---|
| Basal fracture | 2.321 | 1.328–3.752 | <0.001 |
| SAH | 2.147 | 1.936–3.328 | 0.001 |
| Midline shift | 1.908 | 1.322–3.912 | 0.003 |
aStepwise method was used in logistic regression analysis
Table 2: Logistic regression analysis for the risk factors related to HPT.
Discussion
Recently, HPT is reported to occur from 21% to 54% of all patients with head injury [9], even in patients with negative head CT scans [5]. In this study, it was shown that HPT occurred in 46.1% of the subjects enrolled, which was consistent with these previous retrospective reports [9]. Perhaps more importantly, this study provided evidence that patients with early head CT signs showing basal fracture, SAH, EDH, multiple hematoma and midline shift are more likely to develop HPT at the chronic stage, suggesting that patients with these CT signs would be beneficial in testing pituitary hormones as early as possible following injury.
Initially, HPT is confirmed by a series of laboratory tests including pituitary hormones, and generally the dynamic change of these hormones would hinder the accurate and early diagnosis of pituitary dysfunction in clinical sessions. Therefore, the imaging biomarker from tradition and general-used head CT scans would favour the selection for testing hormone status in these TBI patients.
We further evaluated the role of the early CT signs in predicting the occurrence of HPT following TBI by stepwise regression analysis. To the best of our knowledge, the relationship between the early CT signs and the pituitary function in TBI patients has not been reported previously. We found the initial CT signs showed that basal fracture, SAH, and midline shift during the early injury phase were the strongest predictors of HPT. This might provide anatomical radiological evidences contributing to the pituitary dysfunction of TBI patients. Previous studies have noted a relationship between TBI severity and the risk of pituitary dysfunction and the possibility that the relationship between outcome and early CT signs in our study could be a reflection of the injury severity [3,9]. However, studies on larger patient populations are underway to evaluate the relationship between the radiological marker of CT scan and specific hormone status in patients with TBI.
In this study, several limitations have to be mentioned. First, the head CT scan in this study is only performed at the initial stage. Then, we did not observe the dynamic change of CT scans following the onset. Some studies report the progressive injury after the initial injury would have an impact on the chronic neurological outcome [10]. However, our primary goal here is to locate several risk factors might predict the occurrence of HPT following TBI, then a dynamic test for the pituitary hormones would be employed for early clinical intervention. Second, we excluded patients with hormone treatments which would affect the secretion status of pituitary hormones. Therefore, we could not address the effect of hormone replacement on the pituitary function at chronic stage of TBI. Third, the unspecific test for the GH. Other researchers reported that GH deficiency was the most common type in TBI patients with HPT [11]. However, in our study, we found the incidence of GH deficiency was a little less than that of hypogonadism. This discrepancy might be due to the test method itself. The fourth point was that we did not measure hormone levels in the control group, because the definition of HPT was based on the comparison with the normal range according to the laboratory method.
Conclusion
For patients with the initial CT scan shows basal fracture, SAH, and midline shift, dynamic laboratory tests for pituitary hormones would be favoured for detection of HPT as early as possible and the medical intervention would be enforced in time. It can be seen that the early CT signs may become a traditional radiological marker to predict the occurrence of hypopituitarism in TBI patients.
Acknowledgements
This study is supported by the Shanghai Natural Scientific Foundation Council Project -16ZR1431500. All authors declare there is no conflict of interest.
References
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head. Trauma. Rehabil 21(5), 375-378 (2005).
- Wada T, Asano Y, Shinoda J. Decreased fractional anisotropy evaluated using tract-based spatial statistics and correlated with cognitive dysfunction in patients with mild traumatic brain injury in the chronic stage. AJNR. Am. J. Neuroradiol 33(11), 2117-2122 (2012).
- Moreau OOK, Yollin EE, Merlen EE, et al. Lasting pituitary hormone deficiency after traumatic brain injury. J. Neurotrauma 29(1), 81-89 (2011).
- Krahulik D, Zapletalova J, Frysak Z, et al. Dysfunction of hypothalamic-hypophysial axis after traumatic brain injury in adults. J. Neurosurg 113(3), 581-584 (2010).
- Zheng P, He B, Tong WS. Decrease in pituitary apparent diffusion coefficient in normal appearing brain correlates with hypopituitarism following traumatic brain injury. J. Endocrinol. Invest 37(3), 309-312 (2014).
- Zheng P, He B, Tong W. Dynamic pituitary hormones change after traumatic brain injury. Neurol. India 62(3), 280-284 (2014).
- Zheng P, Bin He, Guo Y, et al. Decreased apparent diffusion coefficient in the pituitary and correlation with hypopituitarism in patients with traumatic brain injury. J. Neurosurg 123(1), 75-80 (2015).
- Haddad SH, Arabi YM. Critical care management of severe traumatic brain injury in adults. Scand. J. Trauma. Resusc. Emerg. Med 20(1), 12 (2012).
- Jeong JH, Kim YZ, Cho YW, et al. Negative effect of hypopituitarism following brain trauma in patients with diffuse axonal injury. J. Neurosurg 113(3), 532-538 (2010).
- Tong W-S, Zheng P, Xu J-F, et al. Early CT signs of progressive hemorrhagic injury following acute traumatic brain injury. Neuroradiology 53(5), 305-309 (2011).
- Moreau OK, Cortet-Rudelli C, Yollin E, et al. Growth hormone replacement therapy in patients with traumatic brain injury. J. Neurotrauma 30(11), 998-1006 (2013).