Case Report - Neuropsychiatry (2017) Volume 7, Issue 5
Cortical Aphasia and Apraxia as Main Clinical Presentation of Anti-NMDAR Encephalitis Relapse with a Positive CSF PCR for Cytomegalovirus
- Corresponding Author:
- Mariana Espinola-Nadurille
National Institute of Neurology and Neurosurgery, Insurgentes Sur 3877. ZC 14269 Tlalpan, Mexico City, Mexico
Tel: (55)56063822
Abstract
Objective:
We present a patient with severe aphasic and apraxic syndromes as her clinical manifestation of anti-NMDAR encephalitis. Nineteen months earlier she had a first neurological episode consistent of a first event of the disease. Distinctive paraclinical findings and treatment measures are highlighted.
Materials and Methods:
A 17 years old female with previous history of seizures presented with headache, dizziness, nausea, and vomiting, that progressed to inattention and reduced, non- fluent spontaneous speech, altered repetition and nomination with literal paraphasias and spared comprehension (motor aphasia).
Results:
Initial cerebrospinal fluid (CSF) showed mild pleocytosis. In spite of antiviral treatment with acyclovir, her cognitive performance worsened to severe global aphasia and an apraxic syndrome. MRI, EEG and 18-PET Scan showed left generalized cortical abnormalities. Anti-NMDAR autoantibodies by immunofluorescence were positive in serum and CSF. Pulses of methylprednisolone were started with improvement. Still her cognitive deficits were disabling, so steroids were combined with 5 sessions of plasma exchange, a combination regarded as first-line immunotherapy treatment in anti-NMDAR encephalitis. Prompt and strong improvement was achieved. A pelvic ultrasound and a contrast-enhanced CT scan were negative for ovarian teratoma. Retrospectively we concluded that she had had a first neurological episode of autoimmune encephalitis that went undiagnosed and was self-limited 19 months earlier. Cyclophosphamide was prescribed as an outpatient to reduce the risk of relapses.
Conclusion:
This case widens the clinical spectrum of anti-NMDAR encephalitis in adolescents.
Keywords
Anti-NMDAR, Encephalitis, Cytomegalovirus, Relapse, Apraxia, Aphasia
Introduction
Recently, a group of diseases related to antibodies against cell-surface or synaptic proteins affecting the central nervous system have been described. One of the antigens parts of this group of autoimmune encephalopathies is N-methyl-Daspartate receptor (NMDAR) [1]. Anti-NMDAR encephalitis is characterized by the presence of cerebrospinal fluid (CSF) IgG antibodies against the GluN1 subunit of NMDAR, a neuronal cellsurface synaptic receptor. New efforts are being made to establish the full clinical spectrum of this disorder in diverse gender and age populations. A study involving the largest cohort to date (577 patients) reported that 37% were younger than 18, with a higher frequency of males below 12 years of age. Nevertheless, 220 patients (38%) had an underlying tumor; 213 of them were women and 94% of the tumors were ovarian teratomas [2]. Only recently the parainfectious origin of anti-NMDAR encephalitis has been underscored and linked to previous herpes simplex encephalitis infection in some cases [3,4]. The correct diagnosis of this condition is crucial, as the response rate to immunotherapy can be as high as 70-80% [5]. Tumor removal is necessary in the indicated patients [6].
Here, we present the case of a 17-year-old woman with relapsing anti-NMDAR encephalitis showing severe and isolated cortical aphasia and apraxia with infrequent findings in MRI and a positive PCR in her CSF.
Case Report
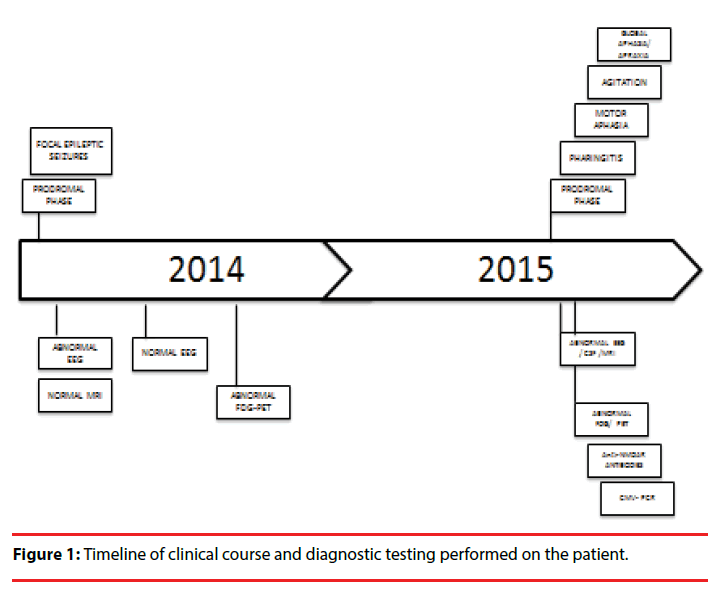
The patient-a 16-year-old right-handed immunocompetent healthy female– arrived for the first time at the emergency room (ER) of the National Institute of Neurology and Neurosurgery of Mexico, in Mexico City, because of two motor focal seizures of the left arm, the second one with generalization (Figure 1). Her symptoms had begun two days earlier with headache, dizziness, anxiety and sleep problems. The patient was a Spanish speaker, had a middle-class upbringing and was then a junior in high school. The neurologists that assessed her decided to complete the diagnostic workup as an outpatient, and discharged her without medication.
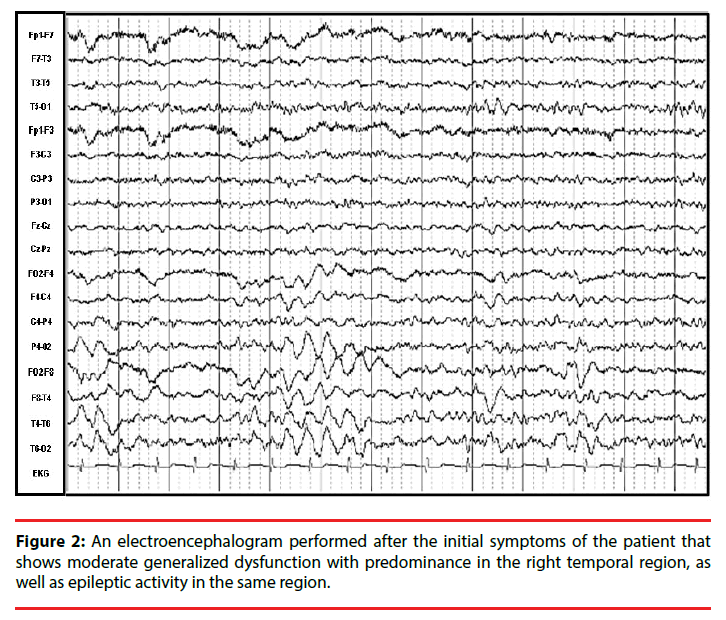
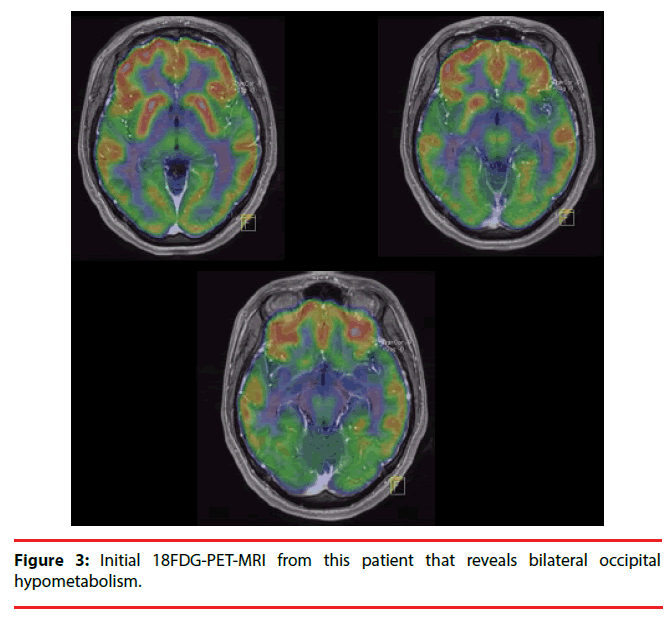
Eleven days later, an MRI was performed, yielding normal results, whereas an electroencephalogram (EEG) showed moderate generalized dysfunction with predominance in the right temporal region as well as epileptic activity in the same region (Figure 2). After 5 days, the headache and dizziness remitted, and seizures did not recur. Antiepileptic medications were prescribed, but due to side-effects, she discontinued this treatment without medical advice. Nevertheless, the patient remained seizure-free. Four months later, an EEG was performed, which revealed no abnormalities, and 8 months later, a positron-emission tomography (PET) with 18-fluorodeoxyglucose (18-FDG) showed bilateral occipital hypometabolism (Figure 3).
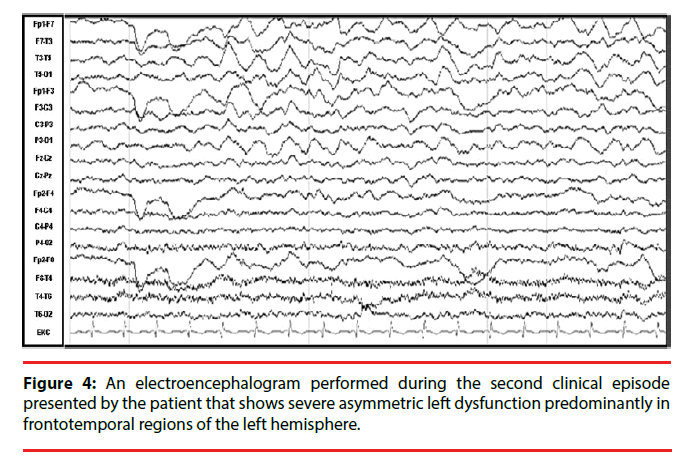
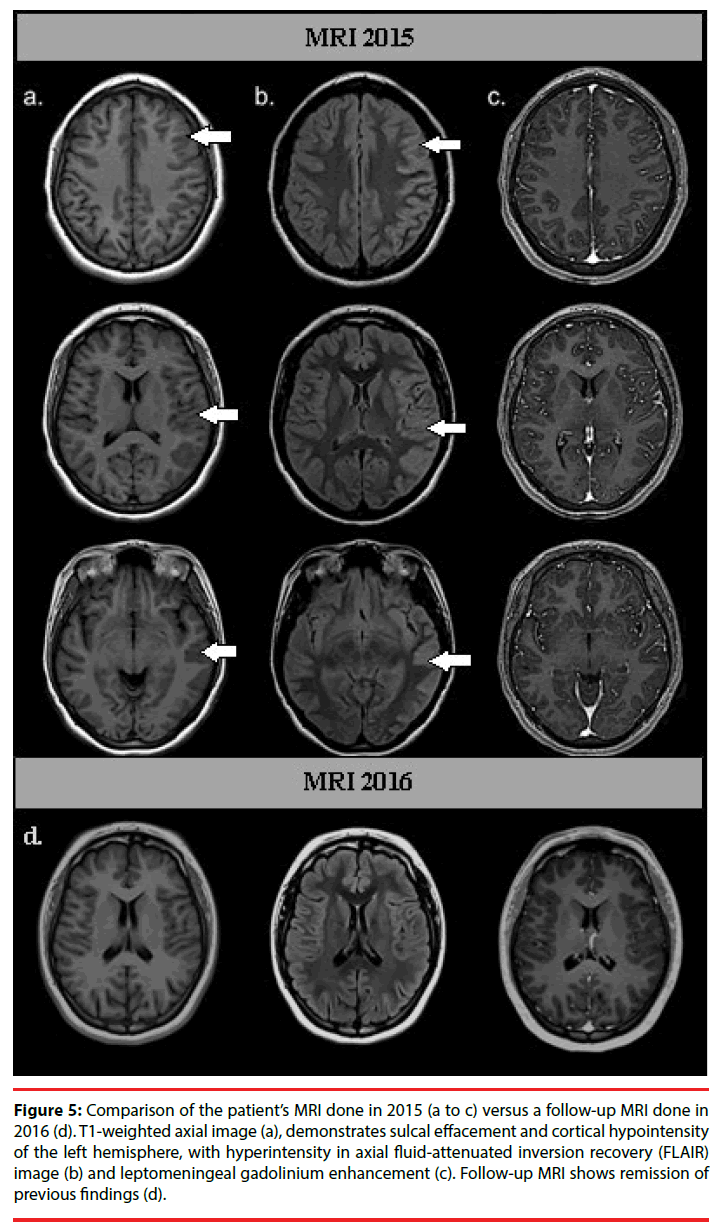
Nineteen months after the initial symptoms, the patient returned to the ER with headache, fever, nausea, and vomiting of 15 days of evolution (Figure 1). Her neurological exam was normal, so she was discharged with analgesics. However fifteen days later, she returned to the ER with difficulty speaking. The patient was found mildly inattentive. She showed no comprehension problems and was able to follow instructions, but had expressive language disturbances, including reduced non-fluent spontaneous speech, altered repetition and nomination with phonemic paraphasias, as in motor aphasia. General laboratory tests and a computed tomography (CT) showed no abnormalities, although her CSF revealed mild pleocytosis with a predominance of mononuclear cells (35cells/mm3, proteins 39 mg/dL, glucose 45 mg/dL). She was then admitted to the neurology department and was started on acyclovir (750 mg tid). Over the following days, a complete diagnostic workup for encephalitis was performed. An initial EEG revealed asymmetry with severe left dysfunction, predominantly in frontal and temporal regions of the left hemisphere (Figure 4). The MRI revealed sulcal effacement and cortical hypointensity of the left hemisphere in the T1 weighted image, left cortical hyperintensity in the axial fluidattenuated inversion recovery (FLAIR) image, and leptomeningeal gadolinium enhancement (Figure 5).
Figure 5: Comparison of the patient’s MRI done in 2015 (a to c) versus a follow-up MRI done in 2016 (d). T1-weighted axial image (a), demonstrates sulcal effacement and cortical hypointensity of the left hemisphere, with hyperintensity in axial fluid-attenuated inversion recovery (FLAIR) image (b) and leptomeningeal gadolinium enhancement (c). Follow-up MRI shows remission of previous findings (d).
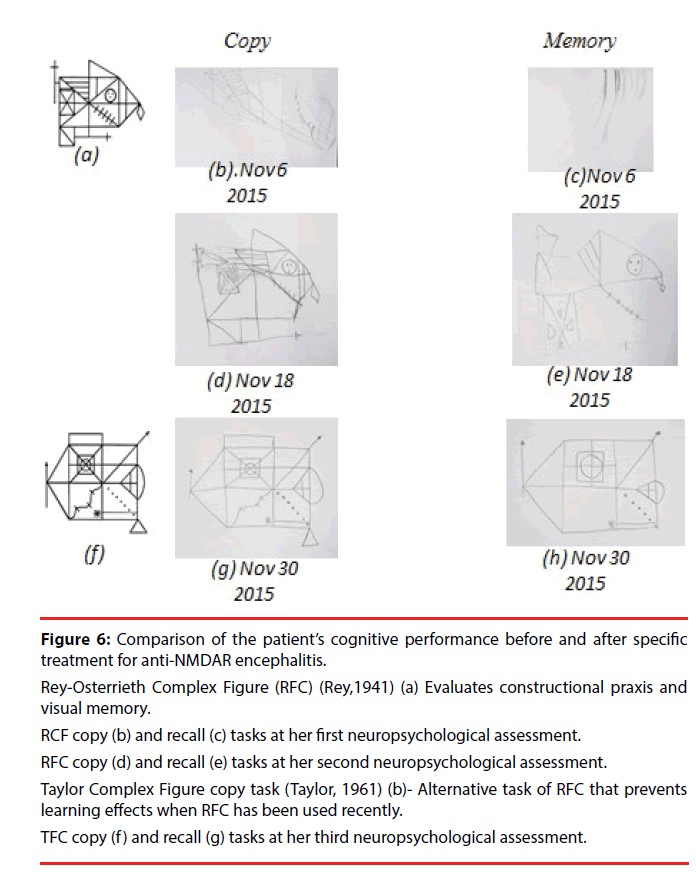
On the seventh day of hospitalization, a neuropsychological assessment revealed worsening of her aphasic syndrome. Regarding automatic language expression, she preserved numerical series (from 1 to 10), but word repetition tests revealed phonemic paraphasias (e.g., “cave” instead of “café”) and omissions (e.g., “aua” instead of “agua”), and for some word repetition commands she showed a total inability to produce words. During phrase repetition tasks, she produced only vocalizations. In naming tests, she exhibited anomia and paraphasic errors. Spontaneous speech (narrative) was severely reduced and agrammatical, and she replied only in monosyllables and isolated words. However, tone, inflection, and rhythm (prosody) were preserved. In addition, language comprehension was severely disturbed, as she was only able to follow instructions consisting of one or two elements. Alexia and agraphia were also present. Due to her difficulties in language comprehension, apraxia was evaluated through verbal commands to perform actions; imitation of actions after the examiner had performed them, and utilization of objects. The patient showed severe disturbances on orofacial praxis examination (e.g., tongue protrusion, lip smacking). As well, she exhibited ideomotor (e.g., pretending to use a comb or a toothbrush, ideational (e.g., eating a meal using cutlery) and constructive apraxias (Figure 6 and Table 1), with the most severe performance defects on the left side of her body. The latter deficits rendered her dependent for feeding, bathing and dressing. She scored 6/30 on the Montreal Cognitive Assessment (MoCA). Her neurological exam showed no additional findings.
Figure 6: Comparison of the patient’s cognitive performance before and after specific treatment for anti-NMDAR encephalitis.
Rey-Osterrieth Complex Figure (RFC) (Rey,1941) (a) Evaluates constructional praxis and visual memory.
RCF copy (b) and recall (c) tasks at her first neuropsychological assessment.
RFC copy (d) and recall (e) tasks at her second neuropsychological assessment.
Taylor Complex Figure copy task (Taylor, 1961) (b)- Alternative task of RFC that prevents learning effects when RFC has been used recently.
TFC copy (f ) and recall (g) tasks at her third neuropsychological assessment.
| Neuropsychological Assessment | MOCA | Token Test | RFC/TFC | ||
|---|---|---|---|---|---|
| Copy | Memory | ||||
| a | 1st evaluation (Nov. 9, 2015) |
6/30 | 4/36 Very severe impairment |
1/36 | 1/36 |
| b | 2nd evaluation (Nov 18, 2015) |
15/30 | 28/36 Mild impairment |
22/36 | 7/36 |
| c | 3rd evaluation (Nov. 30, 2015) |
24/30 | 34/36 Normal |
35/36 | 22/36 |
TOKEN TEST (de Renzi, 1962) Specific assessment of language comprehension
RFC (Rey, 1941): Rey-Osterrieth Complex Figure; TCF: Taylor Complex Figure
Table 1: Comparison of cognitive performance before (a) and after first (pulses of methylprednisolone) (b) and second (plasma exchange) (c) immune treatments.
Tests for systemic autoimmune diseases were negative. We maintained the treatment with acyclovir for 14 days, but due to her clinical worsening despite antiviral treatment, we decided to perform further investigations to rule out an autoimmune cause for the encephalitis and started intravenous methylprednisolone pulses.
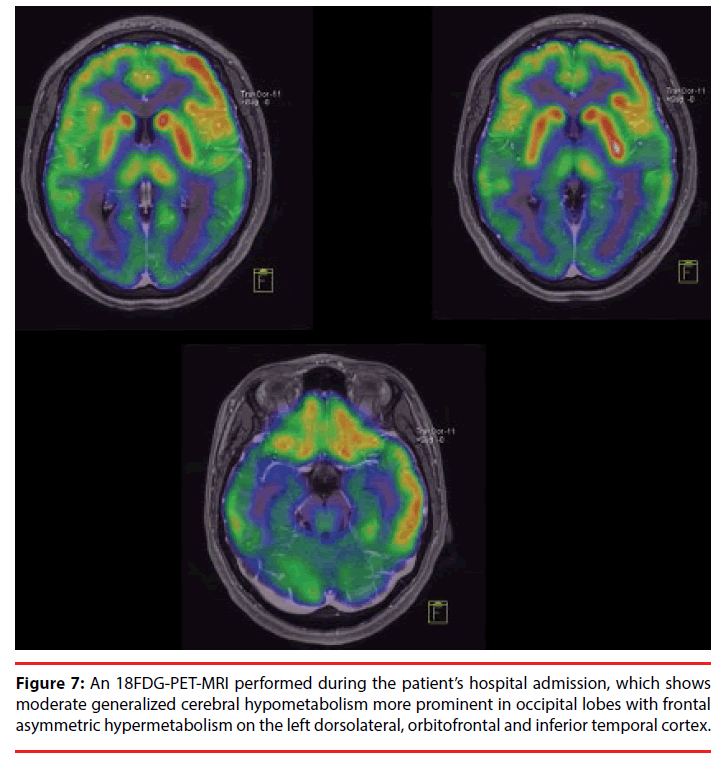
An 18-FDG PET scan exhibited moderate generalized cerebral hypometabolism that was more prominent in the occipital lobes, with frontal asymmetric hypermetabolism in the left dorsolateral, orbitofrontal and inferior-temporal cortex (Figure 7). There was no evidence of extracranial metabolic tumor activity. A pelvic ultrasound and a contrast-enhanced abdominalpelvic CT scan were performed to rule out the presence of an ovarian teratoma. The presence of anti-NMDAR antibodies was confirmed in the serum and CSF by immunofluorescence. After her clinical status had improved following immunosuppressive treatment with IV methylprednisolone, we received the viral CSF PCR results, which were positive for Cytomegalovirus (CMV) and negative for Herpes simplex types 1 and 2, Epstein-Barr, Varicella zoster, Human herpes types 6, 7 and 8, Enterovirus, Toxoplasma, Parvovirus B19 and Lymphocytic choriomeningitis virus.
A second neuropsychological assessment showed significant improvements in expression and language comprehension. Spontaneous speech was reduced and characterized by simple phrases, although they were grammatically correct. She still showed phonemic paraphasias but could name objects. The patient had comprehension deficits for complex grammatical structures, and although she still had praxic difficulties, these were mild. She also showed problems with executive functions that could not be identified previously due to the severity of the aphasic and apraxic syndromes. Her MoCA score increased to 15/30 (Figure 6 and Table 1). Despite the cognitive improvements, the deficits caused significant disabilities, and she was given five sessions of plasma exchange. After one month of hospitalization, a third neuropsychological assessment showed complete remission of the aphasia, although she retained mild symptoms of ideomotor and ideational apraxia, as well as mild dysexecutive symptoms. Her MoCA test score was 24/30 (Figure 6 and Table 1). She was started on cyclophosphamide pulses as an outpatient to reduce the risk of another relapse and discharged for subsequent follow-up as an outpatient.
On following assessments, the patient continued showing improvement in cognition and returned to school one month later with adequate functionality. Five months after her second clinical episode a follow-up MRI showed no evidence of previously described lesions (Figure 5). In addition, a follow-up pelvic ultrasound and a contrast-enhanced abdominal-pelvic CT scan were normal.
Discussion
A teenaged girl with a previous history of epilepsy developed an episode of encephalitis. The clinical features at onset included headache, dizziness, nausea and vomiting. The addition of motor aphasia and inattention to her clinical symptoms led the clinicians to suspect encephalitis, which was corroborated by an inflammatory CSF profile. She was started on acyclovir from day one, as suggested by relevant guidelines [7,8]. Over the next days, her clinical condition worsened to global aphasia, and a severe apraxic syndrome developed despite antiviral treatment. Immunosuppressive treatment was initiated and further studies were performed to identify an autoimmune etiology, which finally led to a positive immunofluorescence test for anti- NMDAR antibodies in the serum and CSF.
In several reports, anti-NMDAR encephalitis has been characterized as a multistage progressive illness, beginning with rapid changes in behavior, psychosis, catatonia and seizures; followed by a wide range of abnormal movements, including oral-facial dyskinesias, chorea, and athetosis, and ultimately resulting in decreased consciousness and autonomic dysregulation [1,6,9,10]. In recent reports, there has been an attempt to categorize these symptoms. In the largest cohort of anti-NMDAR encephalitis patients, Titulaer and colleagues [2] classified symptoms into the following groups: behavior and cognition, memory, speech, seizures, movement disorders, loss of consciousness, autonomic dysfunction and central hypoventilation. Over the first four weeks, most patients develop symptoms of four or more of the described groups [2]. This categorization of symptoms has been adopted by other researchers, leading to an understanding of the disease as more of a clinical spectrum than a multistage illness. [11] [12-14]. However, what remains in common between all case series is that, regardless of the initial symptoms, patients eventually develop a complete clinical presentation with multiple symptoms from the other categories. Approximately 70% of patients develop prodromal symptoms, such as headache, fever or flu-like symptoms, five to ten days before the full syndrome as was observed in our patient [2].
The case described here included the following atypical elements: 1) the patient showed isolated severe cortical aphasia and apraxia, and 2) she did not show the full clinical spectrum described above. 3) A positive CSF PCR for Cytomegalovirus was reported, and 4) she had a first neurological event 19 months earlier that likely corresponds to a first event of anti- NMDAR encephalitis.
What is noticeable from previous reports of anti- NMDAR encephalitis is that neuropsychological deficits in general [15], and aphasia and apraxia in particular, are not well defined or described. On the other hand, speech disturbances have been increasingly described as a salient feature of the illness. In their 2011 review of the disease, Dalmau and colleagues [6] describe speech problems in anti-NMDAR encephalitis as “a rapid disintegration of language, from reduction of verbal output and echolalia (usually with echopraxia) to frank mutism that cannot be attributed to cortical aphasia.” In large case series, such as Florance, et al. [12] that address the disease in children and adolescents, speech deficits are described as reduced verbal output, mutism, mumbling, and perseveration. In the largest cohort described by Titulaer cited above, [2] speech deficits are one of the main clinical signs whereas aphasia and apraxia are not mentioned. The most remarkable distinction of speech dysfunction (described as pressured speech, verbal reduction and mutism) as part of anti-NMDAR encephalitis is in the recent position paper by Graus and colleagues [16]; where the authors include this disturbance in the diagnostic criteria for anti-NMDAR encephalitis along with other 5 major groups of symptoms: abnormal (psychiatric) behavior or cognitive dysfunction, seizures, movement disorders/ dyskinesias or rigidity/abnormal postures, decreased level of consciousness, and autonomic dysfunction/central hypoventilation.
Aphasia is described in some case reports of anti-NMDAR [17] along with other symptoms such as status epilepticus. Interestingly, [18] Deiva, et al. described Broca’s aphasia in a fouryear- old girl as an isolated symptom of this condition, making a differentiation between this phenomenon and other speech disturbances that are more widely recognized. Hayata, et al. [19], recently presented a case report of a patient with anti-Glu2 encephalitis who exhibited global aphasia and swallowing apraxia, along with other more common symptoms such as abnormal behavior, movement disorders and seizures.
To better outline the deficits in our patient, we emphasize that aphasia is a language disorder that is frequently misconceived as a speech disorder. Speech is the “process of language vocalization”, but it is not synonymous with language and should be thought of as distinct [20]. By contrast, language is a “communication system of symbolic expression that has an organized grammar and syntax to convey semantic content” [20]. Aphasia is an “acquired disorder of symbolic language processing [that is] characterized by a combination of naming, fluency, comprehension and repetition deficits that are accompanied by reading and writing impairments” [20]. In our case, the severe deficits in the former functions could have corresponded to the peri-sylvian (including Broca’s and Wernicke’s areas) and extra-sylvian left cortical hemisphere areas [21]. In particular, her deficits were correlated with a nearly exclusive left hemisphere cortical involvement, as demonstrated by the EEG (Figure 4), MRI (Figure 5) and 18-FDG PET scan analyses (Figure 7).
Apraxia is an “acquired disorder of learned, skilled, sequential, motor movements that cannot be accounted for by elementary disturbances of strength, coordination, sensation, or lack of comprehension or attention. It is impairment in selecting and organizing the motor innervations needed to execute an action” [22]. The buccalfacial, ideomotor, ideational, and constructional apraxias [20] demonstrated by our patient could have corresponded to disturbances in her left parietal supramarginal gyrus and/or left premotor frontal area [23], consistent with the left cortical involvement observed in the EEG, MRI, and 18-FDG-PET scan analyses.
The preservation of linguistic and affective prosody, as well as her use of extralinguistic elements (gestures and manners), suggested the preserved function of her right hemisphere [24], which was observable in the EEG, MRI and 18- FDG PET scans.
In a retrospective analysis of the case, we believe that the episode the patient experienced one year and seven months earlier, which was characterized by headache, dizziness, sleep problems and two seizures, was likely the initial episode of anti-NMDAR encephalitis that went undiagnosed. A lumbar puncture was not performed, but an EEG performed thirteen days after the onset of her clinical symptoms revealed generalized moderate dysfunction with predominance in the right temporal region as well as epileptic activity in the same region, consistent with our hypothesis (Figure 2). These symptoms disappeared spontaneously and she never presented with seizures again, despite the lack of a proper antiepileptic treatment regime. Therefore, although this event does not fulfill the criteria for Possible Autoimmune Encephalitis, it could have been a self-limited episode (an EEG was performed 3 months after the episode without treatment and showed normal results). The occipital hypometabolism observed since the first 18-FDG PET scan, a pattern consistently associated with anti-NMDAR encephalitis (Figure 3) [25,26], further supports this hypothesis. In a prospective study of male patients with anti-NMDAR encephalitis, Viaccoz, et al. also described the clinical history of previous neurological and neuropsychiatric episodes in some of their patients, and they also concluded that most were prior undiagnosed instances of the disease [11]. Remission without treatment has been previously documented in anti- NMDAR encephalitis. Gradual improvement can occur within a few weeks [2] or even as late as 3 years after onset [27]. A longitudinal assessment of serum and CSF titers of NMDAR antibodies found a decrease of antibody titers from diagnosis to the last follow-up, regardless of the outcome [28]. The authors suggested a slow spontaneous fading of the immune response could occur, resulting in spontaneous clinical improvement and disappearance of antibodies many months after symptom onset. Although the mechanisms involved in remission and recurrence in anti-NMDAR encephalitis have yet to be described, there is a possible role for CD4+CD25+ regulatory T cells in this context, which suppress the proliferation of conventional T cells, and their production of inflammatory cytokines in experimental autoimmune encephalomyelitis models [29].
In the results of the viral panel, a positive PCR of CMV in CSF was revealed. Qualitative PCR studies are very sensitive and specific for CMV detection (79 and 99%, respectively) [30], but their threshold needs to be carefully calibrated to prevent over-detection [31]. Since these studies do not have the ability to discriminate between a latent DNA and an active viral replication, a qualitative PCR for CMV in immuno-competent hosts without a previously negative PCR make a primary CNS infection unlikely. The absence of IgG and IgM CMV titers at the time was an added limitation in implying a role of CMV in the clinical timeline of the patient.
The parainfectious origin of anti-NMDAR encephalitis has been almost exclusively related to herpes viruses. In this setting, NMDAR antibodies have been associated with herpes simplex encephalitis presenting as prolonged or atypical neurological symptoms with a biphasic course after successful control of the viral infection or relapsing neurologic symptoms after recovery [4]. The time window between herpes viruses’ infection and anti-NMDAR encephalitis is in most cases less than a month [32]. In the context of herpes simplex encephalitis, widespread inflammatory infiltration that induces antigen release has been suggested as a mechanism of autoantibody production [33]. Xu, et al. [34], reported a case of anti-NMDAR encephalitis related to CMV infection in a patient with only a rudimentary spleen suggesting a role of CMV in breaking immune self-tolerance. In the absence of an infectious inflammatory process, it would be more likely that cross-reactive neuronal antigens explain the generation of autoantibodies [35]. Another possible role for CMV in the presented case would be in the enhancement of the inflammatory process. Peripheral infections have been linked to exacerbations of neuroimmune disorders by upregulation of serum acute phase proteins (IL-1β, TNF, and IL-6) causing glial activation after crossing the blood brain barrier or by activation of afferent nerves. Glial activation results in chemokine and cytokine expression, the extravasation of encephalitogenic T-cell, and subsequent T-cell activation with recruitment of professional antigen-presenting cells and production of cytotoxic factors [36]. Moreover, recent studies in animal models have proved that CMV can exacerbate clinical symptoms of experimental autoimmune encephalomyelitis by heightening the activation of disease-specific CD4+ T cells and triggering the expansion of CD4+CD28null T cells [37]. However, further research on the mechanisms by which infections trigger or modulate human autoimmune encephalitis is needed.
Hence, improvement of our patient’s cognitive deficits was achieved with immunosuppressive agents. We started methylprednisolone pulses as a specific treatment for autoimmune encephalitis, achieving partial amelioration of her deficits. Yet, the addition of plasma exchange was needed for a sound response as depicted in Figure 6 and Table 1. In autoimmune encephalopathies these two agents as well as intravenous immunoglobulin, alone or in combination, are regarded as first-line immunotherapy. To date, there is no evidence of superiority on efficacy between IVIg or plasma exchange [5]. Dalmau, et al. recommend the use of immunoglobulin over plasma exchange in anti-NMDAR encephalitis considering that the latter is more difficult to do in the pediatric population, and in patients with autonomic instability or those that are poorly cooperative [6]. However, a recent review shows that plasma exchange is increasingly used in this condition and that it is efficacious and safe even in children [38].
The risk of relapses in anti-NMDAR encephalitis has been calculated to be 12%, being more frequent in patients without underlying tumors. Although multiple relapses are usually less severe than the initial episode, 33% of the patients with anti-NMDAR present recurrent episodes after a relapse [2]. Due to this increased risk, and to the fact that second line immunotherapy during a relapse has proved to provide a significantly decreased incidence of subsequent relapses [2], we decided to add cyclophosphamide to our patient’s regimen even though she had responded well to first-line treatment.
Conclusion
This case broadens the clinical spectrum of anti-NMDAR encephalitis in adolescents. The increased awareness of isolated clinical presentations is important, as early treatment is a predictor of good outcome [18]. We also conclude that the recent trend adopted by several authors of categorizing symptoms into groups has shifted our understanding of this disease from a multistage illness to more of a clinical spectrum, which can be helpful for prompt recognition of this disorder. However, such categorizations require much better descriptions of the symptoms included in each broad category, as they currently lack a detailed delineation of the clinical presentations of the disease. The presence of a positive CSF PCR of Cytomegalovirus does not imply a parainfectious origin of the disease. Further research on parainfectious mechanisms of anti-NMDAR encephalitis is needed.
Acknowledgements
We thank the Molecular Imaging Department of the Institute of Neurology and Neurosurgery of Mexico for their support with the 18FDG-PETMRI images.
We also thank Dr. Katiuzka Casares Cruz for their support with the MRI image.
Fundings
No fundings were received for this manuscript.
Conflict of Interests
The authors declare no conflicts of interest.
References
- Leypoldt F, Armangue Th, Dalmau J. Autoimmune encephalopathies. Ann. NY. Acad. Sci 1338(1), 94-114 (2015).
- Titulaer M, McCraken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-N-Methyl-D-Aspartate (NMDA) receptor encephalitis: a cohort study. Lancet. Neurol 12(2), 157-165 (2013).
- Prüss H, Finke C, Höltje M, et al. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann. Neurol 72(6), 902-911 (2012).
- Armangue T, Moris G, Cantarín-Extremera V, et al. Autoimmune post-herpes simplex encephalitis of adults and teenagers. Neurology 85(20), 1736-1743 (2015).
- Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology 77(2), 179-189 (2011).
- Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet. Neurol 10(1), 63-74 (2011).
- Schott JM. Limbic encephalitis: a clinician’s guideline. Pract. Neurol 6(3), 143-153 (2006).
- Solomon T, Hart I, Beeching N. Viral encephalitis: a clinician’s guide. Pract. Neurol 7(5), 288-305 (2007).
- Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet. Neurol 7(12), 1091-1098 (2008).
- Wandinger KP, Saschenbrecker S, Stoecker W, et al. Anti-NMDA-receptor encephalitis: a severe, multistage, treatable disorder presenting with psychosis. J. Neuroimmunol 231(1-2), 86-91 (2011).
- Viaccoz A, Desestret V, Ducray F, et al. Clinical specificities of adult male patients with NMDA receptor antibodies encephalitis. Neurology 82(7), 556-563 (2014).
- Florance N, Davis R, Lam Ch, et al. Anti-N-Methyl-D-Aspartate Receptor (NMDAR) encephalitis in children and adolescents. Ann. Neurol 66(1), 11-18 (2009).
- Sartori S, Nosadini M, Cesaroni E, et al. Paediatric anti-N-methyl-D-aspartate receptor encephalitis: The first Italian multicenter case series. Eur. J. Paediatr. Neurol 19(4), 453-463 (2015).
- Zekeridou A, Karantoni A, Viaccoz A, et al. Treatment and outcome of children and adolescents with N-methyl-D-aspartate receptor encephalitis. J. Neurol 262(8), 1859-1866 (2015).
- Luna-Lario P, Hernaez-Goñi P, Tirapu-Ustarroz J. Contributions of neuropsychology to encephalitis by anti-NMDA receptor antibodies: A review of the literature. Rev. Neurol 62(9), 415-422 (2016).
- Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet. Neurol 15(4), 391-404 (2016).
- Probasco J, Benavides D, Ciarallo A, et al. Electroencephalographic and fluorodeoxyglucose-positron emission tomography correlates in anti-N-methyl-D-aspartate receptor autoimmune encephalitis. Epilepsy. Behav. Case. Rep 2(1), 174-178 (2014).
- Deiva K, Pera MC, Maurey H, et al. Sudden and isolated Broca’s aphasia: a new clinical phenotype of anti NMDA receptor antibodies encephalitis in children. Eur. J. Paediatr. Neurol 18(6), 790-792 (2014).
- Hayata Y, Hamada K, Sakurai Y, et al. Anti-glutamate ∊2 receptor antibody-positive and anti-N-methyl-d-aspartate receptor antibody-negative lobar encephalitis presenting as global aphasia and swallowing apraxia. Case. Rep. Neurol 6(3), 291-296 (2014).
- Loring D. Ins Dictionary of Neuropsychology. Oxford University Press, New York (1999).
- Diéguez-Vide F, Peña-Casanova J. Brain and language Neuro-linguistic symptomatology. Mexico, Panamericana (2012)
- Strub R, Black W. The mental status examination. 4th ed. FA Davis Company. Philadelphia, USA (2000).
- Benson DF. Aphasia: a clinical perspective. New York: Oxford University Press (1996).
- Heilman KM, Scholes RJ, Watson RT. Auditory affective agnosia. J. Neurol. Neurosurg. Psychiatry 38(1), 69-72 (1975).
- Baumgartner A, Rauer S, Mader I, et al. Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J. Neurol 260(11), 2744-2753 (2013).
- Endres D, Perlov E, Stich O, et al. Hypoglutamatergic state is associated with reduced cerebral glucose metabolism in anti-NMDA receptor encephalitis: a case report. BMC. Psychiatry 15(1), 186 (2015).
- Iizuka T, Sakai F, Ide T, et al. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurol 70(1), 504-511 (2008).
- Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet. Neurol 13(2), 167-177 (2014).
- Fujio K, Okamura T, Sumitomo S, et al. Regulatory T cell-mediated control of autoantibody-induced inflammation. Front. Immunol 3(1), 28 (2012).
- Weinberg A, Hodges TN, Li S, et al. Comparison of PCR, antigenemia assay, and rapid blood culture for detection and prevention of cytomegalovirus disease after lung transplantation. J. Clin. Microbiol 38(2), 768-772 (2000).
- Ross SA, Novak Z, Pati S, et al. Overview of the diagnosis of cytomegalovirus infection. Infect. Disord. Drug. Targets 11(5), 466-474 (2011).
- Venkatesan A, Benavides DR. Autoimmune encephalitis and its relation to infection. Curr. Neurol. Neurosci. Rep 15(3), 3 (2015).
- Höftberger R, Armangue T, Leypoldt F, et al. Clinical Neuropathology practice guide 4-2013: post-herpes simplex encephalitis: N-methyl-D-aspartate receptor antibodies are part of the problem. Clin. Neuropathol 32(07), 251-254 (2013).
- Xu X, Bergman P, Willows T, et al. CMV-associated encephalitis and antineuronal autoantibodies - a case report. BMC. Neurol 12(1), 87 (2012).
- Höftberger R. Neuroimmunology : an expanding frontier in autoimmunity. Front. Immunol 6(1), 1-5 (2015).
- Steelman AJ. Infection as an Environmental Trigger of Multiple Sclerosis Disease Exacerbation. Front. Immunol6(1), 520 (2015).
- Vanheusden M, Broux B, Welten SP, et al. Cytomegalovirus infection exacerbates autoinmune mediated neuroinflammation. Sci. Rep 7(1), 663 (2017).
- Suppiej A, Nosadini M, Zuliani L, et al. Plasma exchange in pediatric anti-NMDAR encephalitis: a systematic review. Brain. Dev 38(7), 613-22 (2016).