Research Article - Neuropsychiatry (2017) Volume 7, Issue 6
Do NeuroD Gene Polymorphisms Predict the Risk of Heroin Dependence or Mediate the Association between Personality Traits and Heroin Dependence?
- Corresponding Author:
- San-Yuan Huang MD, Ph.D
Director, Department of Psychiatry
Tri-Service General Hospital, Taipei
Taiwan. Professor, National Defense Medical Center, Taipei, Taiwan
Tel: (0) + 886-2-87923311, ext 17388
Fax: + 886-2-87926715
Abstract
Abstract
Objective:Heroin dependence (HD) affects brain development and is known to be heritable. The NeuroD gene encodes a neuroge nic differentiation factor, and its expression is essential for the development of the central nervous system. In this genetic case-control study, we aimed to investigate whether NeuroD gene polymorphisms associate with the occurrence of HD and the specific personality traits of patients with HD.
Methods: 1107 unrelated participants (584 patients with HD and 523 controls) were recruited to the study The patients with HD were classified into six clinical subgroups based on their gender, duration, and age of onset to reduce heterogeneity. In total, 539 subjects completed the personality trait assessments.
Results: We found a weak association between the NeuroD 1 rs16867467 locus and HD (p=0.048); this weak association was found only in the male (p=0.039) and late onset (p=0.047) HD subgroups. These findings could not be confirmed after haplotype analysis and Bonferroni corrections for multiple comparisons. Patients with HD had higher novelty seeking (NS) and harm avoidance (HA) scores than healthy subjects. However, none of the polymorphisms in the NeuroD gene affected the NS and HA scores in both patients and healthy subjects (p>0.05). A negative correlation was found between age and novelty seeking scores in both groups, suggesting novelty seeking personality trait as a risk factor for early-onset HD.
Conclusion:This study suggests that the NeuroD gene may neither contribute to the risk of HD nor mediate the relationship between specific personality traits and HD.
Keywords
Heroin dependence, NeuroD, Novelty seeking, Harm avoidance, Personality traits
Acronyms
HD: Heroin Dependence; SADS-L: Schedule Of Affective Disorder And Schizophrenia-Life Time; DSM-IV-TR: Diagnostic And Statistical Manual Of Mental Disorders; 4th Edition; TPQ: Tridimensional Personality Questionnaire; NS: Novelty Seeking; HA: Harm Avoidance; RD: Reward Dependence; SNP: Single Nucleotide Polymorphism; LD: Linkage Disequilibrium; NC: Normal Controls
Introduction
Heroin addiction involves impulsive/compulsive drug seeking, tolerance, and physical dependence [1]. It is associated with high rates of mortality, morbidity, and other adverse consequences, and constitutes a worldwide public health crisis [2]. With dramatic advances in molecular biology during the past decade, heroin dependence (HD) is now considered a chronic, relapsing brain disease with heritable vulnerability. Family twin studies show that the genetic background of an individual accounts for about 50% of the inherited risk to heroin addiction, which suggests substantial heritability [3-5].
Heroin dependence affects the development and differentiation of the brain and changes in neuroactivity [6]. In addition, heroin-induced weakening of brain activity may lead to repeated relapse [7]. The developing brain of adolescent children is particularly susceptible to the effects of drugs abuse [8]; therefore, it is hypothesized to contribute to their increased propensity for drug use [6]. Heroin addiction also has a negative influence on neurogenesis in adults. It has been observed that during self-administration of various drugs and after withdrawal/relapse, there is a reduction in the spontaneous neurogenesis in the brain in animal models, which may result in drug taking/drug seeking behavior [9]. Postmortem human studies of heroin addicts also demonstrated a decreased number and low rates of proliferation of neural precursor cells [10], which suggests an important association between NeuroD development and heroin addiction.
NeuroD is a neurogenic differentiation factor, and is also known as a tissue-specific member of the basic helix–loop–helix (bHLH) family [11,12]. The expression of NeuroD is essential for the development of multiple tissues at different stages, especially in the central nervous system [13]. The NeuroD family is composed of four members, including NeuroD 1, NeuroD 2, NeuroD 4 (also known as neuroM or MATH-3), and NeuroD 6 (also known as Nex1 or MATH-2) [14], each of which exhibits an overlapping but distinct spatiotemporal expression profile [15]. The NeuroD 1 gene is located on chromosome 2q31.3, spanning 4~5 kb; NeuroD 2, on chromosome 17q12, 4~5 kb; NeuroD 4, on chromosome 12q13.2, ~10 kb; and NeuroD 6, on chromosome 7p14.3, 3~4 kb. Previous postmortem studies of the human brain revealed lower NeuroD 2 mRNA levels in the cortex and hippocampus of opiate-dependent individuals, suggesting the possible impact of the NeuroD protein on heroin-abusing behavior and in sustaining addiction [16]. The genetic heterogeneity of the NeuroD gene may have functional consequences, as several studies have linked the genetic variants of NeuroD to diabetes mellitus [17], alcohol dependence [18] and schizophrenia [19]. We postulate that heroin addiction may be associated with NeuroD gene variants owing to the substantial heritability of HD and its possible association with NeuroD development and NeuroD differentiation.
Despite the heritability of vulnerability to HD and the fact that NeuroD variants may play an important role in the development of HD, no studies have reported an association between the genetic variants of NeuroD and HD. In addition, the heterogeneity in the clinical features such as the age of onset of HD and the personality traits of patients may influence the genetic susceptibility [20]. For example, the early onset of HD has been shown to be associated with more frequent drug use, quicker escalation to higher levels of use, greater persistence in using with poorer clinical and functional outcomes [21], and the personality of heroin addicts show higher novelty-seeking and antisocial profiles [22].
This study aimed to determine whether NeuroD gene polymorphisms are associated with the occurrence of HD. In addition, we determined whether the age of onset of HD is related to NeuroD genetic variants and if the relationship between the NeuroD gene and the personality traits of the patients influences the pathogenesis of HD.
Methods
▪ Participant
The study protocol was approved by the Institutional Review Board for the Protection of Human Subjects at the Tri-Service General Hospital (TSGH), a medical teaching hospital that belongs to the National Defense Medical Center in Taipei, Taiwan. The study was carried out in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all the participants. All the participants were unrelated, born and living in Taiwan, and all their biological grandparents were of Han Chinese ancestry. The study participants were all selected from the Han Chinese population to minimize the effect of ethnic differences on gene frequencies.
▪ Group of patients with HD
Patients were recruited from various clinical settings. Each patient was initially examined by an experienced attending psychiatrist and then interviewed by a well-trained psychologist using the Chinese Version of Modified Schedule of Affective Disorder and Schizophrenia-Life Time (SADS-L) test. The SADS-L is designed according to the Research Diagnostic Criteria [23], with inter-rater reliability k values for substance abuse and dependence of 0.82 [24]. The final diagnosis was confirmed with the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV-TR), and all the patients in this study met the DSM-IV-TR criteria for HD.
To reduce the heterogeneity related to confounding variables such as age of onset, duration of drug dependence, and gender, the HD group was further divided into six subgroups: early-onset HD (onset age, ≤25 years) and late-onset HD (onset age, >25 years) subgroups based on age of onset; duration ≤5 years and >5 years based the duration of drug dependence; and male and female based on sex. A Chinese version of the Tridimensional Personality Questionnaire (TPQ) was used for assessing specific personality traits in the HD group. The TPQ is a self-report questionnaire that measures three genetically distinct personality dimensions: novelty seeking (NS), harm avoidance (HA), and reward dependence (RD) [25].
Group of healthy controls
The healthy controls were recruited from the community. A Chinese version of the modified SADS-L was used to screen out individuals in the control group who had psychiatric conditions. All the subjects in the control group were free of past/present major or minor mental disorders. In addition, there was no family history of psychiatric disorders or substance use disorders in the first-degree relatives of the control subjects.
▪ Blood sampling and DNA extraction
After informed consent was obtained from all the participants, a peripheral vein blood sample from each subject was drawn into a vacutainer tube containing the anticoagulant ethylenediaminetetracetic acid (EDTA). Genomic DNA was extracted from the leukocytes using a commercial kit (DNAzol; Invitrogen, Carlsbad, CA, USA).
▪ Selection of neuroD variants
The study variants of NeuroD were selected on the basis of National Center for Biotechnology Information Single Nucleotide Polymorphism (SNP) database (www.ncbi.nlm.nih.gov/ projects/SNP/) and an available TaqMan assay from Applied Biosystems. We selected 7 SNPs with minor allele frequencies of more than 0.1 to represent the NeuroD gene family.
▪ Genotyping methods for NeuroD gene
Genetic polymorphisms of NeuroD 1 (rs1801262, rs16867467, rs2583016), NeuroD 2 (rs12453682), NeuroD 4 (rs2656804, rs1532833), and NeuroD 6 (rs2233404) were genotyped using TaqMan assays (Applied Biosystems, Foster City, CA, USA) employing FAM™ and VIC® dyes as per manufacturer protocol. We used the Applied Biosystems STEPONE™ software and STEPONEPLUS™ real-time PCR systems for thermocycling and data collection. For quality control, genotyping accuracy was confirmed by restriction fragment length polymorphism (RFLP) methods and bidirectional direct sequencing of 50 random samples of DNA with a model 3730 DNA analyzer (Applied Biosystems).
Statistical Analysis
▪ The SPSS statistical software
The independent samples t-test and Pearson’s chi-square analysis were used to compare the clinical and demographical parameters between patients with HD and healthy controls and between subgroups. Hardy–Weinberg equilibrium was assessed for each group, and the allele and genotype frequencies for each individual polymorphism were compared between patients and controls by using the two-tailed Pearson’s chi-square analysis; the Fisher’s exact test was used instead of Pearson’s chi-square test when the sample sizes were smaller than expected (<5 subjects). To assess the influence of age, gender, and NeuroD gene variants on the incidence of HD, we conducted a logistic regression using patient/ control group as the binominal dependent variable (using the dominant model). A oneway analysis of variance (ANOVA) was used to compare the NS and HA scores between polymorphisms in patients with HD and healthy controls. The SPSS (version 20, SPSS Inc., Chicago, IL) statistical software was used for all the analyses, and results with p<0.05 were considered statistically significant.
▪ The HAPLOVIEW software
The linkage disequilibrium (LD) coefficients (D′), haplotype frequency, haplotype block, haplotype association, and the Hardy–Weinberg equilibrium for each SNP were assessed using the HAPLOVIEW software (version 4.2, Broad Institute, Cambridge, MA) [26]. We defined a haplotype block as a set of contiguous SNPs with the four-gamete rule, as there is no evidence of recombination between loci [27]. All tests were two-tailed and the alpha was set at 0.05. In addition, to correct the possible bias and inflation of significance after multiple comparisons, we used Bonferroni corrections and 10000 permutation procedures for the single-marker and haplotype tests, respectively.
The G-POWER software
The power analysis was performed using the G-POWER 3.1 software [28]. Our total sample size (n:1107) had a power of ~0.85 to detect a small effect (effect size:0.1) and 1.00 to detect medium (effect size:0.3) and large (effect size:0.5) effects of genotype distributions. Our study had a power of ~0.91 to detect a small effect in the allele frequencies of these 7 polymorphisms and a power of 1.00 to detect medium and large effects.
Results
▪ Clinical characteristics
The study sample consisted of 1130 unrelated Han Chinese subjects, of which 23 were excluded because of incomplete genotype data. Therefore, the data from 1107 subjects (584 patients and 523 healthy controls) were analyzed in the genetic study. The average age of onset of HD in the patients was 28.4 ± 7.7 years, while the average duration of HD was 10.0 ± 8.8 years. There were no significant differences in the mean age (patients, 38.4 ± 9.3 years; controls, 39.1 ± 12.0 years; p=0.232) or gender ratio (patients, 354:230 male: female; controls, 346=177 male: female; p:0.056) between groups. However, the patients with HD had a lower mean educational level than controls (patients, 11.0 ± 3.8 years; controls, 14.6 ± 2.6 years; p<0.001). Among HD patients in our subjects, the comorbidity of “nicotine dependence”, “amphetamine dependence” or “mood disorders or anxiety disorders” is 84%, 56%, 27%, respectively.”
▪ Allele and genotype frequency analysis
Seven NeuroD SNPs were genotyped in this study, and the allele and genotype frequencies are shown in Table 1. All the genetic variants of the 7 SNPs were in Hardy–Weinberg equilibrium in both patients and controls. The allele frequencies were not significantly associated between the 7 NeuroD SNPs and HD (p>0.05). The genotype frequency analysis revealed a weak association between the rs16867467 polymorphism of NeuroD 1 and HD (χ2:6.082, p=0.048). However, this weak association was only found in the late onset (χ2:6.099, uncorrected p=0.047) and male HD subgroups (χ2:6.477, uncorrected p:0.039); other 6 NeuroD SNPs did not show an association with HD. None of the NeuroD polymorphisms were associated with the duration of HD in the patients (p>0.05, Table 1). When dividing HD patients into subgroups of with or without psychiatric comorbidities, the weak association of NeuroD1 rs16867467 polymorphism and the occurrence of HD remained in patients with psychiatric comorbidities (“nicotine dependence”, “amphetamine dependence” or “mood disorders or anxiety disorders”), but not in patients without psychiatric comorbidities. However, these association findings could not be confirmed by Bonferroni corrections.
| NeuroD Family | Variants | Location in neuroD |
Position reference dbSNP | MAF | pa | Alleleb | Controls (n=523) | Total HD (n=584) | pc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HD | NC | 1 | 2 | Genotype, n (%) | Genotype, n (%) | |||||||||||
| 1/1 | 1/2 | 2/2 | 1/1 | 1/2 | 2/2 | |||||||||||
| NeuroD1 | rs1801262 | Exon | 182543455 | 0.074 | 0.074 | 0.999 | T | C | 5 (1.0) | 67 (12.8) | 451 (86.2) | 5 (0.9) | 76 (13.0) | 503 (86.1) | 0.983 | |
| rs16867467 | Intron | 182544889 | 0.217 | 0.195 | 0.210 | T | C | 26 (5.0) | 152 (29.1) | 345 (66.0) | 22 (3.8) | 209 (35.8) | 353 (60.4) | 0.048* | ||
| rs2583016 | 5’UTR | 182545218 | 0.074 | 0.075 | 0.933 | C | T | 5 (1.0) | 68 (13.0) | 450 (86.0) | 3 (0.5) | 80 (13.7) | 501 (85.8) | 0.652 | ||
| NeuroD2 | rs12453682 | Unknown | 37770005 | 0.395 | 0.404 | 0.642 | T | C | 94(18.0) | 235 (44.9) | 194 (37.1) | 90(15.4) | 281 (48.1) | 213 (36.5) | 0.425 | |
| NeuroD4 | rs2656804 | 3’UTR | 55421250 | 0.378 | 0.360 | 0.381 | T | C | 65(12.4) | 247 (47.2) | 211 (40.3) | 81(13.9) | 280 (47.9) | 223 (38.2) | 0.673 | |
| rs1532833 | Intron | 55416041 | 0.378 | 0.360 | 0.381 | T | G | 65(12.4) | 247 (47.2) | 211 (40.3) | 81(13.9) | 280 (47.9) | 223 (38.2) | 0.673 | ||
| NeuroD6 | rs2233404 | Intron | 31378933 | 0.289 | 0.262 | 0.198 | G | A | 31 (5.9) | 215 (41.1) | 277 (53.0) | 50 (8.6) | 238 (40.8) | 296 (50.7) | 0.234 | |
| NeuroDFamily | Variants | Late onset HD (n=354) |
pc | HD duration > 5 years (n=340) | pc | Male NC (n=346) |
Male HD (n=354) |
pc | ||||||||
| Genotype, n (%) | Genotype, n (%) | Genotype, n (%) | Genotype, n (%) | |||||||||||||
| 1/1 | 1/2 | 2/2 | 1/1 | 1/2 | 2/2 | 1/1 | 1/2 | 2/2 | 1/1 | 1/2 | 2/2 | |||||
| NeuroD1 | rs1801262 | 4(1.1) | 0.418 | 302(85.3) | 0.879 | 1(0.3) | 43(12.6) | 296(87.1) | 0.616 | 2(0.6) | 41(11.8) | 303(87.6) | 1(0.3) | 54(15.3) | 299(84.5) | 0.418 |
| rs16867467 | 16(4.5) | 131(37.0) | 207(58.5) | 0.047* | 15(4.4) | 123(36.2) | 202(59.4) | 0.091 | 18(5.2) | 96(27.7) | 232(67.1) | 17(4.8) | 130(36.7) | 207(58.5) | 0.039* | |
| rs2583016 | 2(0.6) | 52(14.7) | 300(84.7) | 0.698 | 0(0.0) | 44(12.9) | 296(87.1) | 0.262 | 1(0.3) | 43(12.4) | 302(87.3) | 0(0.0) | 56(15.8) | 298(84.2) | 0.233 | |
| NeuroD2 | rs12453682 | 56(15.8) | 168(47.5) | 130(36.7) | 0.646 | 50(14.7) | 168(49.4) | 122(35.9) | 0.319 | 61(17.6) | 150(43.4) | 135(39.0) | 52(14.7) | 168(47.5) | 134(37.9) | 0.439 |
| NeuroD4 | rs2656804 | 53(15.0) | 164(46.3) | 137(38.7) | 0.550 | 50(14.7) | 175(51.5) | 115(33.8) | 0.144 | 43(12.4) | 164(47.4) | 139(40.2) | 53(15.0) | 173(48.9) | 128(36.2) | 0.440 |
| rs1532833 | 53(15.0) | 164(46.3) | 137(38.7) | 0.550 | 50(14.7) | 175(51.5) | 115(33.8) | 0.144 | 42(12.1) | 165(47.7) | 139(40.2) | 53(15.0) | 173(48.9) | 128(36.2) | 0.402 | |
| NeuroD6 | rs2233404 | 29(8.2) | 150(42.4) | 175(49.4) | 0.337 | 34(10.0) | 139(40.9) | 167(49.1) | 0.077 | 20(5.8) | 148(42.8) | 178(51.4) | 35(9.9) | 138(39.0) | 181(51.1) | 0.112 |
Abbreviations: NC, normal controls; HD, heroin dependence.
a Minor allele frequency (MAF) in patients with HD compared with the control group using the Pearson’s χ2 test.
b Allele 1 (bold, italicized) indicates the minor allele, and only alleles with a frequency higher than 5% are shown.
c Genotype frequencies in patients with HD or its subgroups compared with the controls using the Pearson’s χ2 test.
* A p value < 0.0014 (0.05/35) was considered significant after Bonferroni’s correction.
Table 1: Gene location, allele and genotype frequencies of the neuroD gene polymorphisms among patients with HD and controls.
▪ Haplotype analysis
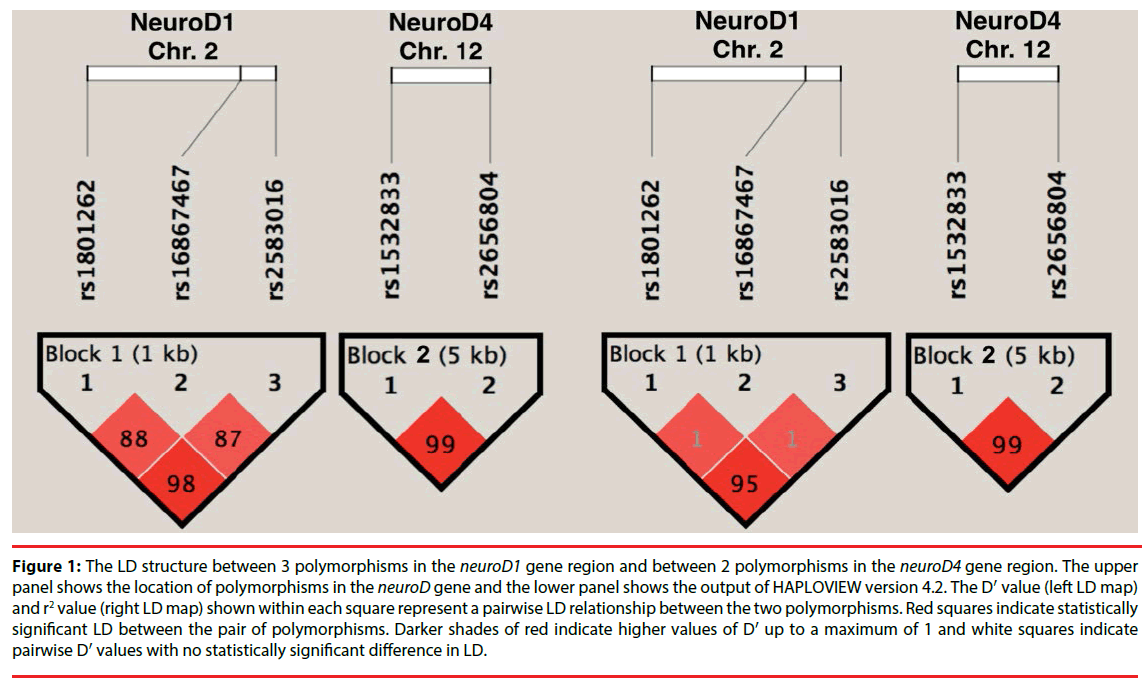
The association of NeuroD variants with HD was further investigated using haplotype analysis. Figure 1 depicts the pairwise linkage disequilibrium (LD) of the SNPs with an LD map and block structure of the studied NeuroD polymorphisms and the D′ values. The haplotype block 1 (rs1801262–rs16867467- rs2583016) includes 3 SNPs in NeuroD 1, while the haplotype block 2 (rs1532833–rs2656804) includes 2 SNPs in NeuroD 4. There was no significant difference between HD and control subjects in the NeuroD 1 and NeuroD 4 haplotype frequencies, respectively (P>0.05 after correcting for multiple comparisons with 10000 permutations). Further analyses in the HD subgroups based on the age of onset, sex, and duration failed to reveal a significant difference in the haplotype frequencies between the HD and control groups (p>0.05; Table 2).
Figure 1: The LD structure between 3 polymorphisms in the neuroD1 gene region and between 2 polymorphisms in the neuroD4 gene region. The upper panel shows the location of polymorphisms in the neuroD gene and the lower panel shows the output of HAPLOVIEW version 4.2. The D′ value (left LD map) and r2 value (right LD map) shown within each square represent a pairwise LD relationship between the two polymorphisms. Red squares indicate statistically significant LD between the pair of polymorphisms. Darker shades of red indicate higher values of D′ up to a maximum of 1 and white squares indicate pairwise D′ values with no statistically significant difference in LD.
| Haplotype block 1 (In NeuroD1) | Frequency | Pa | Pb | Pc | Pd | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs1801262 | rs16867467 | rs2583016 | Total NC |
Total HD |
Early onset HD | Late onset HD |
Male NC | Male HD | ||||
| C | C | T | 0.732 | 0.709 | 0.742 | 0.689 | 0.746 | 0.690 | 0.725 | 1.000 | 0.244 | 0.064 |
| C | T | T | 0.193 | 0.215 | 0.192 | 0.229 | 0.188 | 0.229 | 0.665 | 1.000 | 0.279 | 0.171 |
| T | C | C | 0.070 | 0.071 | 0.062 | 0.075 | 0.060 | 0.076 | 1.000 | 1.000 | 1.000 | 0.950 |
| Haplotype block 2 (In NeuroD4) | Frequency | Pa | Pb | Pc | Pd | |||||||
| rs1532833 rs2656804 | Total NC | Total HD |
Early onsetHD | Late onset HD | Male NC | Male HD | ||||||
| G C | 0.638 | 0.622 | 0.626 | 0.619 | 0.638 | 0.606 | 0.880 | 1.000 | 0.991 | 0.942 | ||
| T T | 0.359 | 0.378 | 0.374 | 0.381 | 0.359 | 0.394 | 0.880 | 1.000 | 0.970 | 0.932 | ||
Abbreviations: NC, normal controls; HD, heroin dependence.
Haplotype frequencies shown in the table were >0.01
All the p values were corrected using the 10000 permutation procedure.
a Healthy controls vs. patients with HD (all)
b Healthy controls vs. patients with early-onset HD (HD onset age ≤ 25 years).
c Healthy controls vs. patients with late-onset HD (HD onset age > 25 years).
d Healthy controls (males) vs. patients with HD (males).
Table 2: Haplotype analysis of the neuroD gene in patients with HD and controls.
▪ Dominant model to reclassify each genotype of the SNPs
We performed logistic regression analyses using age and sex as covariates to assess the influence of each NeuroD gene variant on the pathogenesis of HD (Table 3). We used the dominant model to reclassify each genotype of the NeuroD SNPs as a binominal dependent variable. After correcting for the confounding effects of age and sex, we found no significant influence of these markers on the occurrence of HD (p:0.052). Although the rs16867467-T allele carriers were slightly more likely to develop HD in the late onset (uncorrected p:0.027) and male (uncorrected p:0.017) subgroups, these associations were not statistically significant after Bonferroni corrections (reached a basic significant association, α design was 0.05/7:0.007).
| NeuroD Family | Variants (reference) | Total HD (n = 584) | Late-onset HD (n = 354) | Male HD (n = 354) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR b | 95% CI | p c | OR b | 95% CI | p d | OR b | 95% CI | pe | |||||
| NeuroD1 | rs1801262 | T/T and T/C | (C/C) a | 1.012 | 0.718 – 1.425 | 0.947 | 1.071 | 0.727 – 1.576 | 0.729 | 1.302 | 0.845 – 2.006 | 0.232 | |
| rs16867467 | T/T and T/C | (C/C) a | 1.276 | 0.998 – 1.632 | 0.052 | 1.370 | 1.036 – 1.811 | 0.027* | 1.457 | 1.069 – 1.986 | 0.017* | ||
| rs2583016 | C/C and T/C | (T/T) a | 1.025 | 0.729 – 1.439 | 0.889 | 1.102 | 0.752 – 1.614 | 0.620 | 1.295 | 0.844 – 1.988 | 0.237 | ||
| NeuroD2 | rs12453682 | T/T and T/C | (C/C) a | 1.013 | 0.793 – 1.295 | 0.917 | 1.027 | 0.775 – 1.360 | 0.852 | 1.074 | 0.790 – 1.460 | 0.647 | |
| NeuroD4 | rs2656804 | T/T and T/C | (C/C) a | 1.111 | 0.872 – 1.416 | 0.396 | 1.062 | 0.805 – 1.402 | 0.670 | 1.166 | 0.857 – 1.585 | 0.328 | |
| rs1532833 | T/T and T/G | (G/G) a | 1.111 | 0.872 – 1.417 | 0.394 | 1.061 | 0.804 – 1.400 | 0.675 | 1.164 | 0.856 – 1.582 | 0.333 | ||
| NeuroD6 | rs2233404 | GG and G/A | (A/A) a | 1.103 | 0.871 – 1.398 | 0.416 | 1.149 | 0.876 – 1.506 | 0.315 | 0.997 | 0.740 – 1.343 | 0.983 | |
Abbreviations: HD, heroin dependence; OR, odds ratio; CI, confidence intervals.
a Genotype within parenthesis indicates the reference group of genotype.
b Odds ratio is given with 95% confidence intervals (95% CI) after using a logistic regression analysis.
c Healthy controls vs. patients with HD (all).
d Healthy controls vs. patients with late onset HD (HD onset age > 25 years).
e Healthy controls (males) vs. patients with HD (males).
* A p value < 0.0024 (0.05/21) was considered significant after Bonferroni correction.
Table 3: A logistic regression analysis of the neuroD gene polymorphisms as risk factors for heroin dependence.
▪ NeuroD gene and age in the determination of personality traits
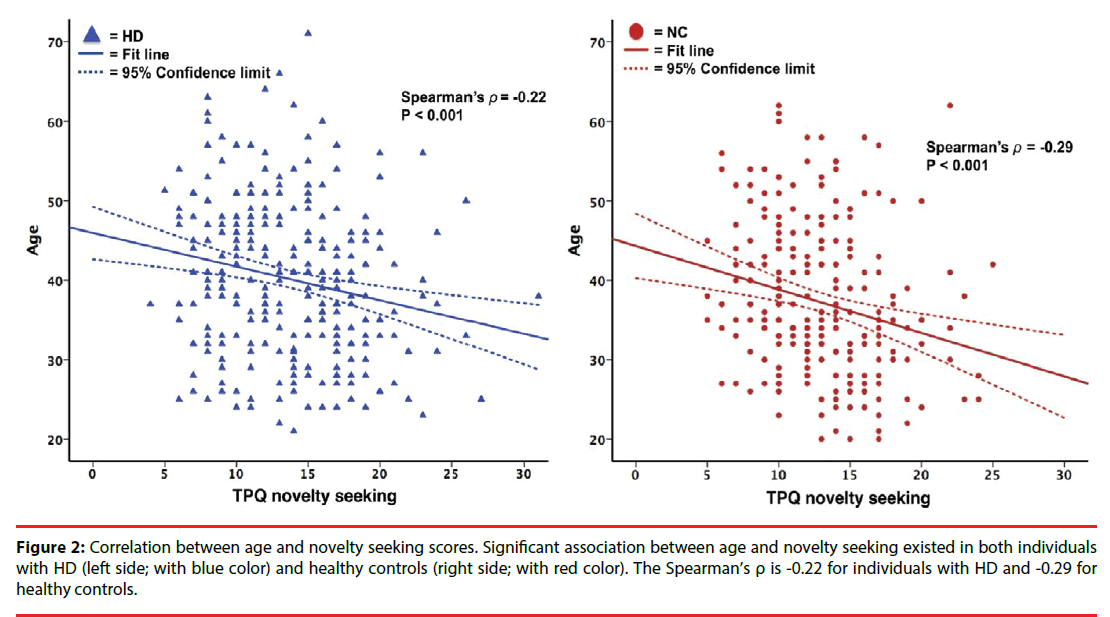
Of the participants, 539 completed the personality assessment with the Chinese version of TPQ (n:292 for patients with HD; n:247 for healthy controls). For TPQ subscores, we only analyzed the NS and HA dimensions in our study given the low inter-reliability of the RD dimension scores in the Han Chinese population of Taiwan. The HA and NS scores were significantly higher in the HD group (mean NS/ HA scores, 13.8± 4.7/12.2 ± 4.9) than in the NC group (mean NS/HA scores, 12.8 ± 4.3/10.8 ± 5.2). However, the subgroup analyses revealed that the NS scores are significantly higher in the early onset HD subgroup (NS scores, 15 ± 4.8; p<0.001) but not in the late onset HD subgroup (NS scores, 13.0 ± 4.4; p=0.621). We found no significant association between the NeuroD genotype and the NS and HA personality traits in both the groups (p>0.05, Table 4). We further analyzed the role of age and sex in determining specific personality traits. We found a negative correlation between age and NS scores in both controls and patients (p<0.001, Figure 2), while no correlation was found between age and HA scores in the two groups (p>0.05, data not shown). In addition, the psychiatric comorbidities interfered with the results of HA and NS scores, that HD patients had higher NS and HA score than normal controls, specifically to HD patients with these comorbidities (“nicotine dependence”, “amphetamine dependence” or “mood disorders or anxiety disorders”) (p<0.05).
| NeuroD family | Variants | Allelea | Total HD (n=292) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | Novelty seeking score | F | p b | Harm avoidance score | F | p b | ||||||
| 1/1 | 1/2 | 2/2 | 1/1 | 1/2 | 2/2 | ||||||||
| NeuroD1 | rs1801262 | T | C | 15.00(±0.00) | 13.38(±4.67) | 13.82(±4.67) | 0.211 | 0.810 | 13.00(±0.00) | 11.47(±4.36) | 12.33(±4.97) | 0.632 | 0.532 |
| rs16867467 | T | C | 12.45(±3.53) | 13.80(±4.57) | 13.82(±4.79) | 0.445 | 0.641 | 13.09(±5.61) | 11.67(±4.57) | 12.47(±5.00) | 1.095 | 0.336 | |
| rs2583016 | C | T | NA | 13.35(±4.60) | 13.84(±4.68) | 0.455 | 0.501 | NA | 11.47(±4.28) | 12.34(±4.98) | 1.310 | 0.253 | |
| NeuroD2 | rs12453682 | T | C | 12.87(±4.06) | 14.03(±4.81) | 13.78(±4.69) | 1.075 | 0.343 | 12.09(±4.46) | 11.93(±4.88) | 12.60(±5.05) | 0.583 | 0.559 |
| NeuroD4 | rs2656804 | T | C | 13.20(±4.54) | 13.76(±4.53) | 13.96(±4.89) | 0.403 | 0.669 | 12.12(±4.50) | 12.33(±4.58) | 12.05(±5.38) | 0.110 | 0.895 |
| rs1532833 | T | G | 13.20(±4.54) | 13.76(±4.53) | 13.96(±4.89) | 0.403 | 0.669 | 12.12(±4.50) | 12.33(±4.58) | 12.05(±5.38) | 0.110 | 0.895 | |
| NeuroD6 | rs2233404 | G | A | 13.66(±4.16) | 13.46(±4.78) | 14.02(±4.68) | 0.477 | 0.621 | 12.38(±5.06) | 12.08(±5.07) | 12.25(±4.69) | 0.064 | 0.938 |
| NeuroD family | Variants | Allelea | Total NC (n=247) | ||||||||||
| 1 | 2 | Novelty seeking score | F | p c | Harm avoidance score | F | p c | ||||||
| 1/1 | 1/2 | 2/2 | 1/1 | 1/2 | 2/2 | ||||||||
| NeuroD1 | rs1801262 | T | C | 10.00(±0.00) | 12.88(±4.29) | 12.79(±4.27) | 0.219 | 0.803 | 6.00(±0.00) | 9.81(±4.83) | 10.99(±5.29) | 1.132 | 0.324 |
| rs16867467 | T | C | 12.76(±3.19) | 12.25(±4.43) | 13.04(±4.28) | 0.873 | 0.419 | 10.53(±5.99) | 10.67(±4.45) | 10.92(±5.51) | 0.082 | 0.921 | |
| rs2583016 | C | T | 10.50(±0.71) | 12.75(±4.41) | 12.82(±4.27) | 0.293 | 0.747 | 8.00(±2.83) | 9.84(±4.83) | 10.99(±5.30) | 0.960 | 0.385 | |
| NeuroD2 | rs12453682 | T | C | 13.32(±4.30) | 13.01(±4.57) | 12.22(±3.78) | 1.297 | 0.275 | 10.34(±4.89) | 11.29(±5.56) | 10.46(±4.98) | 0.862 | 0.424 |
| NeuroD4 | rs2656804 | T | C | 11.72(±3.95) | 13.15(±4.26) | 12.69(±4.35) | 1.470 | 0.232 | 11.38(±4.67) | 10.51(±4.63) | 11.02(±6.10) | 0.456 | 0.634 |
| rs1532833 | T | G | 11.72(±3.95) | 13.15(±4.26) | 12.69(±4.35) | 1.470 | 0.232 | 11.38(±4.67) | 10.51(±4.63) | 11.02(±6.10) | 0.456 | 0.634 | |
| NeuroD6 | rs2233404 | G | A | 13.36(±2.80) | 13.05(±3.84) | 12.54(±4.66) | 0.513 | 0.599 | 10.18(±4.31) | 11.05(±4.95) | 10.69(±5.54) | 0.218 | 0.804 |
Table 4: Association analysis between the neuroD gene polymorphisms and specific personality traits in patients with HD and controls.
Figure 2: Correlation between age and novelty seeking scores. Significant association between age and novelty seeking existed in both individuals with HD (left side; with blue color) and healthy controls (right side; with red color). The Spearman’s ρ is -0.22 for individuals with HD and -0.29 for healthy controls.
Discussion
▪ NeuroD gene family may not predict the occurrence of HD
NeuroD is an important factor in neural differentiation and may influence cognitive function and increase drug abuse in patients with HD. This is the first study using NeuroD genetic variants to approach the etiology of heroin addiction. Of the 7 single markers we studied, NeuroD 1 rs16867467 was possibly associated with the occurrence of HD, while the other variants were not. In addition, this association was only found in the late onset and male subgroups (Table 1) of the 6 HD subgroups defined to reduce the clinical heterogeneity. We also found that a T-allele carrier of NeuroD 1 rs16867467 may be a risk factor for HD in the male and late onset subgroups (Table 3), although these associations could not be confirmed after Bonferroni corrections (at a corrected p threshold of 0.007; 0.005/7:0.007). The haplotype analysis also revealed no significant difference in the haplotype frequencies between the two experimental groups or with the subgroups (Table 3). Our results suggest that the NeuroD gene family (NeuroD 1, NeuroD 2, NeuroD 4, and NeuroD 6) may not play an important role in the occurrence of HD.
NeuroD regulates the differentiation of many neuronal areas, including the dentate gyrus of the hippocampus [29], the cerebellar granule cell layers [30], and the inner ear neurons [31]. Animal studies have shown that the NeuroD pathways would interfere with adult neurogenesis and subsequent contextual memory retention and stability of dendritic spines [32,33]. We postulated that patients carrying a risk genotype in the NeuroD gene locus may have dysfunctional NeuroD proteins leading to impaired neurogenesis, thereby making them more susceptible to the environmental stimuli which raise the risk to develop substance dependence. However, the genetic association study did not support our hypothesis. Although our results indicate no association between the NeuroD gene family and heroin addiction, these results should be verified by further replication studies with different populations or different substance use disorders, since this is the first human genetic study.
The association between NeuroD gene and HD may be obscured by some confounding factors. First, substance use disorder and addictive behavior are multifactorial diseases with multiple genetic contributions, and the interaction between genes could not be overlooked. The existing linkage-based data for addiction has informed the current model for the polygenic genetic architecture of substance dependence in humans; according to this model, variants in each individual gene contribute modest amounts to the overall genetic vulnerability [34]. Hence, our findings should be further verified by well-designed gene-to-gene interaction studies to provide useful information about identifying polygenic mechanisms that mediate the genetic susceptibility to HD, involving genes associated with NeuroD development and NeuroD differentiation. Second, environmental factors such as socioeconomic status, also play an important role in the etiology and pathogenesis of HD [35], that would interfere with the study result. for example, familial socioeconomic status is associated with substance abuse [36]; thus, it must be considered in future gene-association analyses. Third, the NeuroD family consists of four members, each of which is located on a different chromosome. The genetic distance between members of the NeuroD family may imply that each NeuroD member has a unique and distinct expression, and this diversity in location and/or function would increase the complexity of the overall genetic association study.
▪ NeuroD gene, specific personality traits and the occurrence of HD
Since the publication of TPQ, Cloninger’s tridimensional theory of personality and psychopathology has been widely applied to research related to substance use disorders [37]. The study suggested that the NeuroD gene variants may be associated with different personality traits, further influencing the development of HD. We found that the novelty seeking and harm avoidance scores are higher in patients with HD than in healthy controls; however, none of the studied polymorphisms of the NeuroD gene were associated with the novelty seeking or harm avoidance scores. These results imply that the NeuroD gene does not play a role in mediating the relationship between specific personality traits and the occurrence of HD.
▪ Novelty seeking personality trait as a risk factor for early-onset HD
A higher novelty seeking score is one of the most relevant individual factors associated with higher impulsivity, excitability, and behavioral disinhibition [38]; higher novelty seeking would predict the initiation of drug use, subsequent vulnerability to compulsive use, and a higher propensity to relapse [39]. In this study, patients with HD had higher NS traits; this was consistent with our previous studies of patients with amphetamine or alcohol dependence [40,41]. However, when we divided the HD subjects who completed the TPQ into subgroups defined on the basis of the age of onset, and only the patients with early onset HD had significantly higher NS scores than controls. A similar finding has been reported by Li et al., where NS behavior was seen to mediate the association between the catecholamine-O-methyltransferase gene and early-onset HD in the Chinese population [42]. The prevalence of HD has increased over the past decade, especially in young adults (aged 18–25 years) [43,44]. Our study found that a negative correlation between the NS score and age in patients with HD, implying that the novelty seeking personality trait is a risk factor for early-onset heroin dependence [45]. Therefore, an effective prevention strategy is necessary in adolescents with higher novelty seeking traits to prevent heroin addiction in the young population.
▪ Study Limitations
There are several limitations to our study. First, the number of subjects included in each of the HD subgroups may not be sufficient to detect the influence of NeuroD gene polymorphisms on the occurrence of HD; moreover, only 292 of the 584 patients with HD completed the TPQ assessment, further reducing the statistical power to detect a genetic association with specific personality traits. Second, the HD group was clinically heterogeneous in terms of the severity profile, which would interfere with the power of genetic association studies [46]; subdivision of HD patients on the basis of severity profiles may be considered in future studies. Third, owing to the unreliable RD dimension of the Chinese version of TPQ, we did not analyze the RD subscore in the study. In order to address this shortcoming, other personality trait assessment tools should be used to investigate the RD score association. In addition, our study did not exclude heroin addiction with other comorbid substance abuse conditions, the confounding effects of which must not be overlooked.
Conclusion
In conclusion, the polymorphisms in the NeuroD 1 rs16867467 locus showed a trend of association with the occurrence of HD; however, this trend failed to meet the statistical thresholds imposed by the multiple comparison correction algorithms. Our study suggests that the NeuroD gene may neither contribute to the risk of HD nor mediate the relationship between specific personality traits and HD. However, the role of NeuroD in the occurrence of HD and other substance-abuse disorders should be studied further in both animal and human clinical studies.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This research was funded by several grants through the Ministry of Science and Technology (MOST- 102-2325-B-016-002, MOST-104-2314-B- 016-012-MY3) (HSY); Tri-Service General Hospital (TSGH-C103-133, TSGH-C104-129, TSGH-C105-124, TSGH-C106-100) (HSY); and Medical Affairs Bureau, Ministry of National Defense, Taiwan (MAB-104-073 and MAB-106-120) (HSY) for support this study. These funding agencies played no role in the study design, collection, analysis or interpretation of data, the writing of the report, or the decision to submit the paper for publication. We like to thank Miss Mei-Chen Shih, Miss Yun-Hsin Lin, Dr. Che-Hung Yen and Dr. Yi-Wei Yeh for their assistance in the preparing this manuscript.
References
- Levran O, Londono D, O'Hara K, et al. Genetic susceptibility to heroin addiction: a candidate gene association study. Genes. Brain. Behav 7(7), 720-729 (2008).
- Haifeng J, Di L, Jiang D, et al. Gender differences in recovery consequences among heroin dependent patients after compulsory treatment programs. Sci. Rep 5(1), 17974 (2015).
- van den Bree MB, Johnson EO, Neale MC, et al. Genetic and environmental influences on drug use and abuse/dependence in male and female twins. Drug. Alcohol. Depend 52(3), 231-241 (1998).
- Karkowski LM, Prescott CA, Kendler KS. Multivariate assessment of factors influencing illicit substance use in twins from female-female pairs. Am. J. Med. Genet 96(5), 665-670 (2000).
- Kendler KS, Jacobson KC, Prescott CA, et al. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am. J. Psychiatry 160(4), 687-695 (2003).
- Squeglia LM, Jacobus J, Tapert SF. The Influence of Substance Use on Adolescent Brain Development. Clin. EEG. Neurosci 40(1), 31-38 (2009).
- Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. N. Engl. J. Med 374(4), 363-371 (2016).
- Gould TJ. Addiction and Cognition. Addict. Sci. Clin. Pract 5(2), 4-14 (2010).
- Mandyam CD, Koob GF. The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends. Neurosci 35(4), 250-260 (2012).
- Bayer R, Franke H, Ficker C, et al. Alterations of neuronal precursor cells in stages of human adult neurogenesis in heroin addicts. Drug. Alcohol. Depend 156(1), 139-49 (2015).
- Zheng H, Zeng Y, Chu J, et al. Modulations of NeuroD activity contribute to the differential effects of morphine and fentanyl on dendritic spine stability. J. Neurosci 30(24), 8102-8110 (2010).
- Wang F, Li H, Xu M, et al. A homozygous missense mutation in NEUROD1 is associated with nonsyndromic autosomal recessive retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci 56(1), 150-155 (2015).
- Kuwabara T, Hsieh J, Muotri A, et al. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat. Neurosci 12(9), 1097-1105 (2009).
- Uittenbogaard M, Baxter KK, Chiaramello A. NeuroD6 genomic signature bridging neuronal differentiation to survival via the molecular chaperone network. J. Neurosci. Res 88(1), 33-54 (2010).
- Satoh J, Yamamoto Y, Asahina N, et al. RNA-Seq data mining: downregulation of NeuroD6 serves as a possible biomarker for alzheimer's disease brains. Dis. Markers 2014, 123165 (2014).
- Zill P, Buttner A, Eisenmenger W, et al. A possible impact of the neuroD2 transcription factor on the development of drug abusing behavior. Mol. Psychiatry 11(6), 525-527 (2006).
- Gong ZC, Huang Q, Dai XP, et al. NeuroD1 A45T and PAX4 R121W polymorphisms are associated with plasma glucose level of repaglinide monotherapy in Chinese patients with type 2 diabetes. Br. J. Clin. Pharmacol 74(3), 501-509 (2012).
- Zill P, Preuss UW, Koller G, et al. Lack of association between SNPs in the NEUROD2 gene and alcohol dependence in a German patient sample. Psychiatry. Res 187(1-2), 220-223 (2011).
- Spellmann I, Riedel M, Stadtler J, et al. Associations of NEUROD2 polymorphisms and change of cognitive dysfunctions in schizophrenia and schizoaffective disorder after eight weeks of antipsychotic treatment. Cogn. Neuropsychiatry 22(4), 280-297 (2017).
- Kreek MJ, Nielsen DA, Butelman ER, et al. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat. Neurosci 8(11), 1450-1457 (2005).
- Hser YI, Evans E, Huang D, et al. Comparing the dynamic course of heroin, cocaine, and methamphetamine use over 10 years. Addict. Behav 33(12), 1581 (2008).
- Fassino S, Daga GA, Delsedime N, et al. Quality of life and personality disorders in heroin abusers. Drug. Alcohol. Depend 76(1), 73-80 (2004).
- Miller CJ, Johnson SL, Eisner L. Assessment Tools for Adult Bipolar Disorder. Clin. Psychol (New York) 16(2), 188-201 (2009).
- Huang SY, Lin WW, Ko HC, et al. Possible interaction of alcohol dehydrogenase and aldehyde dehydrogenase genes with the dopamine D2 receptor gene in anxiety-depressive alcohol dependence. Alcohol. Clin. Exp. Res 28(3), 374-384 (2004).
- Tikkanen R, Holi M, Lindberg N, et al. Tridimensional Personality Questionnaire data on alcoholic violent offenders: specific connections to severe impulsive cluster B personality disorders and violent criminality. BMC. Psychiatry 7(1), 36 (2007).
- Barrett JC, Fry B, Maller J, et al. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21(2), 263-265 (2005).
- Wang N, Akey JM, Zhang K, et al. Distribution of Recombination Crossovers and the Origin of Haplotype Blocks: The Interplay of Population History, Recombination, and Mutation. Am. J. Hum. Genet 71(5), 1227-1234 (2002).
- Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods 41(4), 1149-1160 (2009).
- Liu M, Pleasure SJ, Collins AE, et al. Loss of BETA2/NeuroD leads to malformation of the dentate gyrus and epilepsy. Proc. Natl. Acad. Sci. U S A 97(2), 865-870 (2000).
- Miyata T, Maeda T, Lee JE. NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Genes. Dev 13(13), 1647-1652 (1999).
- Kim WY. NeuroD Regulates Neuronal Migration. Mol. Cells 35(5), 444-449 (2013).
- Rodriguez RE. Morphine and microRNA Activity: Is There a Relation with Addiction? Front. Genet 3(1), 223 (2012).
- Zheng H, Zhang Y, Li W, et al. NeuroD modulates opioid agonist-selective regulation of adult neurogenesis and contextual memory extinction. Neuropsychopharmacology 38(5), 770-777 (2013).
- Uhl GR, Drgon T, Johnson C, et al. Addiction Genetics and Pleiotropic Effects of Common Haplotypes that Make Polygenic Contributions to Vulnerability to Substance Dependence. J. Neurogenet 23(3), 272-282 (2009).
- El Rawas R, Thiriet N, Lardeux V, et al. Environmental enrichment decreases the rewarding but not the activating effects of heroin. Psychopharmacology (Berl) 203(3), 561-50 (2009).
- Patrick ME, Wightman P, Schoeni RF, et al. Socioeconomic Status and Substance Use Among Young Adults: A Comparison Across Constructs and Drugs. J. Stud. Alcohol. Drugs 73(5), 772-782 (2012).
- Howard MO, Kivlahan D, Walker RD. Cloninger's tridimensional theory of personality and psychopathology: applications to substance use disorders. J. Stud. Alcohol 58(1), 48-66 (1997).
- Prisciandaro JJ, Korte JE, McRae-Clark AL, et al. Associations between behavioral disinhibition and cocaine use history in individuals with cocaine dependence. Addict. Behav 37(10), 1185-1188 (2012).
- Wingo T, Nesil T, Choi JS, et al. Novelty Seeking and Drug Addiction in Humans and Animals: From Behavior to Molecules. J. Neuroimmune. Pharmacol 11(3), 456-470 (2016).
- >Wang TY, Lee SY, Chen SL, et al. Association between DRD2, 5-HTTLPR, and ALDH2 genes and specific personality traits in alcohol- and opiate-dependent patients. Behav. Brain. Res 250(1), 285-292 (2013).
- Tzeng NS, Lu RB, Yeh HW, et al. The dopamine transporter gene may not contribute to susceptibility and the specific personality traits of amphetamine dependence. Drug. Alcohol. Depend 149(1), 100-107 (2015).
- Li T, Yu S, Du J, et al. Role of Novelty Seeking Personality Traits as Mediator of the Association between COMT and Onset Age of Drug Use in Chinese Heroin Dependent Patients. PLoS. One 6(8) (2011).
- Ihongbe TO, Masho SW. Prevalence, correlates and patterns of heroin use among young adults in the United States. Addict. Behav 63(1), 74-81 (2016).
- Hopfer CJ, Khuri E, Crowley TJ, et al. Adolescent heroin use: a review of the descriptive and treatment literature. J. Subst. Abuse. Treat 23(3), 231-237 (2002).
- Palmer RHC, Knopik VS, Rhee SH, et al. Prospective Effects of Adolescent Indicators of Behavioral Disinhibition on DSM-IV Alcohol, Tobacco, and Illicit Drug Dependence in Young Adulthood. Addict. Behav 38(9), 2415-2421 (2013).
- Hasin DS, O'Brien CP, Auriacombe M, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. Am. J. Psychiatry 170(8), 834-851 (2013).