Research Article - (2024) Volume 14, Issue 5
A Comparative Pharmacokinetic Evaluation of Paliperidone Palmitate Extended-Release Injectable Suspensions: An Open Label, Randomized, Two treatment, Two-Period, Two-Sequence, Multiple Dose, Steady State Crossover Bioequivalence Study in Patients with Schizophrenia and/or Schizoaffective Disorder
- Corresponding Author:
- Arthik Shetty
Department of Medical Affairs and Clinical Research, Sun Pharma Laboratories Limited, Mumbai, Maharashtra, India
E-mail: [email protected]
Received date: 23-Sep-2024, Manuscript No. NPY-24-148606; Editor assigned: 25-Sep-2024, PreQC No. NPY-24-148606 (PQ); Reviewed date: 09-Oct-2024, QC No. NPY-24-148606; Revised date: 16-Oct-2024, Manuscript No. NPY-24-148606 (R); Published date: 23-Oct-2024, DOI: 10.37532/1758-2008.2024.14(5).728
Abstract
Background: Schizophrenia (SZ) and Schizoaffective (SA) disorders are severe psychiatric conditions affecting millions globally. Paliperidone, administered intramuscularly once a month is a long-acting injectable antipsychotic, effective in symptom control and relapse prevention. This study assessed bioequivalence and safety of paliperidone palmitate Extended-Release (ER) Injectable suspension (Paliris LA®) (Test(T)) manufactured by Sun Pharmaceuticals Industries Limited compared to paliperidone palmitate ER Injectable suspension (Invega Sustenna®) (Reference(R)) manufactured by Janssen Pharmaceuticals in patients with SZ and or SA disorder.
Methods: This open-label, multi-center, randomized, two-treatment, two-period, two-sequence, steady-state, cross-over, multiple-dose bioequivalence study enrolled 108 patients. Patients received 156 mg/ml of T or R on Day-1, Day-29, Day-57, Day-85 and Day-113 (Period-01), followed by other treatment on Day-141, Day-169, Day-197, Day-225 and Day-253 (Period-02), per randomization schedule. Pharmacokinetic parameters Maximum concentration at steady state (CmaxSS), Area under concentration-time curve (AUC0-τ) AND mean plasma concentration-time profiles at steady-state) were assessed. Safety was monitored through Treatment-Emergent Adverse Events (TEAEs).
Results: Both formulations demonstrated comparable pharmacokinetic profiles meeting the criteria for bioequivalence within acceptable limits (80.00-125.00%). Ratio of Least-Squares (LS) mean difference was 95.64 (90% Confidence Interval (CI): 90.28-101.32) for AUC0-τ, 91.47 (90% CI: 84.61-98.89) for CmaxSS and 91.27 (90% CI: 82.25-101.29) for percentage of fluctuation. Plasma concentration-time profiles were similar and no serious Adverse Events (AE) was reported. Commonly reported TEAEs for T included leukopenia, neutropenia AND dyspepsia while R commonly caused vomiting, injection site pain, urinary tract infection, hypertriglyceridemia, dizziness, orthostatic hypotension and headache.
Conclusion: Paliris LA® and Invega Sustenna® are bioequivalent in terms of pharmacokinetic profile and well-tolerated, supporting their interchangeability in treating SZ and SA disorders.
Keywords
Bioequivalence, long-acting injectables, paliperidone palmitate, schizoaffective disorder, schizophrenia
Introduction
Schizophrenia (SZ), one of the leading causes of disability, significantly contributes to the prevalence of mental disorders constituting a persistent and severe psychiatric condition characterized by a spectrum of symptoms [1]. The symptoms include hallucinations and delusions, collectively referred to as “positive symptoms” as well as diminished motivation, flattened emotions (termed “negative symptoms”), cognitive deficits including impaired memory and reduced cognitive flexibility, alongside motor and mood disturbances [2,3]. Globally, SZ has affected approximately 23 million individuals, with 3.5 million cases specifically in India [4,5]. Schizoaffective Disorder (SA), another psychotic condition is characterized by the manifestation of psychotic symptoms typical of schizophrenia, along with mood symptoms such as manic or depressive episodes [6]. Studies indicate that SA is about one-third as common as SZ, with an estimated lifetime prevalence of around 0.3% [7]. It affects approximately 30% of individuals aged between 25 and 35, with a higher prevalence among women and accounting for 10% to 30% of hospital admissions for psychosis [7].
The existing pharmacological treatments available for the treatment of SZ comprise firstgeneration or typical antipsychotics (dopamine receptor antagonists) and second-generation or a typical antipsychotic (serotonin-dopamine antagonists) [8]. Treatment strategy for SA also involves the use of antipsychotic medication, either standalone or in conjunction with mood stabilizers or antidepressants [9]. In recent years, growing research has recommended utilizing second-generation antipsychotics as they have shown decreased extrapyramidal side effects in comparison to first-generation antipsychotics [10,11]. While maintaining continuous long-term pharmacological treatment is an objective in SZ or SA management, real-world studies have revealed that adherence to oral antipsychotics often falls below 50% with a high rate of treatment discontinuation exceeding 50% leading to re-hospitalization and relapse [12,13]. The primary reason for relapse in treated schizophrenia often arises from inadequate adherence to oral medication [14]. The adherence issues are addressed effectively by Long-Acting Injectable (LAI) antipsychotics, which are concentrated formulations that release the drug slowly after Intra-Muscular (IM) injection, enabling effective maintenance doses with intervals from 2 weeks to several months [15].
Materials and Methods
Study protocol
Findings suggest that LAIs eliminate the need for daily dosing and reduce the risk of accidental or intentional overdose. Their use not only maintains consistent plasma levels but also minimizes dose-related side effects, improves positive symptoms, alleviates depressive symptoms and promotes social functioning [16,17]. In comparison to oral antipsychotics, LAIs have been shown to significantly improve treatment adherence, restore social functionality and decrease the likelihood of relapse, hospitalization, emergency department visits and overall cost savings, particularly for high-risk hospitalized SZ patients. Paliperidone, a member of the benzisoxazole derivatives chemical class, is the principal active metabolite of the secondgeneration antipsychotic, risperidone [18].
Acting as a centrally-active antagonist of the Dopamine D2 and Serotonin 2A (5HT2A) receptors, paliperidone is one of the longacting, antipsychotic therapeutic, to treat adult patients with SZ or SA disorder [19-22].
Oral paliperidone Extended Release (ER) was approved by the United States Food and Drug Administration (USFDA) for SZ in 2006 and for SA disorder in 2009 [23,24]. It has also received approval in India for both acute and maintenance treatment of SZ [25]. Paliperidone palmitate is available in oral as well as Longacting Injectable (LAI) formulations [26,27].
Notably, studies have highlighted its efficacy in improving symptom control, functional outcomes and relapse prevention making it rank among the most frequently prescribed antipsychotic medications [28,29].
This study aimed to evaluate the bioequivalence and safety of paliperidone palmitate Extended Release (ER) injectable suspension (Paliris LA®) (Test (T)) manufactured by Sun Pharmaceuticals Industries Limited, in comparison to paliperidone palmitate ER injectable suspension product (Invega Sustenna®) (Reference (R)) manufactured by Janssen Pharmaceuticals in patients diagnosed with SZ and or SA disorder [30,31].
Study Design
An open label, multi-center, randomized, twotreatment, two-period, two-sequence, multiple dose, steady state cross-over, bioequivalence study was conducted to compare Paliris LA® (Paliperidone palmitate ER injectable suspension 156 mg/ml) of Sun Pharmaceutical Industries Limited (Test product or T) with Invega Sustenna® (Paliperidone palmitate ER injectable suspension 156 mg/ml) of Janssen Pharmaceuticals, Inc., (Reference product or R), administered intramuscularly in patients with SZ and or SA disorder. The study complies with the declaration of Helsinki. The study obtained written approval from the Indian regulatory body (Central Drugs Standard Control Organization (CDSCO)).
Study objectives
The primary objective of the study was to evaluate the steady-state bioequivalence of paliperidone palmitate ER injectable suspension 156mg/ml from Sun Pharmaceutical Industries Limited with Invega Sustenna® ER injectable suspension 156 mg/ml from Janssen Pharmaceuticals, Inc., in patients diagnosed with SZ and or SA disorder. The secondary objective of the study was to monitor the Adverse Events (AEs) and to ensure the safety of patients after Investigational Product (IP) administration.
Study participants
Subjects were selected based on predefined inclusion and exclusion criteria, the details of which are provided in the supplementary file. Clinically stable patients aged between 18 years and 65 years with a Body Mass Index (BMI ≥ 18.00 kg/m2) weight of at least 50 kg for males or 48 kg for females, diagnosed with SZ and or SA disorder (as per Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-V criteria) and maintained on a stable dose (at least two prior maintenance doses) of paliperidone palmitate ER injectable suspension 156 mg were eligible.
Study treatments
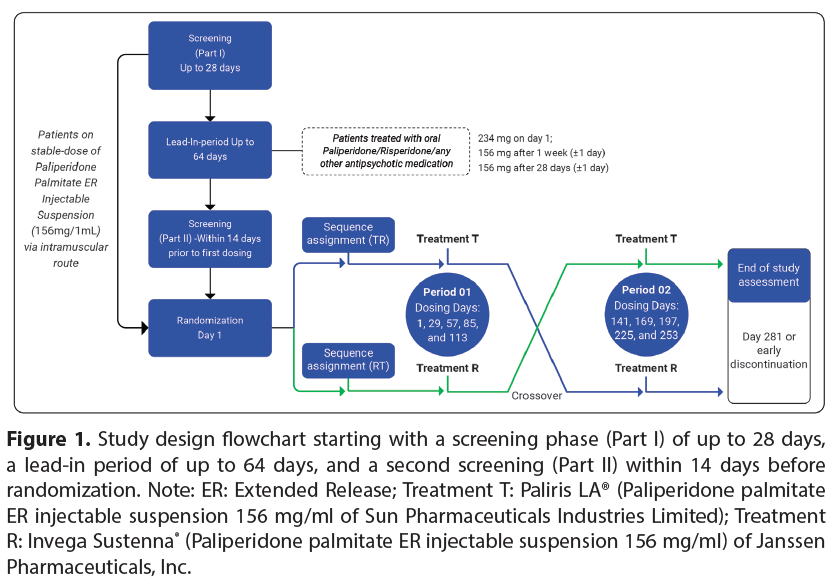
Screening: Patients underwent demographic assessments, physical examinations such as vital signs and assessment for COVID-19 symptoms, evaluation of orthostatic hypotension thorough medical and medication histories, 12-Lead Electrocardiogram (ECG), serum pregnancy tests (for females capable of childbearing), Columbia Suicide Severity Rating Scale (C-SSRS) assessments, urine screens for drugs of abuse, urine pregnancy tests, alcohol breath tests and safety laboratory tests (covering hematology, biochemistry, serology AND urine analysis). The study design flowchart is provided in (Figure 1). Patients were screened for eligibility to enter either the lead-in period or treatment period. Patients on a stable dose of paliperidone palmitate ER 156 mg/ml injectable suspension entered directly into the treatment period (without the lead-in period).
However, non-naive transition patients (who were treated with oral paliperidone, oral risperidone or any other oral antipsychotic medication) were included in the lead-in period based on the clinical judgement of the investigator. The lead-in period allowed the patients to reach a stable dose of paliperidone palmitate ER injectable suspension 156 mg via the Intra-Muscular (IM) route before entering the treatment period. Patients were given paliperidone palmitate ER 234 mg/ml on day 1 in the deltoid muscle, followed by a 156 mg/ ml dose in the deltoid muscle one week later (± 1 day) AND another 156 mg/ml dose after 28 days (±1 day) administered either in the deltoid or gluteal muscle. Patients who completed leadin period entered into final screening period (Part-II) within 14 days of 1st Intra-Peritoneal (IP) dosing.
Drug administration during the treatment period
Randomization schedule was generated using Statistical Analysis System (SAS®) (SAS Institute Inc., United States of America) software version 9.4 according to which the patients were administered the designated IP (either T or R) via IM injection in deltoid or gluteal muscle. The dose of paliperidone palmitate ER injectable suspension 156mg/ ml was selected as per USFDA s Office of Generic Drugs (OGD) recommendations. The treatment assignment was done using Interactive Web Responsive System (IWRS). Patients received either T or R for consecutive five dosing periods on day 1, day 29, day 57, day 85 and day 113 (Period 01). Subsequently, patients were switched over to the other treatment based on the randomization schedule for the next consecutive five doses on day 141, day 169, day 197, day 225 and day 253 (Period 02) (Figure 1).
Figure 1: Study design flowchart starting with a screening phase (Part I) of up to 28 days, a leadin period of up to 64 days, and a second screening (Part II) within 14 days before randomization. Note: ER: Extended Release; Treatment T: Paliperidone palmitate ER injectable suspension 156 mg/ml of Sun Pharmaceuticals Industries Limited; Treatment R: Invega Sustenna® (Paliperidone palmitate ER injectable suspension 156 mg/ml) of Janssen Pharmaceuticals, Inc.
Blood sample processing and storage
During the study a total of 40 blood samples (6 pre-dose samples and 34 post-dose samples), each measuring 4.0 ml, were collected at different time-points for 281 days from each patient for pharmacokinetic analysis. Details of blood collection time-points are provided in the supplementary file. Blood samples were collected at different study centers in Dipotassium Ethylenediaminetetraacetic Acid (K2-EDTA) vacutainers and centrifuged at 3300 rpm for 15 minutes at 4°C ± 2°C within one hour of collection and stored at -20°C ± 5°C at the clinical facility. Upon receipt at the pharmacokinetics department (Sun Pharmaceutical Industries Limited, Tandalja, Vadodara, Gujarat), samples were stored at -65°C ± 10°C. Quantification of paliperidone in human plasma was performed using a validated Liquid Chromatography-Mass Spectrometry (LC-MS) method.
Pharmacokinetic analysis
Primary pharmacokinetic parameters included Maximum Concentration at Steady State (CmaxSS) and area under the concentration-time curve (AUC0-τ). Secondary pharmacokinetic parameters included Minimum Concentration at Steady State (CminSS), Time to Reach Maximum Concentration at Steady State (Tmaxss), percentage of fluctuation, Average Concentration at Steady State (Cavss), Trough Concentration at Steady State (Ctroughss) and swing (difference between peak and trough concentrations). Trough concentration data on day 57, 85 and 113 of period 01 and on day 197, 225 and 253 of period 02 were analyzed statistically to verify the attainment of steady state prior to pharmacokinetic sampling after day 113 and 253 respectively in each individual. The analysis was done using the non-compartmental model with Phoenix® WinNonlin® software (Version 6.4).
Safety analysis criteria
The criteria for evaluating safety in the study included vigilant monitoring and documentation of Adverse Events (AEs), Serious Adverse Events (SAEs) AND Treatment-Emergent Adverse Events (TEAEs). To ensure a thorough safety evaluation, vital signs were recorded and physical examination and routine clinical and laboratory investigations were performed. Urine samples were screened for the presence of drugs of abuse and alcohol breath tests were administered.
Statistical analysis
Arithmetic means, minimum and maximum values, standard deviations and coefficients of variation were calculated for the demographic data. The statistical methods were employed to assess the efficacy of pharmacokinetics, specifically focusing on parameters such as Cmaxss, AUC0-τ and percentage of fluctuation. Parametric Analysis of Variance (ANOVA) was utilized to compare these parameters between different groups and treatments with 90% Confidence Intervals (CI) calculated for each parameter to provide a measure of precision. Additionally, to meet the assumptions of normality and homogeneity of variance, the parameters were subjected to Natural Logarithm (Ln) transformation before analysis. The results were evaluated using the SAS® mixed procedure (Version 9.4).
90% confidence intervals and ratio analysis: The 90% CI of the Geometric Least Square Mean Ratio (GMR) of CmaxSS and AUC0-τ of T and R product of paliperidone was required to be between 80% and 125% (inclusive) for logtransformed data. Additionally, the fluctuation for the T product was evaluated for comparability with the fluctuation of the R product.
Sample size justification: For expected mean difference between T and R formulation about ± 10% and anticipated intra-subject Coefficient of Variation (CV) of around 30% for CmaxSS, a sample size of 80 patients was found to be sufficient to ensure 80% power for demonstrating bioequivalence for this cross over study design. Additional patients were recruited who continued until completion or withdrawal and included in pharmacokinetic and statistical analysis as applicable.
Results
Subjects
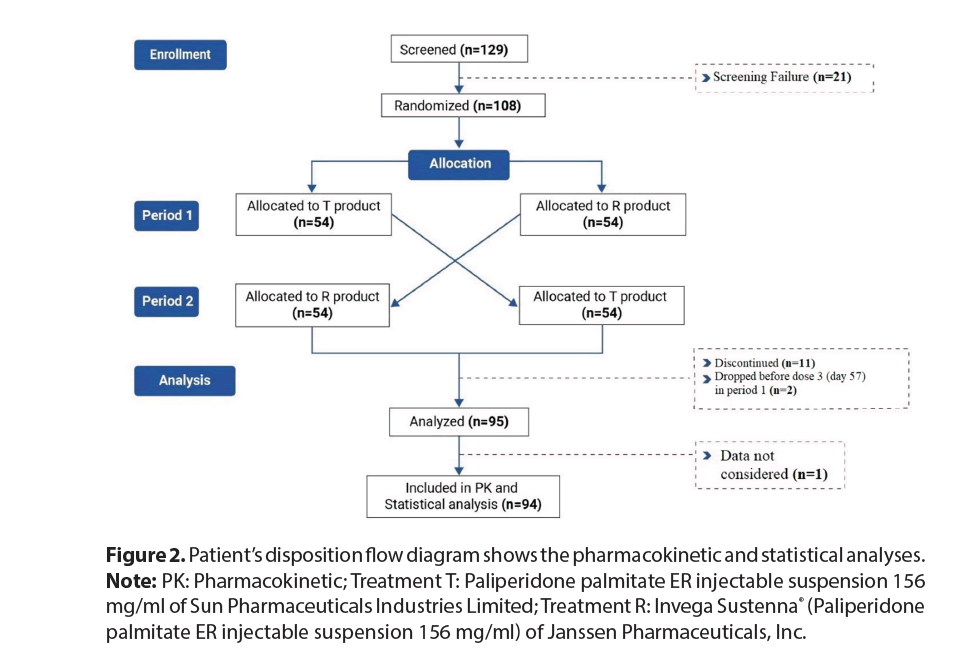
The study was conducted from 10 July, 2021 to 12 September, 2022. Out of 129 patients screened, 21 did not match the screening criteria. Eventually, 108 patients were randomized forming the safety population. Among them, 13 discontinued the study prematurely and the remaining 95 patients completed the study, with 94 included in subsequent pharmacokinetic and statistical analyses (Figure 2).
Figure 2: Patient’s disposition flow diagram shows the pharmacokinetic and statistical analyses. Note: PK: Pharmacokinetic; Treatment T: Paliperidone palmitate ER injectable suspension 156 mg/ml of Sun Pharmaceuticals Industries Limited; Treatment R: Invega Sustenna® (Paliperidone palmitate ER injectable suspension 156 mg/ml) of Janssen Pharmaceuticals, Inc.
Demographic or baseline characteristics
The study consisted of 54 participants each in the sequence TR and sequence RT groups. Overall, males accounted for 70.4% and females accounted for 29.6%. Mean age ranged from 34.8 years to 35.4 years and height, weight and Body Mass Index (BMI) varied within relatively narrow ranges across both groups. All the participants were Asian and identified as Not Hispanic or Latino. Demographic characteristics are given in (Table 1). No abnormalities were found in the hematology and biochemistry assessment, physical examination AND other tests performed during screening and end of the study period.
| Characteristic | Statistics | Sequence TR | Sequence RT |
|---|---|---|---|
| (N=54) | (N=54) | ||
| Age (years) | Mean (SD) | 35.4 (9.28) | 34.8 (8.67) |
| Range | 18-55 | 18-56 | |
| Height (cm) | Mean (SD) | 161.63 (9.407) | 162.78 (8.329) |
| Range | 143.0-185.6 | 144.0-183.0 | |
| Weight (kg) | Mean (SD) | 63.487 (10.3584) | 63.186 (9.0011) |
| Range | 49.40-92.90 | 49.10-81.20 | |
| BMI (kg/m²) | Mean (SD) | 24.277 (3.2056) | 23.897 (2.9551) |
| Range | 18.74-33.30 | 19.10-31.30 | |
| Ethnicity | |||
| Not Hispanic or Latino | n (%) | 54 (100.0) | 54 (100.0) |
| Race | |||
| Asian | n (%) | 54 (100.0) | 54 (100.0) |
| Gender | |||
| Male | n (%) | 35 (64.8) | 41 (75.9) |
| Female | n (%) | 19 (35.2) | 13 (24.1) |
Table 1: Demographic details of the study participants.
Pharmacokinetic and statistical results
The pharmacokinetic analysis of T and R products revealed comparable profiles in key parameters such as AUC0-τ, CmaxSS, CminSS, CavSS and Ctrough as demonstrated in (Table 2). The mean AUC0-τ values were 29998.15 ng.h/ml for T and 30626.24 ng.h/ml for R, while mean CmaxSS values were 59.25 ng/ml and 65.98 ng/ ml and mean TmaxSS values were 207.32 hours and 177.46 hours respectively. However, slight differences were observed in CminSS, CavSS and Ctrough. Notably, R product exhibited higher percentage of fluctuation (70.17% versus 58.35%) and swing (1.11 versus 0.87) than T product, indicating greater variability in plasma concentrations over the dosing interval compared to T product (Table 2).
| Test | Reference | |||
|---|---|---|---|---|
| Parameters | Mean ± SD | CV% | Mean ± SD | CV% |
| AUC0-τ(ng.h/ml) | 29998.15 ± 12014.58 | 40.05 | 30626.24 ± 10819.64 | 35.33 |
| Cmaxss(ng/ml) | 59.25 ± 27.01 | 45.59 | 65.98 ± 39.64 | 60.08 |
| Cminss(ng/ml) | 32.43 ± 12.76 | 39.36 | 33.24 ± 12.61 | 37.93 |
| Cavss (ng/ml) | 44.64 ± 17.88 | 40.05 | 45.58 ± 16.10 | 35.33 |
| Ctrough(ng/ml) | 39.39 ± 15.64 | 39.7 | 38.91 ± 16.20 | 41.62 |
| Tmaxss (h) | 207.32 ± 155.21 | 74.87 | 177.46 ± 145.80 | 82.16 |
| *TmaxSS (h) | 168.00 (0.00-672.00) | - | 144.00 (0.00-672.00) | - |
| Percentage (%) of Fluctuation | 58.35 ± 27.02 | 46.3 | 70.17 ± 69.20 | 98.61 |
| Swing | 0.87 ± 0.58 | 66.93 | 1.11 ± 1.80 | 162.47 |
| Note: *Median values (range) are presented; N=93 for reference treatment as 672 hours’ sample (after dose-5 in period-2) of one patient was not collected. Standard Deviation (SD); Coefficient of Variation (CV); Area under the concentration-time curve during a dosing interval (AUC0-τ); Maximum plasma concentration at steady state (CmaxSS); Minimum plasma concentration at steady state (CminSS); Average plasma concentration at steady state (CavSS); Concentration at the end of the dosing interval (Ctrough); Time to reach maximum plasma concentration at steady state (TmaxSS); Time to reach maximum plasma concentration at steady state (median and range) (*TmaxSS); Percent fluctuation between peak and trough concentrations (% of Fluctuation); Swing: Peak-to-trough fluctuation ratio. | ||||
Table 2: Summary of results for pharmacokinetic parameters of T and R products in patients with SZ or SA disorder (N=94), mean values ± SD and CV% are reported for each parameter.
The statistical analysis revealed comparable results across key pharmacokinetic parameters. For AUC0-τ, the least squares means were 10.26 for T and 10.30 for R, with corresponding geometric means of 28440.08 and 29736.04 respectively. The ratios of the least-squares geometric means (and 90% geometric CIs) of T to R product for paliperidone were 95.64% (90.28 to 101.32) for AUC0-τ, 91.47% (84.61 to 98.89) for Cmaxss and for fluctuation 91.27% (82.25 to 101.29). The 90% geometric CIs for all the pharmacokinetic variables were between the acceptable range of 80% and 125% (Table 3). The intra-patient CV of AUC0-τ, Cmaxss and percentage of fluctuation was 23.99%, 33.00% and 44.79% respectively, demonstrating consistency in pharmacokinetic profiles.
| PK variables | Least Squares Means | Least Squares Geometric | Ratio of Least-Squares Geometric Means1 | 90% Geometric CI2 | Intra-patient CV% | ||
|---|---|---|---|---|---|---|---|
| Means3 | % | ||||||
| Test | Reference | Test | Reference | ||||
| AUC0-τ | 10.26 | 10.3 | 28440.08 | 29736.04 | 95.64 | 90.28-101.32 | 23.99 |
| Cmaxss | 4.01 | 4.1 | 55.13 | 60.27 | 91.47 | 84.61-98.89 | 33 |
| Percentage of fluctuation | 3.94 | 4.03 | 51.17 | 56.07 | 91.27 | 82.25-101.29 | 44.79 |
| Note: Pharmacokinetic (PK); Area under the concentration-time curve during a dosing interval (AUC0-τ); Maximum plasma concentration at steady state (CmaxSS); Confidence Interval (CI); Coefficient of Variation (CV); N=93 for reference treatment as 672 hour sample (after dose-5 in period-2) of one patient was not collected. 1Least Squares Geometric Means Ratio Calculated according to the formula: e (LSM Test (T) – LSM Reference (R)) X 100. 290% Geometric Confidence Interval using Ln-transformed data. 3Least-square geometric means calculated from least-squares mean as e (least-square mean) | |||||||
Table 3: Summary of statistical analysis of paliperidone (N=94) - Ln-transformed data.
Mean plasma concentration–time profile at steady state
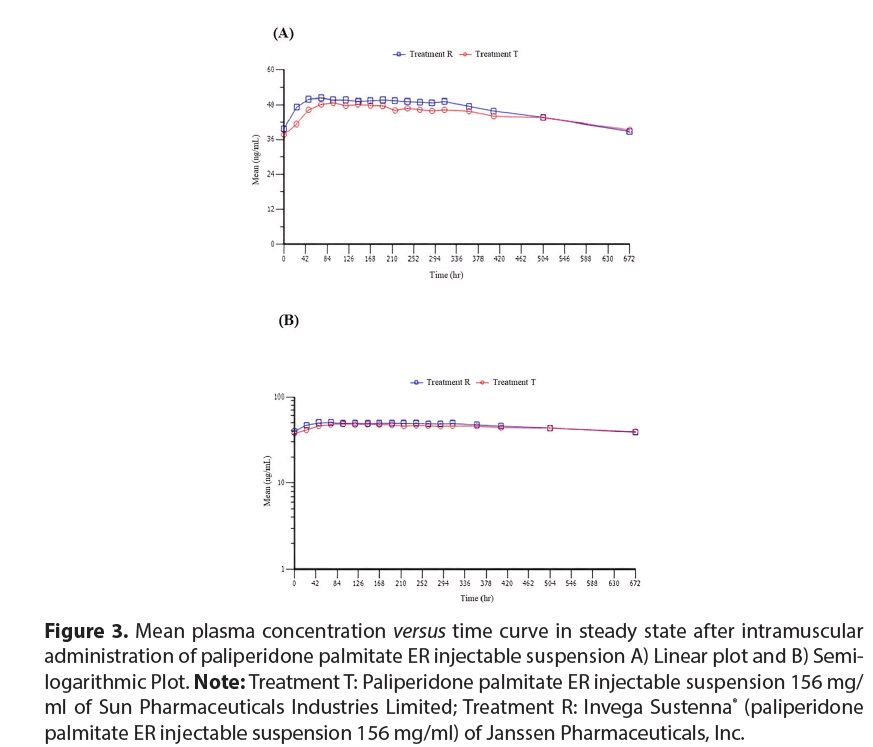
The mean plasma concentration-time profiles at steady state were analyzed. Paliperidone administration was followed by a rapid increase in plasma concentrations over 24 to 48 hours from administration of the first dose administration of both T and R products. Initially (0 hours), the plasma concentrations were 37.7185 ng/ml and 39.7797 ng/ml that increased to 46.2285 ng/ml and 49.8729 ng/ ml at 48 hours in T and R groups, respectively. However, sustained plasma concentrationtime profile for 312 hours (i.e., 13 days) was observed in both the groups. By the end of 672 hours (i.e., 28 days), the mean concentrations were 39.4 ng/ml and 38.8 ng/ml for T and R respectively. Both products exhibited similar pharmacokinetic profiles, as shown in mean concentration versus time plots (linear and semi logarithmic) for paliperidone in (Figure 3 (A) and (B)).
Figure 3: Mean plasma concentration versus time curve in steady state after intramuscular administration of paliperidone palmitate ER injectable suspension A) Linear plot and B) Semilogarithmic Plot. Note: Treatment T: Paliperidone palmitate ER injectable suspension 156 mg/ ml of Sun Pharmaceuticals Industries Limited; Treatment R: Invega Sustenna® (paliperidone palmitate ER injectable suspension 156 mg/ml) of Janssen Pharmaceuticals, Inc.
Safety results
A total of 108 patients randomzied were included in the safety population. Overall, 64 Treatment-Emergent Adverse Events (TEAEs) were reported among 38 patients (35.2%) throughout the study duration and the majority of the reported TEAEs were mild to moderate in nature. Out of 64 TEAEs, 37 AEs reported by 29 patients were mild, 25 AEs reported by 14 patients were moderate and 2 AEs reported by 2 patients were severe or medically significant but not immediately life threatening.
Out of 64 TEAEs, 2 AEs reported by 1 patient was certainly related, 15 AEs reported by 11 patients were possibly related, 10 AEs reported by 8 patients were likely related and 37 AEs reported by 21 patients were unlikely related to study drug. The commonly reported TEAEs for the T product included leukopenia, neutropenia and dyspepsia, while the R product commonly caused vomiting, injection site pain, urinary tract infection, hypertriglyceridemia, dizziness, orthostatic hypotension and headache. No SAE or death was reported during the study period. No TEAE led to discontinuation of the study drug. The safety results are demonstrated in (Table 4).
| Test | Reference % | n(count of TEAEs) | |
|---|---|---|---|
| n(%) count of AEs | n(%) count of AEs | ||
| (N=106) | (N=103) | ||
| Total TEAEs | 20 (18.9) 25 | 29 (28.2) 39 | 38 (64) |
| Commonly (> 1%) reported TEAEs and their occurrence rates in patients (%) | |||
| Leukopenia | 2(1.9)2 | - | 2(2) |
| Neutropenia | 2(1.9)2 | - | 2(2) |
| Dyspepsia | 2(1.9)2 | - | 2(2) |
| Schizophrenia (Exacerbation of Schizophrenia) | 2(1.9)2 | 2(1.9)3 | 4(5) |
| Vomiting | - | 2(1.9)2 | 2(2) |
| Injection site pain | 1(0.9)1 | 2(1.9)2 | 3(3) |
| Urinary tract infection | 1(0.9)1 | 2(1.9)2 | 3(3) |
| Hypertriglyceridaemia | - | 2(1.9)2 | 2(2) |
| Dizziness | - | 2(1.9)2 | 2(2) |
| Orthostatic hypotension | 1(0.9)1 | 3(2.9)4 | 4(5) |
| Headache | 1 (0.9)1 | 3(2.9)3 | 4 (4) |
| Others (< 1%) | 8(7.5)13 | 11(10.7)19 | 19(32) |
| Severity | |||
| Mild | 12(11.3)14 | 20(19.4)23 | 29(37) |
| Moderate | 9(8.5)10 | 11(10.7)15 | 14(25) |
| Severe or medically significant | 1(0.9)1 | 1(1.0)1 | 2(2) |
| Relation to study drug | |||
| Certainly related | 1(0.9)1 | 1(1.0)1 | 1(2) |
| Possibly related | 6(5.7)6 | 8(7.8)9 | 11(15) |
| Probably or likely related | 1(0.9)1 | 8(7.8)9 | 8(10) |
| Unlikely related | 13(12.3)17 | 13(12.6)20 | 21(37) |
| Note: N: Total number of patients; n: Number of patients; TEAEs:Treatment-Emergent Adverse Events | |||
Table 4: Total number of TEAEs, their severity, relationship to the study drug and their distribution across treatment groups T and R.
Discussion
The present study assessed the bioequivalence of paliperidone palmitate ER injectable suspension 156 mg/ml from Sun Pharmaceutical Industries Limited (T) and Janssen Pharmaceuticals Inc., (R) in patients with SZ and or SA disorder. Antipsychotic medications are available in both oral and Long-Acting Injectable (LAI) forms. The most frequent reason for relapse in SZ is poor adherence to oral antipsychotics. Discontinuation rates for these medications range from 26% to 44% and up to two-thirds of patients exhibit at least partial non-adherence, consequently elevating the risk of hospitalization [32]. The need for inpatient care among SZ or SA patients significantly impacts the overall cost associated with managing this condition. The research findings indicate that LAIs, including paliperidone palmitate offer potential advantages over oral antipsychotics in terms of decreasing relapse and hospitalization rates among SZ or SA patients, suggesting considerable potential for expense reduction [33-36]. Patients diagnosed with SZ (N=5638) who started taking LAIs, such as paliperidone palmitate indicated improved medication adherence, with a 5% higher mean adherence and 20% lower likelihood of LAIs discontinuation [37]. It has also been demonstrated that compared to oral medications, paliperidone palmitate injection significantly extends the relapse-free period for patients, with individuals experiencing an extension of over 200 days without relapse [38]. LAIs require less frequent drug dosing as compared to oral antipsychotics because of their ER formulation [39].
Oral antipsychotics require daily administration which can lead to adherence issues. The LAI antipsychotics address this by eliminating the necessity for daily dosing, ensuring consistent therapeutic drug levels over extended periods thus, diminishing the likelihood of relapse and re-hospitalization [40]. LAIs enhance adherence by ensuring stable pharmacokinetics, resulting in reliable medication delivery and reduced dosing frequency [41]. While first-generation LAIs present opportunities for improved adherence and reduced rates of discontinuation and readmission, their clinical advantages are tempered by elevated occurrences of extrapyramidal adverse effects and tardive dyskinesia, often leading to treatment discontinuation [42]. The emergence of second-generation antipsychotic drugs, such as paliperidone, in LAI form holds potential due to their potential to exhibit fewer movement-related side effects compared to first-generation antipsychotic drugs [43]. Paliperidone is preferred over other LAIs (such as risperidone) based on several advantages, including enhanced compliance due to longer dosing intervals, decreased nurse time required and simpler storage and preparation needs [44].
Paliperidone palmitate has been compared to placebo and oral antipsychotics and has demonstrated superiority in improving outcomes in SZ or SA patients [33,45,46]. A study reported that patients receiving paliperidone palmitate LAI experienced a reduction in the number of inpatient days compared to those receiving oral atypical antipsychotics (incidence rate ratio of 0.78; p=0.004) [47]. Furthermore, Kim et al., demonstrated a notable 29% reduction in readmission rates of paliperidone palmitate as compared to oral medication and a substantial 58% decrease in re-admission rates among patients with recurrent admissions [48]. Moreover, paliperidone palmitate exhibits positive impacts on functionality, improving negative symptoms and efficacy in preventing relapses in SZ as well as SA disorder [49-52]. The Personal and Social Performance (PSP) test has demonstrated that paliperidone palmitate LAI improves functional outcomes. It has been observed that 29.4% of patients initially exhibiting an unfavorable level of functioning reached a favorable level after paliperidone palmitate administration (PSP score greater than 70) [53]. Patients treated with paliperidone palmitate also showed consistent improvement in Brief Negative Symptom Scale (BNSS) and Positive and Negative Syndrome Scale (PANSS) scores.
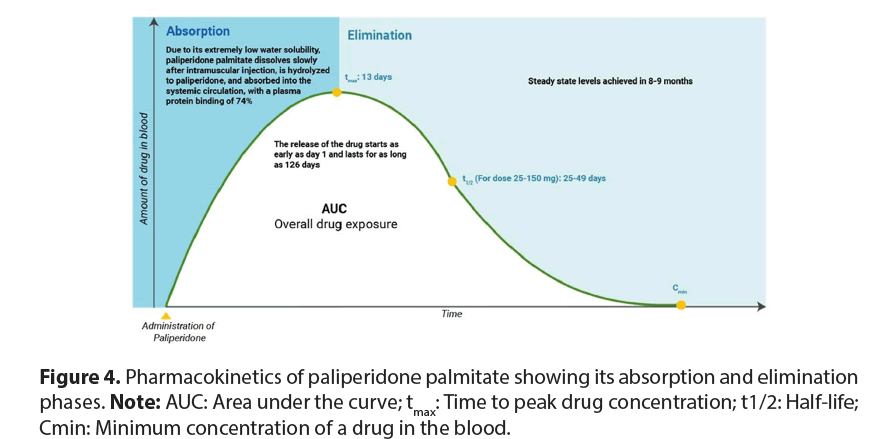
The mean Standard Deviation (SD) values were observed to drop from 17.2(4.31) to 15.9(4.93) for negative subscale scores indicating improvement in negative symptoms (mild to severe) of SZ [54]. An Indian study involving 109 SZ patients receiving paliperidone ER, resulted in a significant reduction in the total Positive and Negative Syndrome Scale (PANSS) score from 96.1 ± 12.5 at baseline to 54.7 ± 15.9 at the end of 6 weeks [55]. By the end of the trial, the percentage of patients classified as markedly ill in the paliperidone ER group significantly reduced from 47.2% to 2.8%. Additionally, in terms of preventing relapses, among 367 SZ patients who took paliperidone palmitate, 84.6% remained free from relapse during the 13-week treatment period [56]. Paliperidone serves as the main active metabolite found in risperidone. This ER medication is formulated as a palmitate ester of paliperidone within an aqueous suspension of nanocrystals, providing a gradual and continuous release pattern [57,58]. Nanocrystals are typically around one-tenth the size smaller than the particles in conventional drug powders, having an average diameter of 20 μm. This innovation enhances drug solubility, absorption and bioavailability [59]. Paliperidone palmitate is nearly insoluble in water and after its IM administration it dissolves slowly at the injection site but is then hydrolyzed rapidly to paliperidone before being systemically absorbed.
This medication is formulated to rapidly achieve stable paliperidone concentrations at the onset of treatment, eliminating the need for oral supplementation. Peak plasma concentrations of paliperidone palmitate once monthly are typically reached around 13 days after a single injection and its observed half-life of 25-49 days allows for monthly administration (Figure 4). Since paliperidone is mainly cleared renally and minimally metabolized in the liver, it poses low potential for drug interactions and serum concentrations are unlikely to be affected by the Cytochrome P450 2D6 (CYP2D6) genotype [60,61]. In this study, the pharmacokinetic analysis of T and R products revealed comparable profiles in AUC0-τ, CmaxSS and CavSS and overall findings aligned with those of previous report [62,63].
The R product exhibited higher percentage of fluctuation and swing compared to T, indicating greater variability in plasma concentrations over the dosing interval. The median Tmax was recorded at a median of 7 days for T and 6 days for R, aligning with previous studies on the pharmacokinetics of paliperidone palmitate [64,65]. It is important to note that the consistency in pharmacokinetic profiles of the current study, as indicated by the intra-patient coefficients of variation, suggests therapeutic similarities between T and R throughout the dosing period.
The statistical analysis confirmed the bioequivalence of T and R as evidenced by the narrow 90% geometric confidence intervals and the ratios of the least-squares geometric means falling within the standard bioequivalence range (80%-125%) [66]. Examination of plasma concentration-time profiles at steady state revealed that both T and R products exhibited similar trends. Administration of paliperidone resulted in a rapid rise in plasma concentrations within 24 hours to 48 hours following the initial doses of both the T and R formulations. For both the products, plasma concentrations were sustained throughout the study duration. The common TEAEs reported in this study were consistent with known side effects of paliperidone such as leukopenia, neutropenia and vomiting [67]. The most of the TEAEs observed in this study were mild to moderate in nature similar to previously reported TEAEs [68]. While this study suggests bioequivalence of the T and R products, factors such as patientspecific characteristics treatment adherence and individual response variations were not extensively explored.
Conclusion
Long-term management of SZ and SA disorders is significantly challenged by high rates of relapse and hospitalization, often due to poor adherence to oral antipsychotics. Paliperidone palmitate once a month formulation offers stable pharmacokinetics and reliable drug delivery due to its ER formulation which addresses significant adherence issues often encountered with oral medications. By maintaining consistent drug levels over time, it effectively prevents relapses of SZ or SA potentially leading to reduced hospitalizations and fewer re-admissions.
This study demonstrates comparable pharmacokinetic and safety profiles between paliperidone palmitate ER injectable suspension (administered once a month) from Sun Pharmaceutical Industries Limited, India. with that of Invega Sustenna® paliperidone palmitate ER injectable suspension 156 mg (administered once a month) of Janssen Pharmaceuticals, Inc.
These findings support the interchangeability of these products providing additional option for individualized treatment approaches in patients with SZ.
Acknowledgement
The authors thank all the subjects who participated in the study. The authors thank Amaresh Chakra for providing assistance for statistical analysis. The authors would also like to thank NeoCrest Life Sciences Consulting Private Limited for providing medical writing assistance for this manuscript.
Ethical Committee Consent
The site Institutional Ethics Committee (IEC). All subjects signed the informed consent form prior to participation in the study. The study was registered on Clinical Trials Registry India (CTRI) (Registration No: CTRI/2021/06/033960).
Conflict of Interest
Authors are employees of Sun Pharma that sponsored the study.
Financial Support
The study was sponsored by Sun Pharmaceutical Industries Limited, Mumbai, India
References
- Solmi M, Seitidis G, Mavridis D et al. Incidence, prevalence and global burden of schizophrenia-data, with critical appraisal, from the Global Burden of Disease (GBD) 2019. Mol Psychiatry 28(12), 5319-5327 (2023).
- Granger KT, Sand M, Caswell S, et al. A new era for schizophrenia drug development-lessons for the future. Drug Discov Today 28(7), 103603 (2023).
- Mandal PK, Gaur S, Roy RG, et al. Schizophrenia, bipolar and major depressive disorders: Overview of clinical features, neurotransmitter alterations, pharmacological interventions and impact of oxidative stress in the disease process. ACS Chem Neurosci 13(19), 2784-2802 (2022).
- GBD Mental disorders collaborators Global, regional and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. The Lancet Psychiatry 9(2), 137-150 (2022).
- Bondre AP, Shrivastava R, Raghuram H, et al. A qualitative exploration of perceived needs and barriers of individuals with schizophrenia, caregivers and clinicians in using mental health applications in Madhya Pradesh, India. SSM Ment Health 2:100063 (2022).
- Souto DR, Espinosa JP, Vieta E, et al. Clozapine in patients with schizoaffective disorder: A systematic review. Rev Psiquiatr Salud Ment (Engl Ed) 14(3), 148-156 (2021).
- Tom Joshua P. AS. Schizoaffective Disorder. Statpearls Publishing (2023)
- Chokhawala K, Stevens L. Antipsychotic medications. StatPearls (2023).
- Lintunen J, Taipale H, Tanskanen A, et al. Long-term real-world effectiveness of pharmacotherapies for schizoaffective disorder. Schizophr Bull 47(4), 1099-1107 (2021).
- De Filippis R, De Fazio P, Gaetano R, et al. Current and emerging long-acting antipsychotics for the treatment of schizophrenia. Expert Opin Drug Saf 20(7), 771-790 (2021).
- Rosso G, Pessina E, Martini A, et al. Paliperidone palmitate and metabolic syndrome in patients with schizophrenia: A 12-month observational prospective cohort study. J Clin Psychopharmacol 36(3), 206-212 (2016).
- Martin A, Bessonova L, Hughes R, et al. Systematic review of real-world treatment patterns of oral antipsychotics and associated economic burden in patients with schizophrenia in the United States. Adv Ther 39(9), 3933-3956 (2022).
- Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: A systematic review and comparative meta-analysis of randomised, cohort and pre–post studies. Lancet Psychiatry 8(5), 387-404 (2021).
- Lafeuille MH, Dean J, Carter V, et al. Systematic review of long-acting injectables versus oral atypical antipsychotics on hospitalization in schizophrenia. Curr Med Res Opin 30(8), 1643-1655 (2014).
- Zolezzi M, Abouelhassan R, Eltorki Y, et al. Long-acting injectable antipsychotics: A systematic review of their non-systemic adverse effect profile. Neuropsychiatr Dis Treat 1917-1926 (2021).
- Ma N, Zhang L, Zhang W, et al. Long-acting injectable antipsychotic treatment for schizophrenia in Asian population: A scoping review. Neuropsychiatr Dis Treat 31,1987-2006 (2023).
- Lin CH, Chen FC, Chan HY, et al. A comparison of long-acting injectable antipsychotics with oral antipsychotics on time to rehospitalization within 1 year of discharge in elderly patients with schizophrenia. Am J Geriatr Psychiatry 28(1), 23-30 (2020).
- Valsecchi P, Barlati S, Garozzo A, et al. Paliperidone palmitate in short-and long-term treatment of schizophrenia. Riv Psichiatr 54(6), 235-248 (2019).
- Kramer M, Litman R, Hough D, et al. Paliperidone palmitate, a potential long-acting treatment for patients with schizophrenia results of a randomized, double-blind, placebo-controlled efficacy and safety study. Int J Neuropsychopharmacol 13(5), 635-647 (2010).
- Dlugosz H, Nasrallah HA. Paliperidone: A new extended-release oral atypical antipsychotic. Expert Opin Pharmacother 8(14), 2307-2313 (2007).
[Crossref][Google Scholar][Pubmed]
- Mathews M, Gopal S, Nuamah I, et al. Clinical relevance of paliperidone palmitate 3-monthly in treating schizophrenia. Neuropsychiatr Dis Treat 1365-1379 (2019).
- Janssen Pharmaceuticals Invega Sustenna (paliperidone palmitate) extended release injectable suspension. Prescribing Info (2012).
- Wang SM, Han C, Lee SJ, et al. Paliperidone: A review of clinical trial data and clinical implications. Clin Drug Investig 32, 497-512 (2012).
- Chue P, Chue J. A critical appraisal of paliperidone long-acting injection in the treatment of schizoaffective disorder. Ther Clin Risk Manag 27, 109-116 (2016).
- Shah M, Parikh D, Karia S. Depot preparation in schizophrenia: Indian outlook. Ann Ind Psych 2(2), 152-157 (2018).
- Chen CY, Tang TC, Chen TT, et al. Efficacy, tolerability and safety of oral paliperidone extended release in the treatment of schizophrenia: A 24-week, open-label, prospective switch study in different settings in Taiwan. Neuropsychiatr Dis Treat 8, 725-732 (2018).
- Emsley R, Berwaerts J, Eerdekens M, et al. Efficacy and safety of oral paliperidone extended-release tablets in the treatment of acute schizophrenia: Pooled data from three 52-week open-label studies. Int Clin Psychopharmacol 23(6),343-356 (2008).
- Najarian D, Sanga P, Wang S, et al. A randomized, double-blind, multicenter, noninferiority study comparing paliperidone palmitate 6-month versus the 3-month long-acting injectable in patients with schizophrenia. Int J Neuropsychopharmacol 25(3),238-251(2022).
- Kim S, Koh M, Choi G, et al. Effects of long-acting injectable paliperidone palmitate on clinical and functional outcomes in patients with schizophrenia based on illness duration. J Clin Psychiatry 82(1),5842 (2021).
- Fernández-Miranda JJ, Díaz-Fernández S, De Berardis D, et al. Paliperidone palmitate every three months (PP3M) 2-year treatment compliance, effectiveness and satisfaction compared with Paliperidone Palmitate-Once Monthly (PP1M) in people with severe schizophrenia. J Clin Med 10(7), 1408 (2021).
- Meltzer HY, Bobo WV, Nuamah IF, et al. Efficacy and tolerability of oral paliperidone extended-release tablets in the treatment of acute schizophrenia: Pooled data from three 6-week, placebo-controlled studies. J Clin Psychiatry 69(5), 817-829 (2008).
- Kaplan G, Casoy J, Zummo J. Impact of long-acting injectable antipsychotics on medication adherence and clinical, functional and economic outcomes of schizophrenia. Patient Prefer Adherence 1171-1180 (2013).
- Kane JM, Schooler NR, Marcy P, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: A randomized clinical trial. JAMA psychiatry 77(12), 1217-12124 (2020).
- Lian L, Kim DD, Procyshyn RM, et al. Efficacy of longâÂÂacting injectable versus oral antipsychotic drugs in early psychosis: A systematic review and metaâÂÂanalysis. Early Interv Psychiatry 16(6):589-599(2022).
- Dickson MC, Nguyen MM, Patel C, et al. Adherence, persistence, readmissions and costs in medicaid members with schizophrenia or schizoaffective disorder initiating paliperidone palmitate versus switching oral antipsychotics: A real-world retrospective investigation. Adv Ther 40(1), 349-366 (2023).
- Girardi P, Del Casale A, Rapinesi C, et al. Predictive factors of overall functioning improvement in patients with chronic schizophrenia and schizoaffective disorder treated with paliperidone palmitate and aripiprazole monohydrate. Hum Psychopharmacol 33(3), e2658 (2018).
- Greene M, Yan T, Chang E, et al. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ 21(2), 127-134 (2018).
- Schreiner A, Aadamsoo K, Altamura AC, et al. Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr Res169(3), 393-399 (2015) .
- Mahabaleshwarkar R, Lin D, Fishman J, et al. The impact of once-monthly paliperidone palmitate on healthcare utilization among patients with schizophrenia treated in an integrated healthcare system: A retrospective mirror-image study. Adv Ther 38, 1958-1974 (2021).
- Blackwood C, Sanga P, Nuamah I, et al. Patients’ preference for long-acting injectable versus oral antipsychotics in schizophrenia: Results from the patient-reported medication preference questionnaire. Patient Prefer Adherence 1093-1102 (2020).
- Darville N, Van Heerden M, Mariën D, et al. The effect of macrophage and angiogenesis inhibition on the drug release and absorption from an intramuscular sustained-release paliperidone palmitate suspension. J Control Release 230, 95-108 (2016).
- Milz R, Benson C, Knight K, et al. The effect of longer dosing intervals for long-acting injectable antipsychotics on outcomes in schizophrenia. Neuropsychiatr Dis Treat 31, 531-545 (2023).
- Stone JM, Roux S, Taylor D, et al. First-generation versus second-generation long-acting injectable antipsychotic drugs and time to relapse. Ther Adv Psychopharmacol 8(12), 333-336 (2018).
- NHS. Long-Acting Injectable (LAIs) antipsychotics: Guidance for prescribing, administration and medicines management (2023).
- Nasrallah HA, Gopal S, Gassmann-Mayer C, et al. A controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic in schizophrenia. Neuropsychopharmacology 35(10), 2072-2082 (2010).
- Alphs L, Benson C, Cheshire-Kinney K, et al. Real-world outcomes of paliperidone palmitate compared to daily oral antipsychotic therapy in schizophrenia: A randomized, open-label, review board-blinded 15-month study. J Clin Psychiatry 76(5), 16960 (2015).
- Pilon D, Tandon N, Lafeuille MH, et al. Treatment patterns, health care resource utilization and spending in Medicaid beneficiaries initiating second-generation long-acting injectable agents versus oral atypical antipsychotics. Clin Ther 39(10), 1972-1985 (2017).
- Kim HO, Seo GH, Lee BC. Real-world effectiveness of long-acting injections for reducing recurrent hospitalizations in patients with schizophrenia. Ann Gen Psychiatry 19, 1-7 (2020).
- Ohnishi T, Kobayashi H, Yamaoka T, et al. The effects of paliperidone palmitate 1 month on the employment status and social functioning of patients with schizophrenia. Innov Clin Neurosci 17(1-3), 36-44 (2020).
- Devrimci-Ozguven H, Atmaca M, Baran Z, et al. Efficacy and safety of paliperidone palmitate treatment in patients with schizophrenia: A real-world multicenter, retrospective, mirror-image study. J Clin Psychopharmacol 39(6), 604-610 (2019).
- Gu Y, Peng H, Dai J, et al. Evaluation of paliperidone on social function in patients with chronic schizophrenia. Gen Psychiatr 31(2), e000011 (2018).
- Fu DJ, Turkoz I, Simonson RB, et al. Paliperidone palmitate once-monthly reduces risk of relapse of psychotic, depressive and manic symptoms and maintains functioning in a double-blind, randomized study of schizoaffective disorder. J Clin Psychiatry 76(3), 13729 (2014).
- Zhang H, Turkoz I, Zhuo J, et al. Paliperidone palmitate improves and maintains functioning in Asia–Pacific patients with schizophrenia. Adv Ther 34, 2503-2517 (2017).
- Gopal S, Gogate J, Pungor K, et al. Improvement of negative symptoms in schizophrenia with paliperidone palmitate 1-month and 3-month long-acting injectables: Results from a phase 3 non-inferiority study. Neuropsychiatr Dis Treat 6, 681-690 (2020).
- Shah S, Joshi D. Tolerability and efficacy of paliperidone extended release compared to olanzapine in the treatment of schizophrenia: A randomized, double–blind, multicentric trial. Ind Psychiatry J 20(1), 25-31 (2011).
- Si T, Li N, Lu H, et al. Impact of paliperidone palmitate one-month formulation on relapse prevention in patients with schizophrenia: A post-hoc analysis of a one-year, open-label study stratified by medication adherence. J Psychopharmacol 32(6), 691-701 (2018).
- Cleton A, Rossenu S, Crauwels H, et al. A singleâÂÂdose, openâÂÂlabel, parallel, randomized, doseâÂÂproportionality study of paliperidone after intramuscular injections of paliperidone palmitate in the deltoid or gluteal muscle in patients with schizophrenia. J Clin Pharmacol 54(9), 1048-1057 (2014).
- Toja-Camba FJ, Gesto-Antelo N, Maronas O, et al. Review of pharmacokinetics and pharmacogenetics in atypical long-acting injectable antipsychotics. Pharmaceutics 13(7), 935 (2021).
- Chue P, Chue J. A review of paliperidone palmitate. Expert Rev Neurother 12(12), 1383-1397 (2012).
- Helland A, Spigset O. Serum concentrations of paliperidone after administration of the long-acting injectable formulation. Ther Drug Monit 39(6), 659-662 (2017).
- Vermeir M, Naessens I, Remmerie B, et al. Absorption, metabolism and excretion of paliperidone, a new monoaminergic antagonist in humans. Drug Metab Dispos 36(4), 769-779 (2008).
- Shimizu H, Neyens M, De Meulder M, et al. Population pharmacokinetics of paliperidone palmitate (onceâÂÂmonthly formulation) in Japanese, Korean and Taiwanese patients with schizophrenia. Clin Pharmacol Drug Dev 9(2), 224-234. (2020)
- Rossenu S, Cleton A, Hough D, et al. Pharmacokinetic profile after multiple deltoid or gluteal intramuscular injections of paliperidone palmitate in patients with schizophrenia. Clin Pharmacol Drug Dev 4(4), 270-278 (2015).
- Reyntigens AJ, Heykants JJ, Woestenborghs RJ, et al. Pharmacokinetics of haloperidol decanoate a 2-year follow-up. Int Pharmacopsychiatry 17(4):238-246 (1982).
[Crossref][Google Scholar][Pubmed]
- Jørgensen A, Andersen J, Bjørndal N, et al. Serum concentrations of cis (Z)-flupentixol and prolactin in chronic schizophrenic patients treated with flupentixol and cis (Z)-flupentixol decanoate. Psychopharmacology 77, 58-65 (1982).
- Du P, Li P, Liu H, et al. OpenâÂÂlabel, randomized, singleâÂÂdose, 2âÂÂperiod, 2âÂÂsequence crossover, comparative pharmacokinetic study to evaluate bioequivalence of 2 oral formulations of olanzapine under fasting and fed conditions. Clin Pharmacol Drug Dev 9(5), 621-628 (2020).
- Alvarez-Herrera S, Escamilla R, Medina-Contreras O, et al. Immunoendocrine peripheral effects induced by atypical antipsychotics. Front Endocrinol (Lausanne) 11,195 (2020).
- Pandina GJ, Lindenmayer JP, Lull J, et al. A randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. J Clin Psychopharmacol 30(3), 235-244 (2010).