Case Report - Neuropsychiatry (2017) Volume 7, Issue 6
Deep Brain Stimulation of the Medial Forebrain Bundle in a Patient with Treatment-Resistant Bipolar Depression and Comorbid OCD: Acute and 12-Month Follow-Up Results
- Corresponding Author:
- Bernardo Dell’Osso, M.D.
Department of Psychiatry, University of Milan, Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Milano., Via Francesco Sforza 35,20122 Milano, Italy
Tel: +39 02 55035206-5994
Fax: +39 02 55033140
Abstract
Limited but encouraging evidence exists on the efficacy of Deep Brain Stimulation (DBS) of the Medial Forebrain Bundle (MBF) in otherwise intractable patients with Major Depressive and Obsessive-Compulsive Disorder (OCD). Subject and Methods: Herein, we present acute and follow-up results (up to 12-months) of the first Italian patient, a 34 year old man with a diagnosis of treatment-resistant Bipolar Depression (BD) and comorbid OCD, successfully treated with DBS of the MFB. Periodic follow-up visits with psychometric evaluations highlighted a remarkable improvement of patient’s depressive and OC symptoms at 3 months (50-80% scores reduction), that was maintained at 6 and 12 months. In particular, suicidal ideation, which was found to be pervasive before the implant as well as patient’s overall disability, showed a rapid and significant acute response to DBS. Over the 12 months of stimulation, pre-implant pharmacological treatment could be gradually decreased, while the patient remained clinically stable. According to the limited, reported experience, we support the efficacy and tolerability of DBS of the MFB as a promising intervention in patients with treatment-resistant BD and comorbid OCD, particularly in relation to acute and post-acute outcome. Larger controlled trials are needed to confirm our findings and assess DBS therapeutic properties in the long-term.
Keywords
Treatment-resistant depression, Deep brain stimulation, Medial forebrain bundle, Obsessive compulsive disorder, Bipolar disorder, Psycho-motor alterations
Introduction
Deep Brain Stimulation (DBS) as therapeutic option for treatment-resistant Obsessive Compulsive Disorder (OCD) has been supported by systematic reviews and meta-analyses [1-3], approved by the FDA and the EMA [4] and currently acknowledged by international treatment guidelines [5,6]. On the other hand, the use of DBS in treatment-resistant depression (TRD) has shown mixed results. The reasons for such contrasting outcomes might be traced back to some factors including: insufficient randomized controlled data, different followup intervals, heterogeneity of patients (in terms of age, comorbidity, past response to medical and other treatments, etc.), inconsistent tools for evaluation (psychometric questionnaires), uneven definition of response and remission; different targets of stimulation including: the subgenual cingulate gyrus, the anterior limbs of the capsula interna, and the nucleus accumbens (NAcc), and the lack of a meticulous postoperative DBS programming data [7-10].
A new promising stimulation target has been investigated by Schlaepfer and colleagues – the medial forebrain bundle (MFB) – an area responsible of interconnecting the NAcc, the ventral tegmental area, the ventromedial and lateral nuclei of the hypothalamus, and the amygdala. Such structures play a crucial role in regulating the reward pathways and have been reportedly indicated as effective DBS targets in patients with TRD [11-13]. Few follow-up studies exist on the DBS-implanted patients, particularly with respect to the MFB target. A recent meta-analysis identified 12-month response and remission rates of about 39% and 26% for TRD patients treated with DBS of the subgenual cingulate cortex. Previously, rates of 62.5% and 18.8% respectively, were reported for subcallosal cingulate gyrus stimulation [14,15]. Herein, we present acute and follow-up results of the first Italian patient with treatment-resistant bipolar depression and comorbid OCD, treated with DBS of the supero-lateral branch of the MFB (slMFB).
Case Review
From 18 years of age, M.O. had suffered from several recurrent major depressive episodes (MDEs) and two hypomanic episodes (with increased energy levels and work activity, engagement in risky projects, inflated selfesteem, and insomnia). In addition, at 25, M.O. received a diagnosis of OCD. The subtype of OCD consisted of intrusive thoughts, i.e. of carrying out violent acts against himself or his loved ones, followed by the compulsion of storing away potentially harmful items (e.g. knives or toxic liquids) or walking far from windows or elevated spots. A relapse of the obsessive symptomatology used to occur independently from the affective phases of the mood disorder he was also suffering from, as detected by the SCID-I [16], negative for an ongoing depressive or (hypo)manic episode, while the Yale-Brown obsessive-compulsive scale (Y-BOCS) [17] score was compatible with a moderate-severe symptomatology (on average: 25).
He started his first pharmacological treatment at 24 and, in light of a Bipolar Disorder II (BD-II) diagnosis with comorbid OCD, from 24 to 34 years, he was treated with several antidepressants (including SSRI, atypical antidepressants, tricyclics) in mono- and poly-therapy, and augmentative mood-stabilizers including: lithium up to 900 mg per day for up to 2 years – with an average dosed lithium in the plasma equivalent to 0.85 mEq/l – and sodium valproate up to 1500 mg per day for 5 years – with plasma concentrations of sodium valproate of 80 mcg/ mL) used for the treatment of OC symptoms, as recommended by International treatment guidelines, with poor response (i.e., <25% at Y-BOCS score) [18].
When M.O. came to our Clinic at the age of 34 (March 2012), he received a lifetime diagnosis of BD-II with treatment-resistance characteristics [19,20] and comorbid OCD, and a cross-sectional diagnosis of MDE “with Anxious Distress” (the latter ongoing for more than 2 years) and moderate (Y-BOCS=12) [18] obsessive symptoms (according to the SCID-5-CV) [21].
Lamotrigine (100 mg/day) and aripiprazole (5 mg/day) were prescribed in order to treat both the depressive and the obsessive dimension. After 4 months of treatment and no significant clinical change, the patient discontinued the follow-up visits. In the subsequent 3 years, he underwent other pharmacological and brain stimulation interventions, including 10 sessions of bilateral ECT, followed by 5 additional maintenance sessions and a 4-week (5 applications/week, 20 applications in total) low frequency rTMS (targeting the left dorso-lateral prefrontal cortex), with only limited benefit [20].
M.O. returned to our Clinic in March 2015 (baseline), for further evaluation. He was assessed with different psychometric scales including the Hamilton Depression Rating Scale, 21-item version (HDRS-21): 33, the Hamilton Anxiety Rating Scale (HARS): 38, the Montgomery– Åsberg Depression Rating Scale (MADRS): 38, the Clinical Global Impressions Scale, severity (CGI-S): 6, the Y-BOCS: 12. His current therapy consisted of: sertraline 300 mg/day, bupropion 600 mg/day, trazodone 75 mg/day, lamotrigine 300 mg/day, delorazepam 2 mg/day. He complained about a poor socio-relational and familiar life and, due to severe anxiety with agoraphobia, he had recently ceased working and travelling (Sheehan Disability Scale, SDS score=22/30). The patient, in particular, reported significant asthenia and psychomotor retardation with loss of energy and slowness of movement end he had pervasive suicidal ideation (scoring 3/4 at the related HDRS-21 item). Due to severe sexual side effects, likely linked to the antidepressant therapy, the Arizona Sexual Experience Scale (ASEX) [22] was administered (score: 21/30).
In light of his clinical history, M.O. was considered a candidate patient for DBS and underwent the implant in May 2015.
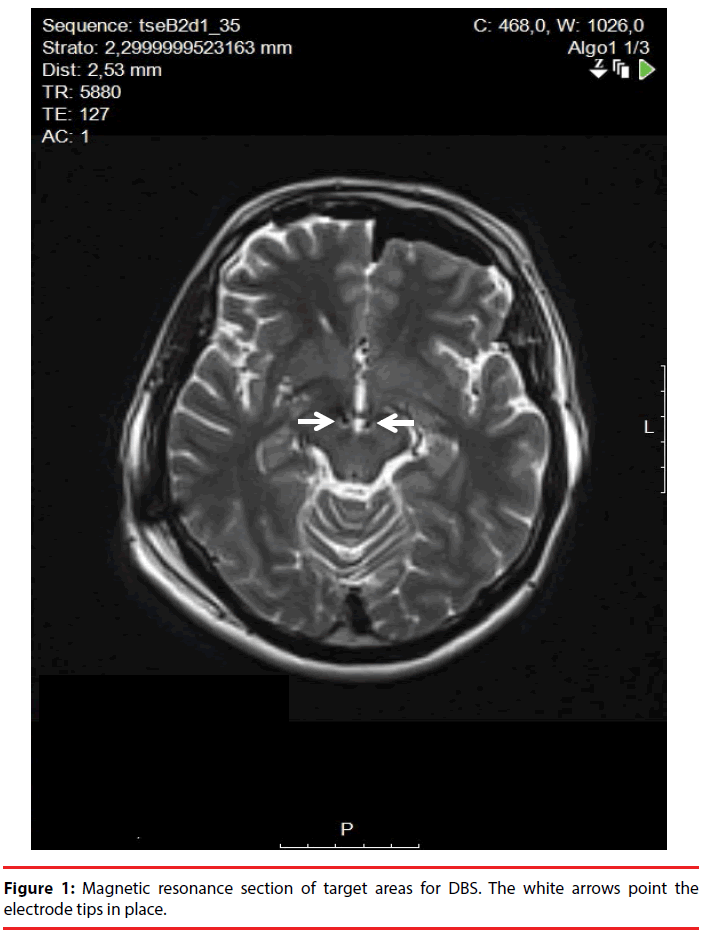
The day before surgery, a 1.5 Tesla brain MRI-scan was performed acquiring a T2 FSE sequence, a 1 mm slice contrast-enhancement T1 sequence, and a DTI sequence (used to detect the MFB spanning from the ventral tegmental area through the nucleus accumbens to the prefrontal cortex) [11]. The day of surgical implant, a stereotactic CT scan was performed. The final target was the same of that described by Schlaepfer, et al. aiming anteriorly to the red nucleus boundaries and 6 mm beneath the AC-PC plane (Figure 1). After the implant of a bilateral 3389 Medtronic DBS-lead, intraoperative macrostimulation was performed to assess stimulation effect and choose the final DBS-lead position. On the left side, the central trajectory was chosen, whereas, on the right side, the DBS-lead was placed 2 mm anteriorly to the initial target, due to the onset of acute anxiety during intraoperative macrostimulation. In the best response point, we observed a mood improvement without collateral anxiety.
After the implant, M.O. was monthly assessed with clinical interviews and psychometric evaluations. After 3 months, his scores were: HDRS-21: 9, HARS: 8, MADRS: 9, CGI-S: 2, Y-BOCS: 5, corresponding to a 50- 80% reduction, compared to baseline. The pharmacological treatment was maintained stable. Six months after the implant, the patient showed a further improvement: HDRS-21: 8, HARS: 8, MADRS: 7, CGI-S: 2, Y-BOCS: 5. The pharmacological treatment was then reduced to: sertraline 100 mg/day, bupropion 450 mg/day, trazodone 75 mg/day, lamotrigine 300 mg/day, delorazepam 1.4 mg/day. The ASEX scored 13 (38% decrease). Twelve months after the implant, patient’s conditions were unmodified, and his pharmacological treatment, with the exception of delorazepam, gradually reduced. Over the 12 months of follow-up, M.O. experienced 1 brief depressive episode (10 days duration), at 6 months from implant. No hypomanic/manic episodes were observed. At the last follow-up visit the implanted pulse generator parameters were: monopolar stimulation 0-, Case + 130 Hz, 60 Msec 1.8 V, 4- Case +, 130 Hz, 60 Msec, 1.8 V.
Discussion
The present case-report supports the available, growing evidence about the efficacy and tolerability of slMFB-DBS for patients with TRD and TR-OCD [12,13]. This target was already found to determine a rapid clinical improvement [11], as observed in our patient, who also showed a sustained remission at 12 months. In fact, a remarkable reduction of depressive symptoms was achieved within 3 months from device activation. Suicidal thoughts acutely responded to DBS (HDRS-21 item 3: from 3/4 to 0/4, 3 weeks after the implant). Such improvement remained stable at 6 and 12 months after the implant. Comorbid anxiety showed a rapid and sustained response, as shown by the reduction of benzodiazepines, agoraphobic symptoms and avoidance behaviors. M.O. went back to work and re-established some friendship bonds (SDS: 8). Six months after the implant, DBS allowed a gradual reduction of the remaining pharmacotherapy, with overall better tolerability and improved sexual health. M.O. experienced only 1 brief depressive episode over the 12 months following implant, with an overall duration of 10 days. This finding supports the hypothesis that DBS might reduce the number and duration of depressive recurrences, showing a potential stabilizing activity.
According to our limited, open-label experience, DBS seems a promising intervention deserving further investigation in cases of bipolar TRD, particularly in patients with severe anxious symptomatology, and comorbid OCD. Controlled trials with follow-up design need to be conducted to confirm present open-label findings and assess DBS therapeutic actions as a multi-dimensional stabilizing technique.
Acknowledgments
This study was conducted according to the principles expressed in the Declaration of Helsinki. We would like to thank the colleagues who contributed to the surgical procedures and data collection: Drs. Alberto Bona, Carlotta Zanaboni, Beatrice Benatti, Benedetta Grancini.
Conflict of interest
All authors report no other affiliation or economic interest in any organization that may imply a conflict of interest with the present work.
References
- Dell’Osso B, Altamura AC, Allen A, et al. Brain stimulation techniques in the treatment of obsessive-compulsive disorder: current and future directions. CNS. Spectr 10(12), 966-979, 983 (2005).
- Hamani C, Pilitsis J, Rughani AI, et al. Deep brain stimulation for obsessive-compulsive disorder: systematic review and evidence-based guideline sponsored by the American Society for Stereotactic and Functional Neurosurgery and the Congress of Neurological Surgeons (CNS) and endorsed by the CNS. Neurosurgery 75(4), 327-333 (2014).
- Kisely S, Hall K, Siskind D, et al. Deep brain stimulation for obsessive-compulsive disorder: a systematic review and meta-analysis. Psychol. Med 44(16), 3533-3542 (2014).
- Malone DA. Use of deep brain stimulation in treatment-resistant depression. Cleve. Clin. J. Med 77(s3), S77-S80 (2010).
- Koran LM, Simpson HB. Guideline Watch (March 2013): practice guideline for the treatment of patients with obsessive-compulsive disorder (2013).
- Schlaepfer TE, George MS, Mayberg H, et al. WFSBP Guidelines on Brain Stimulation Treatments in Psychiatry. World. J. Biol. Psychiatry 11(1), 2-18 (2010).
- Rizvi SJ, Donovan M, Giacobbe P, et al. Neurostimulation therapies for treatment resistant depression: a focus on vagus nerve stimulation and deep brain stimulation. Int. Rev. Psychiatry 23(5), 424-436 (2011).
- Quraan MA, Protzner AB, Daskalakis ZJ, et al. EEG power asymmetry and functional connectivity as a marker of treatment effectiveness in DBS surgery for depression. Neuropsychopharmacology 39(5), 1270-1281 (2014).
- Naesström M, Blomstedt P, Bodlund O. A systematic review of psychiatric indications for deep brain stimulation, with focus on major depressive and obsessive-compulsive disorder. Nord. J. Psychiatry 70(7), 483-491 (2016).
- Morishita T, Fayad SM, Higuchi M, et al. Deep Brain Stimulation for Treatment-resistant Depression: Systematic Review of Clinical Outcomes. Neurotherapeutics 11(3), 475-484 (2014).
- Schlaepfer TE, Bewernick BH, Kayser S, et al. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol. Psychiatry73(12), 1204-1212 (2013).
- Coenen VA, Schlaepfer TE, Goll P, et al. The medial forebrain bundle as a target for deep brain stimulation for obsessive-compulsive disorder. CNS. Spect 22(3), 282-289 (2017).
- Fenoy AJ, Schulz P, Selvaraj S, et al. Deep brain stimulation of the medial forebrain bundle: Distinctive responses in resistant depression. J. Affect. Disord 203(1), 143-151 (2016).
- Kennedy SH, Giacobbe P, Rizvi SJ, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am. J. Psychiatry 168(5), 502-510 (2011).
- Berlim MT, McGirr A, Van den Eynde F, et al. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J. Affect. Disord 159(1), 31-38 (2014).
- First M, Spitzer R, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Clinician Version (SCID-CV). American Psychiatric Press, Inc., Washington, DC, USA.
- Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch. Gen. Psychiatry 46(11), 1012-1016 (1989).
- Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch. Gen. Psychiatry 46(11), 1006-1011 (1989).
- Pacchiarotti I, Mazzarini L, Colom F, et al. Treatment-resistant bipolar depression: towards a new definition. Acta. Psychiatr. Scand 120(6), 429-440 (2009).
- Hirschfeld RM, Calabrese JR, Frye MA, et al. Defining the clinical course of bipolar disorder: response, remission, relapse, recurrence, and roughening. Psychopharmacol. Bull 40(3), 7-14 (2007).
- First MB, Williams JB, Karg RS, et al. SCID-5-CV : structured clinical interview for DSM-5 disorders, clinician version. American Psychiatric Association (2016).
- McGahuey CA, Gelenberg AJ, Laukes CA, et al. The Arizona Sexual Experience Scale (ASEX): reliability and validity. J. Sex. Marital. Ther 26(1), 25-40 (2000).