Research Article - Neuropsychiatry (2017) Volume 7, Issue 4
Does Parkinsons Disease Dementia Reduce Cancer Risk more than Alzheimers Disease Alone?
- Corresponding Author:
- Yi-Hwei Li
Department of Public Health, Tzu-Chi University, No.701
Chung Yang Rd., Sec .3, Hualien 97004, Taiwan
Tel: +886-3-8565301 ext. 2271
Fax: +886-3-8564041
Abstract
Abstract
Objective:
Both Alzheimer’s disease (AD) and Parkinson’s disease (PD) are associated with lower cancer risk. However, the cancer risk in Parkinson’s disease dementia (PDD) has never been discussed. We aimed to test whether PDD is cancer protective, and to compare the types and risks of incident cancer between AD and PDD.
Methods:
We used Taiwan National Health Insurance Database. From 1997 to 2010, a total of 2,527 PD dementia and 25,557 AD patients were enrolled and followed up for cancer by record linkage. Age and sex standardized incidence ratios (SIRs) of overall and site-specific cancers were calculated. Cox proportional hazards model was used to compare the cancer risk of AD relative to PDD.
Results:
With an average 4.4 years of follow-up period, both AD and PDD were associated with lower overall cancer risk (SIR and 95% confidence interval (CI): 0.83 (0.77-0.89) and 0.70 (0.55-0.89), respectively). The adjusted overall cancer risk of AD was significantly higher than that of PDD (hazard ratio (HR) and 95% CI: 1.26 (1.04-2.53), p=0.02). As for the site-specific cancer risk, the colorectal cancer risk of AD was also significantly higher than that of PDD (SIR: 0.95 (0.80-1.12) and 0.53 (0.80-1.00); HR: 1.83 (1.07-3.14), p=0.029).
Conclusions:
Both AD and PDD are inversely associated with incident cancer. Compared to AD, PDD is associated with even lower cancer risk. The decreased cancer risk in PDD may be contributed by the combined effect from both AD and PD. The mechanism deserves further investigations.
Keywords
Alzheimer’s disease; Parkinson’s disease dementia; Cancer risk
Introduction
In the last decade, two neurodegenerative diseases, Alzheimer’s disease (AD) for cortical disorder and Parkinson’s disease (PD) for subcortical, have been shown to be associated with reduced cancer risk [1-20]. AD is widely reported to reduce cancer risk given its high prevalence [6-14]. Similarly, PD has been found with lower incidences of all cancers except melanoma [15-21]. In terms of cancer risk, AD and PD have never been viewed jointly. In any report on AD, symptoms of parkinsonism were never documented. Likewise, dementia status was also undetermined in PD researches.
Parkinson’s disease dementia (PDD) is a heterogeneous entity with PD and AD intertwined with each other [22]. Jellinger found PDD is significantly associated with AD pathology [23-25]. However, PDD is not merely a crude sum of PD and AD pathology. In the report from Papapetropoulos et al., over one third of their PD dementia patients were short of AD pathology. Conversely, almost 30% of the PD patients with marked AD pathology did not fulfill the diagnostic criteria for AD [26]. Nonetheless, PDD serves as a platform where cortical and subcortical dementias encounter.
No prior study ever probes into the cancer risk in PDD. It is intriguing to investigate whether the cancer-protectiveness can be added-on from cortical and subcortical dementias. The first aim of our study was to test whether PDD is generally cancer protective like most other neurodegenerative diseases. The second was to compare the overall and site-specific cancer risks between AD and PDD.
Methods
▪ Data sources
A section of National Health Insurance Research Database (NHIRD), Registry for Catastrophic Illness Patients (HV), was used in the study. HV included all relevant information about any “catastrophic illness” such as diagnosis, date of diagnosis, date of death, and outpatient/ inpatient claimed data during 1995 and 2010. The diagnoses in NHIRD database were coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). “Catastrophic illnesses” include senile dementia, end-stage renal disease, severe liver cirrhosis, cancers and etc. Patients with a “catastrophic illness card” (CIC) get complete medical coverage when it’s valid. Taiwan launched its compulsory social insurance, National Health Insurance (NHI) in 1995. The annual coverage rate of NHI ranged from 96.1% to 99.6%, with more than 20 million residents enrolled since 1997.
▪ Study population
Patients older than 65, newly diagnosed with Alzheimer’s disease, and without pre-existing cancer were eligible for enrollment. There were 76,689 patients with dementia (ICD-9-CM code: 290 or 294) CIC from 1995 to 2010. The NHI scheme requires that any patient applying for a dementia CIC should submit a medical certificate and disease summary given by certified neurologists or psychiatrists. The whole reviewing process is stringent enough to allow for no doubt concerning diagnosis.
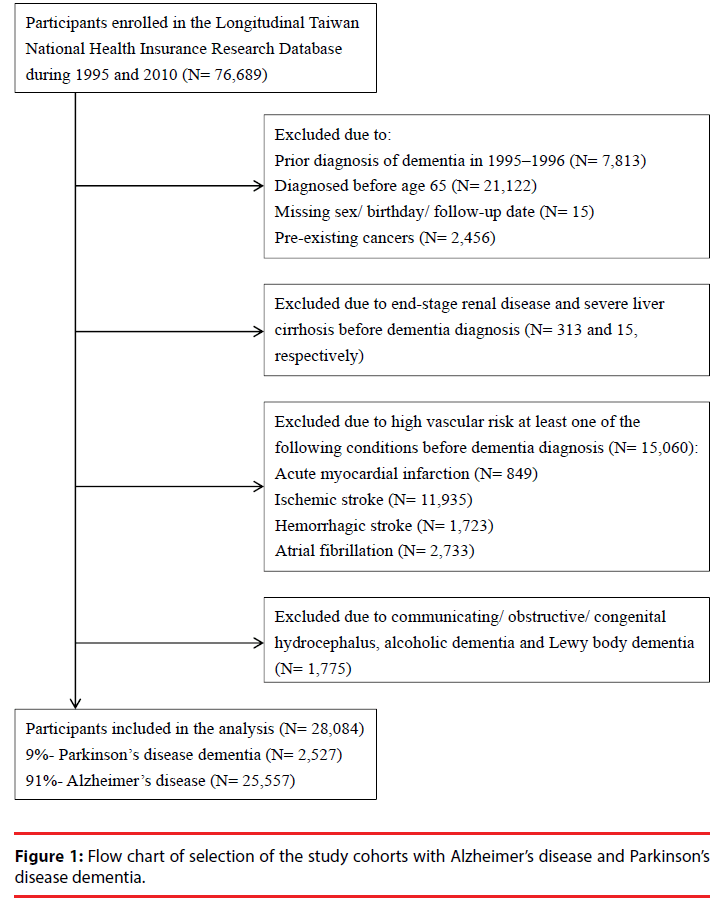
To identify newly diagnosed senile dementia, early onset dementia was excluded. The first 2 years (1995-1996) of the database were also skipped. Hence, we excluded 21,122 patients diagnosed before age 65, 7,813 patients diagnosed before 1997, 15 patients with missing sex/birthday/follow-up date, and 2,456 patients with pre-existing cancers.
Of the remaining 45,283 senile dementia patients, 313 with end-stage renal disease (ICD- 9-CM code: 585.6) and 51 with severe liver cirrhosis (ICD-9-CM codes: 571.2, 571.5, 571.6) before dementia diagnosis were excluded.
Following the diagnostic guidelines for AD recommended by National Institute on Aging- Alzheimer’s Association [27], we excluded patients with high vascular risk before dementia onset to elicit “probable AD”. Thus, 15,060 patients were removed. They met at least one of the following conditions before dementia diagnosis: acute myocardial infarction (ICD-9- CM code: 410, n=849), ischemic stroke (ICD-9- CM codes: 433, 434, 436, 437, 438, n=11,935, transient ischemia attack (TIA) not included), hemorrhagic stroke (ICD-9-CM codes: 430, 431, 432, n=1,723), and atrial fibrillation (ICD- 9-CM code: 427, n=2,733). Any comorbidity was ascertained by at least two ambulatory visits or one hospitalization record denoting the corresponding ICD-9-CM code.
Among the 29,859 senile dementia patients, 1,775 diagnosed with communicating hydrocephalus (ICD-9-CM code: 331.3), obstructive hydrocephalus (ICD-9-CM code: 331.4), alcoholic dementia, congenital hydrocephalus or Lewy body dementia (ICD-9- CM codes: 331.7, 331.8) were further excluded. Leaving out the most common organic brain syndromes, 28,084 with probable AD were included and classified into two groups : 2,527 (9%) as “PDD” with pre-existing PD (ICD-9-CM code: 332), and 25,557 (91%) as “AD” without pre-existing PD. The flowchart of the selection process of the study cohorts was summarized in Figure 1.
▪ Cancer status and background cancer incidence
The first-ever cancer status (ICD-9-CM codes: 140-208) including diagnosis date and primary site was obtained by linkage to HV. Cytological and/or other pathological reports would be required to apply for a cancer CIC. Benign tumors, in situ malignancies, Kaposi’s sarcoma, and metastatic cancers were excluded from further analysis.
Background cancer incidence rates for general population were calculated from cancer registry provided by Health Promotion Administration, Ministry of Health and Welfare in Taiwan. “Cancer Control Act” rules any medical institution with over 50 beds should report incident cancer information including patient’s age, sex, birth date, age at diagnosis, diagnosis date, primary cancer site, histology, grading and stage [28]. In the present study, age- and sex-specific cancer incidence rates in general population were retrieved during the study period.
Because the data set used in this study consisted of de-identified secondary data released for research purposes, the study was exempt from full review by the Institutional Review Board.
▪ Statistical analysis
The patients’ baseline characteristics were presented as numbers and percentages for categorical data; as means ± SDs for continuous data. To test differences between the groups (AD and PDD), chi-square tests were used for categorical variables, 2-sample t-tests for continuous variables, and log-rank tests for survival data.
The follow-up time for cancer, defined as person-years at risk, began on the issuance of dementia CIC and ended on either the issuance of cancer CIC, the date of death, or December 31, 2010.
We calculated expected number of cancers via multiplying the number of person-years accumulated in each age/ sex stratum by the corresponding background incidence rate. The standardized incidence ratio (SIR), taken as the observed/expected number of cancer cases, was used to measure relative risk. Its corresponding 95% confidence interval (CI) was calculated assuming a Poisson distribution of the observed number of cancers. Cox proportional hazards model was used to derive cancer hazard ratios (HRs) for AD relative to PDD while adjusting for comorbidities (listed in Table 1). SAS statistical software (SAS System for Windows, version 9.4; SAS Institute, Cary, NC, U.S.A.) was used for statistical analysis.
| Overall (n=28084) | Alzheimer's disease (n=25557) | Parkinson's disease dementia (n=2527) | P-value | |
|---|---|---|---|---|
| Sex | <.001 | |||
| Female | 17241 (61.4) | 15800 (61.8) | 1441 (57.0) | |
| Male | 10843 (38.6) | 9757 (38.2) | 1086 (43.0) | |
| Mean age at diagnosis, years | 78.1 ± 6.8 | 78.2 ± 6.9 | 77.1 ± 6.4 | <.001 |
| Comorbidity | ||||
| Depression | 3358 (12.0) | 2856 (11.2) | 502 (19.9) | <.001 |
| Hypertension | 7790 (27.7) | 6966 (27.3) | 824 (32.6) | <.001 |
| Diabetes | 5179 (18.4) | 4688 (18.3) | 491 (19.4) | 0.179 |
| Coronary artery disease without AMI | 3700 (13.2) | 3281 (12.8) | 419 (16.6) | <.001 |
| Congestive heart failure | 1350 (4.8) | 1210 (4.7) | 140 (5.5) | 0.071 |
| Anemia | 3078 (11.0) | 2732 (10.7) | 346 (13.7) | <.001 |
| Chronic obstructive pulmonary disease | 3894 (13.9) | 3487 (13.6) | 407 (16.1) | <.001 |
| Chronic liver disease | 2400 (8.5) | 2119 (8.3) | 281 (11.1) | <.001 |
| Chronic kidney disease | 593 (2.1) | 524 (2.1) | 69 (2.7) | 0.029 |
| Follow-Up | ||||
| Mean (median) follow-up years | 4.43 (3.78) | 4.44 (3.77) | 4.37 (3.71) | |
| Mean (median) survival years | 4.51 (3.88) | 4.52 (3.90) | 4.42 (3.76) | <.001 |
| Died during follow-up | 11805 | 10574 | 1231 | |
| Annual mortality, % | 9.32 | 9.16 | 11.01 |
Table 1: Baseline characteristics and follow-up information for elderly patients with Alzheimer’s disease and Parkinson’s disease dementia.
Results
Table 1 summarizes the characteristics of our study sample. A total of 25,557 patients were AD (without PD), and 2,527 were PDD. In both AD and PDD, women outnumbered men (61.8% and 57%, respectively). The mean agesat- diagnosis were 78.2 and 77.1 in AD and PDD respectively. In general, patients with PDD had more comorbidities than AD group. The prevalence of depression (19.9%), hypertension (32.6%), coronary artery disease (16.6%), anemia (13.7%), chronic obstructive pulmonary disease (16.1%), and chronic liver (11.1%) and chronic renal diseases (2.7%) were all higher in the PDD group.
Table 1 also shows the follow-up information for the two study groups. A total of 10,157 AD patients and 1,231 PDD patients died during the follow-up period; the annual mortality was 9.16% and 11.01% respectively. The median survival time for AD was 3.90 years, significantly longer than that of PDD (3.76 years). This could be partially explained by the higher prevalence of comorbidities in the PDD group. The mean follow-up time was 4.4 years for both AD and PDD.
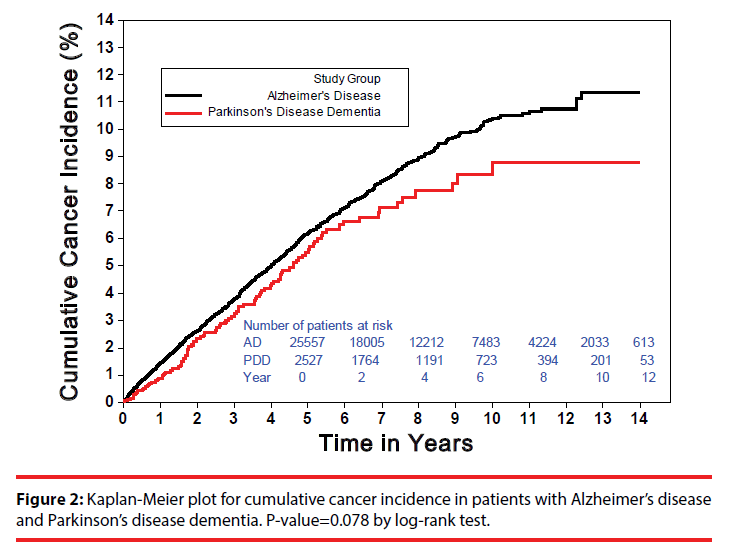
Figure 2 demonstrates the Kaplan-Meier plots for cumulative cancer incidence in patients with AD and PDD. Up to 1,341 incident cancers (annual incidence: 1.18%) were observed in AD, and 110 (annual incidence: 1.00%) were in PDD (Table 2). The difference for the overall incident cancers between AD and PDD was marginally significant (P-value=0.078 by log-rank test). Notably, for the initial 5 years, the cumulative cancer incidence of AD had slightly exceeded that of PDD. After 5 years, however, the Kaplan- Meier curve in PDD began to flatten, rendering the risk difference enlarged (Figure 2).
| Alzheimer's disease | Parkinson's disease dementia | |||||
|---|---|---|---|---|---|---|
| Category | Number of cancers (annual incidence) | SIR (95% CI) | Number of cancers (annual incidence) | SIR (95% CI) | Hazard Ratio (95% CI) |
P-value |
| Overall cancer (140-208) | 1341 (1.18%) | 0.83 (0.77-0.89)*** | 110 (1.00%) | 0.70 (0.55-0.89)** | 1.26 (1.04-1.53) | 0.020 |
| Age at diagnosis | ||||||
| 65-79 years | 818 (1.14%) | 0.86 (0.78-0.94)** | 80 (1.03%) | 0.76 (0.57-1.02) | 1.16 (0.92-1.46) | 0.210 |
| >= 80 years | 523 (1.25%) | 0.78 (0.70-0.88)** | 30 (0.92%) | 0.57 (0.36-0.89)* | 1.37 (0.95-1.99) | 0.093 |
| Years since diagnosis | ||||||
| During years 1-5 | 1083 (1.29%) | 0.89 (0.82-0.97)** | 91 (1.10%) | 0.76 (0.58-1.00)* | 1.19 (0.96-1.47) | 0.118 |
| After year 5 | 258 (0.88%) | 0.63 (0.54-0.73)*** | 19 (0.68%) | 0.49 (0.28-0.85)* | 1.34 (0.84-2.14) | 0.224 |
Table 2: Comparison of overall cancer risks between Alzheimer’s disease and Parkinson’s disease dementia according to age at diagnosis and disease chronicity.
Table 2 presents the overall cancer SIRs in the “age-at-diagnosis” and “years-since-diagnosis” subgroups of AD and PDD. The overall cancer SIR was 0.83 (95% CI: 0.77-0.89, P<0.001) in AD; 0.70 (95% CI: 0.55-0.89, P=0.004) in PDD, indicating cancer reduction exists in both groups. The adjusted cancer risk of AD was significantly higher than that of PDD (Hazard Ratio (HR):1.26, 95% CI: 1.04-1.53, P=0.02), suggesting cancer risk reduction is more prominent in PDD than in AD.
In both AD and PDD, nearly all the subgroup cancer SIRs were significantly lower than those of general population. AD patients with older age-at-diagnosis (≥ 80 years of age) had slightly less cancer risk (SIR: 0.78, 95% CI: 0.70-0.88) than those diagnosed between age 65 and 79 (SIR: 0.86, 95% CI: 0.78-0.94). Also, it seems the longer after dementia diagnosis, the stronger was the cancer-reduction effect (within-5-years SIR: 0.89, 95% CI: 0.82-0.97; after-5-years SIR: 0.63, 95% CI: 0.54-0.73). A similar trend could be seen for PDD. The overall cancer SIR in PDD patients diagnosed between age 65 and 79 was 0.76 (95% CI: 0.57-1.02) whereas the SIR in patients diagnosed at 80 or later was 0.57 (95% CI: 0.36-0.89). Likewise, the overall cancer SIR in PDD patients within the first 5 years of diagnosis was 0.76 (95% CI: 0.58-1.00) and the SIR decreased to 0.49 (95% CI: 0.28-0.85) after year 5. This finding was in line with the Kaplan- Meier plots in Figure 2 where the cancer risk difference between AD and PDD widened after year 5. Because the overall cancer HRs (of AD in reference to PDD) all exceeded 1, PDD patients were generally less likely to develop cancers than AD patients.
Table 3 displays the site-specific cancer SIRs respectively in AD and PDD as well as their corresponding HRs. Most of the SIRs were less than 1, meaning both the AD and PDD cohorts were less likely to develop cancers than general population. Patients with AD were significantly protected from digestive cancers (SIR: 0.82, 95% CI: 0.73-0.91), respiratory cancers (SIR: 0.67, 95% CI: 0.54-0.83) and genitourinary cancers (SIR: 0.79, 95% CI: 0.67-0.94). Among digestive cancers, liver cancers were particularly less frequent in AD (SIR: 0.67, 95% CI: 0.54- 0.83) than in normal controls. In PDD cohort, due to limited sample size, most SIRs did not reach significance level. Only respiratory cancers were found to be reduced in PDD (SIR: 0.53, 95% CI: 0.28-0.99). Skin cancers seemed somewhat increased (SIR: 1.09, 95% CI: 0.40- 2.97), yet the cancer case number was too few (n=8) to draw any conclusion. In terms of HR, patients with AD generally confer more cancer risk than patients with PDD, although only the HR for colorectal cancer was significant (HR: 1.83, 95% CI: 1.07-3.14). Compared to PDD, AD seemed to have less risk for skin cancers, but again the lower HR was not statistically significant (HR: 0.74, 95% CI: 0.35-1.57).
| Alzheimer's disease | Parkinson's disease dementia |
|||||
|---|---|---|---|---|---|---|
| Site (ICD9 codes) |
Number of cancers | SIR (95% CI) | Number of cancers | SIR (95% CI) | Hazard Ratio (95% CI) |
P-value |
| Overall cancer (140-208) | 1341 | 0.83 (0.77-0.89)*** | 110 | 0.70 (0.55-0.89)** | 1.26 (1.04-1.53)* | 0.020 |
| Oral cavity (140-149) | 36 | 0.83 (0.53-1.29) | 2 | 0.45 (0.08-2.38) | 1.88 (0.45-7.84) | 0.388 |
| Digestive | ||||||
| All (150-159) | 596 | 0.82 (0.73-0.91)*** | 53 | 0.75 (0.53-1.07) | 1.25 (0.95-1.66) | 0.117 |
| Stomach (151) | 109 | 0.84 (0.65-1.09) | 8 | 0.64 (0.27-1.57) | 1.29 (0.63-2.66) | 0.486 |
| Colorectal (153-154) | 262 | 0.95 (0.80-1.12) | 14 | 0.53 (0.28-1.00) | 1.83 (1.07-3.14)* | 0.029 |
| Liver (155) | 144 | 0.67 (0.54-0.83)*** | 21 | 0.98 (0.54-1.79) | 1.40 (0.84-2.33) | 0.198 |
| Respiratory | ||||||
| All (160-165) | 219 | 0.76 (0.64-0.91)** | 15 | 0.53 (0.28-0.99)* | 1.43 (0.85-2.42) | 0.182 |
| Lung (162) | 208 | 0.77 (0.65-0.92)** | 15 | 0.56 (0.30-1.06) | 1.36 (0.81-2.31) | 0.250 |
| Bone, skin, and breast | ||||||
| All (170-175) | 138 | 0.98 (0.78-1.24) | 14 | 1.07 (0.50-2.26) | 0.94 (0.54-1.63) | 0.821 |
| Skin (172-173) | 60 | 0.75 (0.54-1.05) | 8 | 1.09 (0.40-2.97) | 0.74 (0.35-1.57) | 0.435 |
| Breast (174), female only | 68 | 1.29 (0.90-1.85) | 3 | 0.60 (0.14-2.51) | 2.01 (0.63-6.42) | 0.237 |
| Genitourinary | ||||||
| All (179-189) | 235 | 0.79 (0.67-0.94)** | 18 | 0.62 (0.34-1.11) | 1.26 (0.78-2.05) | 0.341 |
| Prostate (185), male only | 85 | 0.88 (0.66-1.18) | 6 | 0.60 (0.22-1.66) | 1.50 (0.65-3.44) | 0.341 |
| Other and unspecified | ||||||
| All (190-199) | 51 | 0.84 (0.58-1.21) | 2 | 0.34 (0.07-1.71) | 2.44 (0.59-10.05) | 0.218 |
| Brain (191) | 11 | 1.43 (0.57-3.58) | 0 | NA | NA | |
| Haemopoietic | ||||||
| All (200-208) | 66 | 1.04 (0.74-1.47) | 6 | 0.98 (0.32-3.01) | 1.14 (0.49-2.65) | 0.758 |
Table 3: Comparison of site-specific cancer risks between Alzheimer’s disease and Parkinson’s disease dementia.
Discussion
In this study, our principal finding was that PDD, a mixture of PD and AD phenotype, may be more cancer protective than AD alone. The cancerprotectiveness prevailed in nearly all cancer types, although only gastric cancers, respiratory cancers and genitourinary cancers in AD and respiratory cancers in PDD were significantly less. Besides, the overall cancer risk became even lower if dementia patients were diagnosed later in life or followed up for a longer period.
Many previous studies have demonstrated the inverse association between PD and the overall cancer risk. Olsen et al. analyzed 14,088 PD patients from Danish registry data and estimated the overall cancer SIR to be 0.88 (95% CI: 0.8-0.9) [16]; Wirdefeldt et al. based on a Swedish matched cohort study to give a HR of 0.87 (95% CI: 0.79-0.96) [19]. In Asian populations, however, there are conflicting results. In a nationwide population-based study in Taiwan by Sun et al, the cancer risk (HR) in PD patients was estimated to be 0.88 (95% CI: 0.78-0.99) [18]. In contrast, Lin et al. reported that there was an increased cancer risk in PD patients [29]. Besides, Freedman and Pfeiffer used Medicare data to elucidate null association between PD and prevalent cancers in the Asian American patients [30]. Notably, the reports with an increased or null overall cancer risk for PD had some pitfalls in their methodology. In Lin et al.’s study in Taiwan, the cancer risk may be overestimated because the first year of follow-up was not removed from the analysis of associations. As compared with PD-free controls, there was about a four-fold higher risk of brain cancers in PD. This may be caused by more brain magnetic resonance imaging exams were evaluated for patients with PD. The enhanced scrutiny might as well result in early detection of brain cancers in the first year of follow-up. In the Freedman and Pfeiffer study, the study design was cross-sectional and thus insufficient to elicit the temporal association between PD and incident overall cancers.
Our report adds to the consistent finding: AD patients develop cancers at a slower rate than the general population. In our report, the SIR for all cancers in AD was 0.83 (95% CI: 0.77-0.89), which approximates the estimate (0.88 (95% CI: 0.80-0.97)) by Ou et al. using NHIRD in Taiwan.9 Two most recent meta-analyses suggest stronger cancer reduction in AD with similar pooled RRs (0.55 (95% CI: 0.41-0.75) and 0.58 (95% CI: 0.40-0.86)) [13,14]. However, heterogeneity among the included studies makes the pooled estimates hard to interpret. Without stringent sample exclusion, some of the studies might have recruited dementia patients associated with PDD or vascular dementia, rendering their estimates seemingly more on the cancer-reduction side.
Both late-onset AD and longer follow-up time (or more chronic AD) were associated with less cancer risk in our study. Musicco et al. demonstrated late-onset AD is associated with less cancer occurrence, but Ou et al. found null age-cancer association in their AD sample [9]. The lack of significance was probably due to an inadequate number of very old patients (aged ≥ 80 years) in Ou et al’s study. Consistent to our report, however, Ou et al. did reveal a lower overall cancer risk in AD with longer follow-up time. Like AD, PD and some other neurodegenerative diseases [1-5], schizophrenia has the same bearing on reduced cancer risk [31-35]. Our group observed a further reduction in cancer risk among late-onset or chronic schizophrenia patients [31]. Generally senescence is regarded as a carcinogen; very advanced age (80 years or older) may ironically become an anti-carcinogen due to decreased cancer-proliferating potential [36]. However, further reduction of cancer risk in elderly AD or PDD could not be accounted for by age alone because the SIRs were derived from contrasting risks in reference to agematched controls. Nevertheless, age modifies cancer risk in AD, PDD, and probably other neurodegenerative diseases as well. In the future, it is worthwhile clarifying whether the additional cancer-reduction effect (from further aging/ chronicity of AD or PDD) is related to a more severe neurodegenerative state.
The underlying mechanisms are still unknown concerning the lower cancer risk in neurodegenerative diseases such as AD and PD. The etiologies could be genetic or environmental, yet most of the studies emphasize the genetic aspects [36-40]. For instance, some genes which are upregulated in neurodegenerative diseases are concurrently downregulated in cancers, and vice versa [37]. With regard to PD, genes related with familial PD were tested for their expressions in different cancers. Parkin, Ubiquitin C-terminal hydroxylase, PINK1, and LRRK2 all have been found to be associated with cell-cycle control [39,40]. As far as hormone is concerned, circulating melatonin level was proposed to be high in PD patients but low in cancer patients [41]. Do PD-related genes and AD-related genes interact synergistically with each other to reduce cancer risk? Or it is the encoded proteins that actually matter in the pathogenesis of cancers? The exact mechanisms must be verified through extensive research.
Some strengths and limitations of our report need to be addressed. First, the selection criteria for patients with AD or PDD were stringent. Our AD phenotype was strictly selected from patients with catastrophic certificates denoting dementia. Any possibility of vascular dementia or organic brain syndrome was carefully excluded. Specifically, Lewy-body dementia (LBD) which mimics PD in many aspects was deliberately excluded. Furthermore, the PDD cohort was selected from dementia patients who had an existing PD diagnosis which was confirmed by clinical neurologists. Any inclusion or exclusion of AD or PDD subjects was largely unbiased. Second, as a population-based cohort study, our AD sample is by far the largest one, thus providing quite reliable cancer risk estimates. For an elderly population where attrition rate is high and follow-up time can be hardly long, sample size should be as large as possible. Third, most identifiable comorbidities that may confound the relative cancer risk between AD and PDD were carefully adjusted.
As for limitations, first, although all the AD and PDD patients were diagnosed carefully in the clinical sense, we did not have pathological confirmation. Identification of probable AD patients may have included those with frontotemporal dementia. Besides, excluding vascular risk factors removes patients with multiinfarct dementia but probably not patients with severe white matter disease. Despite the imperfections in verification of dementia etiologies, for a population-based study with large sample size, it is not feasible to have pathological validation of each single subject. Second, some potential confounding factors could not be adjusted inexhaustibly owing to the intrinsic limitations of NHIRD. For instance, smoking or statin use may be associated with both the risk of dementia and cancer, yet the information was not available in our database.
In conclusion, both patients with AD and PDD are protected from incident overall cancers compared to the general population. In addition, patients with PDD are less prone to cancer development than those with AD, corresponding well to our research hypothesis. For both dementias, late-onset and disease chronicity also incur further cancer reduction. The precise mechanisms and pathophysiology deserve more research attention.
References
- Driver JA. Inverse association between cancer and neurodegenerative disease: review of the epidemiologic and biological evidence. Biogerontology15(6),547-557 (2014).
- Catala-Lopez F, Suarez-Pinilla M, Suarez-Pinilla P, et al. Inverse and direct cancer comorbidity in people with central nervous system disorders: a meta-analysis of cancer incidence in 577,013 participants of 50 observational studies. Psychother. Psychosom83(2), 89-105(2014).
- Tabares-Seisdedos R, Rubenstein JL. Inverse cancer comorbidity: a serendipitous opportunity to gain insight into CNS disorders. Nat. Rev. Neurosci14(4), 293-304(2013).
- Tabares-Seisdedos R, Dumont N, Baudot A, et al. No paradox, no progress: inverse cancer comorbidity in people with other complex diseases. Lancet. Oncol12(6), 604-608(2011).
- Turner MR, Goldacre R, Goldacre MJ. Reduced cancer incidence in Huntington's disease: record linkage study clue to an evolutionary trade-off? Clin. Genet83(6), 588-590 (2013).
- Romero JP, Benito-Leon J, Louis ED, et al. Alzheimer's disease is associated with decreased risk of cancer-specific mortality: a prospective study (NEDICES). J. Alzheimers. Dis40(2), 465-473 (2014).
- Roe CM, Fitzpatrick AL, Xiong C, et al. Cancer linked to Alzheimer disease but not vascular dementia. Neurology 74(2),106-112 (2010).
- Roe CM, Behrens MI, Xiong C, et al. Alzheimer disease and cancer. Neurology64(5), 895-898 (2005).
- Ou SM, Lee YJ, Hu YW, et al. Does Alzheimer's disease protect against cancers? A nationwide population-based study. Neuroepidemiology40(1), 42-49 (2013).
- Realmuto S, Cinturino A, Arnao V, et al. Tumor diagnosis preceding Alzheimer's disease onset: is there a link between cancer and Alzheimer's disease? J. Alzheimers. Dis31(1), 177-182 (2012).
- Musicco M, Adorni F, Di Santo S, et al. Inverse occurrence of cancer and Alzheimer disease: a population-based incidence study. Neurology81(4):322-328 (2013).
- Driver JA, Beiser A, Au R, et al. Inverse association between cancer and Alzheimer's disease: results from the Framingham Heart Study.BMJ344, e1442 (2012).
- Ma LL, Yu JT, Wang HF, et al. Association between cancer and Alzheimer's disease: systematic review and meta-analysis. J. Alzheimers. Dis42(2), 565-573 (2014).
- Shi HB, Tang B, Liu YW, et al. Alzheimer disease and cancer risk: a meta-analysis. J. Cancer. Res. Clin. Oncol141(3), 485-494 (2015).
- Minami Y, Yamamoto R, Nishikouri M, et al. Mortality and cancer incidence in patients with Parkinson's disease. J. Neurol.247(6), 429-434 (2000).
- Olsen JH, Friis S, Frederiksen K, et al. Atypical cancer pattern in patients with Parkinson's disease. Br. J. Cancer92(1),201-205 (2005).
- Driver JA, Logroscino G, Buring JE, et al. A prospective cohort study of cancer incidence following the diagnosis of Parkinson's disease. Cancer. Epidemiol. Biomarkers. Prev16(6), 1260-1265 (2007).
- Sun LM, Liang JA, Chang SN, et al. Analysis of Parkinson's disease and subsequent cancer risk in Taiwan: a nationwide population-based cohort study. Neuroepidemiology37(2), 114-119 (2011).
- Wirdefeldt K, Weibull CE, Chen H, et al. Parkinson's disease and cancer: A register-based family study. Am. J. Epidemiol179(1), 85-94 (2014).
- Bajaj A, Driver JA, Schernhammer ES. Parkinson's disease and cancer risk: a systematic review and meta-analysis. Cancer Causes & Control : CCC 21(5), 697-707 (2010).
- Rugbjerg K, Friis S, Lassen CF, et al. Malignant melanoma, breast cancer and other cancers in patients with Parkinson's disease. Int. J. Cancer131(8), 1904-1911 (2012).
- Quinn NP, Rossor MN, Marsden CD. Dementia and Parkinson's disease--pathological and neurochemical considerations. Br. Med. Bull42(1), 86-90 (1986).
- Compta Y, Parkkinen L, O'Sullivan SS, et al. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain134(Pt 5), 1493-1505 (2011).
- Lennox G. Dementia with Lewy bodies. In: Growdon J, Rossor M, eds. The dementias. Boston: Butterworth-Heinemann, 67-80 (1998).
- Jellinger KA. Prevalence of Alzheimer lesions in Parkinson's disease. Mov Disord18(10), 1207-1208 (2003).
- Papapetropoulos S, Lieberman A, Gonzalez J, et al. Can Alzheimer's type pathology influence the clinical phenotype of Parkinson's disease? Acta neurologica Scandinavica111(6), 353-359 (2005).
- McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement7(3), 263-269 (2011).
- Cancer Control Act. http://www.hpa.gov.tw/English/ClassShow.aspx?No=200803260008 Accessed 2016/9/2, 2016.
- Lin PY, Chang SN, Hsiao TH, et al. Association Between Parkinson Disease and Risk of Cancer in Taiwan. JAMA Oncology1(5), 633-640 (2015).
- Freedman DM, Pfeiffer RM. Associations Between Parkinson Disease and Cancer in US Asian Americans. JAMA Oncology2(8), 1093-1094 (2016).
- Lin GM, Chen YJ, Kuo DJ, et al. Cancer incidence in patients with schizophrenia or bipolar disorder: a nationwide population-based study in Taiwan, 1997-2009. Schizophr. Bull39(2), 407-416 (2013).
- Lin CY, Lane HY, Chen TT, et al. Inverse association between cancer risks and age in schizophrenic patients: a 12-year nationwide cohort study. Cancer. Sci104(3), 383-390 (2013).
- Chou FH, Tsai KY, Su CY, et al. The incidence and relative risk factors for developing cancer among patients with schizophrenia: a nine-year follow-up study.Schizophr.Res129(2-3), 97-103 (2011).
- Barak Y, Achiron A, Mandel M, et al. Reduced cancer incidence among patients with schizophrenia. Cancer 104(12), 2817-2821 (2005).
- Grinshpoon A, Barchana M, Ponizovsky A, et al. Cancer in schizophrenia: is the risk higher or lower? Schizophr Res73(2-3), 333-341 (2005).
- Harding C, Pompei F, Lee EE, et al. Cancer suppression at old age. Cancer Res68(11), 4465-4478 (2008).
- West AB, Dawson VL, Dawson TM. To die or grow: Parkinson's disease and cancer.Trends. Neurosci28(7), 348-352 (2005).
- Driver JA, Lu KP. Pin1: a new genetic link between Alzheimer's disease, cancer and aging. Curr Aging Sci3(3), 158-165 (2010).
- Ibanez K, Boullosa C, Tabares-Seisdedos R, et al. Molecular evidence for the inverse comorbidity between central nervous system disorders and cancers detected by transcriptomic meta-analyses. PLoS. Genet10(2), e1004173 (2014).
- Ruiz-Martinez J, de la Riva P, Rodriguez-Oroz MC, et al. Prevalence of cancer in Parkinson's disease related to R1441G and G2019S mutations in LRRK2. Mov. Disord29(6), 750-755 (2014).
- Schernhammer E, Chen H, Ritz B. Circulating melatonin levels: possible link between Parkinson's disease and cancer risk? Cancer Causes &Control: CCC17(4), 577-582 (2006).