Research Article - (2019) Volume 9, Issue 2
Chronic Kidney Disease Diminishing Mini-Mental State Examination Score in Older Patients with Type 2 Diabetes and Low Normal Ankle-Brachial Index
- *Corresponding Author:
- I-Te Lee
No. 1650 Taiwan Boulevard, Sect. 4, Taichung 40705, Taiwan
Tel: +886-4-23741300
Fax: +886-4-23741318
Abstract
Objective
Dementia is an aging-related process and highly prevalent in patients with type 2 diabetes.
Mini-mental state examination (MMSE) is a useful tool to screen dementia. The present study
assesses whether a low MMSE score is associated with micro- and macro-vascular diseases in
older patients with type 2 diabetes.
Methods
Among patients aged ≥ 65 years with type 2 diabetes, subjects were enrolled if they had
undergone assessments of MMSE score and ankle-brachial index (ABI) during the annual
evaluation of pay-for-performance program. Peripheral artery disease (PAD) was defined as
ABI ≤ 0.90, low normal ABI was defined as a value>0.90 and<1.10, and normal ABI was defined
as a value between 1.10 and 1.40. Chronic kidney disease (CKD) was defined as estimated
glomerular filtration rate (eGFR)<60 mL/min/1.73 m2.
Results
MMSE score was significantly lower in patients with PAD than in those without PAD (23.4 ±
5.2 vs. 25.9 ± 3.7; P<0.001). In addition to PAD, both CKD and the low normal ABI value were
associated with a low MMSE score (24.9 ± 4.2 in CKD vs. 26.4 ± 3.3 in non-CKD, P<0.001; 25.5
± 3.9 in low normal ABI vs. 26.2 ± 3.5 in normal ABI, P=0.021; respectively). On multivariate
regression analysis, the lowest MMSE score was observed in patients with PAD (95%
confidence interval: -3.401 to -1.182, P<0.001) among all patients, and the MMSE score was
also significantly lower in those with CKD and low normal ABI than those with normal ABI and
without CKD (95% confidence interval: -1.778 to -0.051, P=0.038).
Conclusion
PAD is an independent factor for low MMSE scores in older patients with type 2 diabetes. In
those without PAD, CKD and low normal ABI exert synergistic effects on the low MMSE score.
Keywords
Ankle-brachial index, Chronic kidney disease, Cognitive impairment, Mini-mental state examination, Older, Peripheral artery disease, Type 2 Diabetes
Introduction
Dementia is an age-related process, and remains a heavy burden on public healthcare [1-3]. In a nationwide population-based survey in Taiwan, where individuals aged ≥ 65 years were interviewed, the prevalence rates of dementia and mild cognitive impairment were 8.04% and 18.76%, respectively [4]. However, the National Health Insurance database of individuals aged ≥ 65 years in Taiwan indicated that the rate of dementia treatment was relatively low [5].
Dementia has exhibited a strong association with type 2 Diabetes [6]. In the Rotterdam Study, type 2 diabetes predicted 1.9-fold risk of dementia in compared to those without diabetes during the 2.1 years of follow up [7]. Cheng et al. [8] reported that diabetes had a 1.5-fold risk of all-cause dementia in a meta-analysis study. Based on the data from the National Health and Nutrition Examination Surveys (NHANES), the prevalence of type 2 diabetes has rapidly increased, particularly from 18.4% in 1999/2000 to 24.6% in 2013/2014 among those aged ≥ 65 years [9]. In the Taiwan population aged ≥ 65 years, partly due to the reduction in the mortality rate in patients with diabetes [10], the prevalence of diabetes has increased from 16.41% in 1993/1996 to 25.89% in 2005/2008 [11]. Thus, dementia will become an important problem as the older population with diabetes increases.
Cognitive decline can be considered as a chronic complication of diabetes in the central nervous system, and may also be associated with other micro- or macro-vascular diseases [12]. The association between chronic kidney disease (CKD) and cognitive impairment is evident [13], and longitudinal cognitive function decline has been found to be correlated with the estimated glomerular filtration rate (eGFR) in the older population without dementia or stroke [14].
Peripheral artery disease (PAD), defined as anklebrachial index (ABI)<0.9, has been reported to be associated with cognitive impairment, determined by a mini-mental state examination (MMSE) score<24, in a diabetes subgroup of Chinese patients without stroke [15]. Compared with the brachial-ankle pulse wave velocity (baPWV), ABI exhibited a better association with MMSE score [16]. Furthermore, low normal ABI, with a value>0.90 and<1.10, has been found to be related to a higher cardiovascular mortality risk as compared to ABI≥1.10 [17]. Moreover, Foster et al. [18]. reported that low normal ABI was associated with a rapid eGFR decline, as compared to normal ABI with value between 1.10 and 1.40. Therefore, the present study aimed to assess whether a low MMSE score is associated with PAD in older Chinese patients with type 2 diabetes, and we also assessed whether CKD exerted a synergistic effect on a low MMSE score in the older Chinese patients with type 2 diabetes and low normal ABI.
Materials and Methods
▪ Study design and subjects
This cross-sectional study was conducted at Taichung Veterans General Hospital, Taiwan. The diabetes pay-for-performance (P4P) program is a landmark national health policy in Taiwan. Through incentive payments, comprehensive care and completed assessments for diabetes are administered. Laboratory assessments, including fasting glucose, glycated hemoglobin (HbA1c), liver enzyme, serum creatinine, and urinary creatinine-to-albumin ratio (UACR) measurements are required to be completed during the annual evaluation of the diabetes P4P program [19]. In our clinical practice, the MMSE is also administered during the annual evaluation if the patient is aged ≥ 65 years. ABI examination is indicated in this older population with diabetes based on the Practice Guidelines for PAD from the American College of Cardiology Foundation/American Heart Association Task Force [20], and was automatically recommended by the hospital information system during the annual evaluation if no record of ABI was available in their electrical medical record.
The study included type 2 diabetes patients who had enrolled in the diabetes P4P program and completed MMSE assessments during the annual evaluation between August 01, 2016 and July 31, 2017. The exclusion criteria were as follows (1) ABI assessment was not completed within three months after the MMSE assessment, (2) laboratory biochemical assessments during the annual P4P program evaluation were not completed within three months after the MMSE assessment, (3) a history of dementia or stroke, (4) a history of lower-extremity surgery, (5) the presence of end-stage renal disease, and (6) an evidence of non-compressible vessels as indicated by ABI values>1.40 in both the lower limbs. Data collection was performed by reviewing the electronic medical records. This study complied with the tenets of the Declaration of Helsinki, and the research protocol was approved by the Institutional Review Board of Taichung Veterans General Hospital.
▪ Biochemistry assessments
Biochemical data including fasting glucose, HbA1c, fasting lipoprotein, alanine aminotransferase, serum creatinine, and UACR were collected. Glucose levels were measured using an oxidase‒peroxidase method (Wako Diagnostics, Tokyo, Japan). HbA1c was measured using the cation-exchange HPLC (NGSP certificated; G8, TOSOH, Tokyo, Japan). Concentrations of lipoprotein, alanine aminotransferase, and creatinine were measured using commercial kits (Beckman Coulter, Fullerton, USA). Albumin concentrations in spot urine samples were assessed using the immune-turbidimetric method (Advia 1800, Siemens, New York, USA).
The eGFR was calculated as 186 × [serum creatinine (mg/dL)]-1.154 × [age (years)]-0.203 (× 0.742, if female), and CKD was defined as an eGFR<60 mL/min/1.73 m2 based on the Modification of Diet in Renal Disease (MDRD) equation [21]. UACR was calculated using the following formula: albumin (mg)/creatinine (g), and increased urinary albumin excretion was defined as UACR ≥ 30 mg/g [22].
▪ Ankle-brachial profiles
ABI values were measured using a validated automatic device (VP-1000 Plus; Omron healthcare Co. Ltd., Kyoto, Japan). Patients were examined with cuffs on both arms and ankles after rest in the supine position for at least 5 min. The cuffs were connected to a plethysmographic sensor and an oscillometric pressure sensor to measure blood pressure [23]. The baPWV values were calculated as the ratio of path distance of brachial – ankle to pulse transmission time of brachial – ankle. The lower ABI value and higher baPWV value between the lower limbs of the same patient were recorded for the analyses [24]. PAD is defined as ABI value ≤ 0.90 [23]. Normal ABI is defined as ABI value ≥ 1.10, and low normal ABI is defined as ABI value<1.10 in the patients without PAD [17,18].
We assessed the reproducibility of the ankle-brachial profiles in a group of 30 subjects. A high linear correlationwas observed in the ABI (r=0.836, P<0.001) and in the baPWV (r=0.941, P<0.001) between the first and repeated measurements; the 95% confidence interval (95%CI) for bias ranged from -0.013 to 0.022 for ABI and from -17 to 68 for baPWV between repeated measurements.
▪MMSE
The MMSE is a convenient tool for cognitive assessment and screening of dementia, with a total score ranging from 0 to 30. The MMSE consists of orientation (5 points for time and 5 points for place), short-term memory (3 points for registry and 3 points for recall), calculating with attention (5 points), language (5 points), comprehension/executive function (3 points), and copying interlocking pentagons (1 point). A lower score indicates worse cognitive function [25]. The Chinese version of MMSE is available from the Taiwan Dementia Society. The MMSE was administered by a trained study assistant in the outpatient of Endocrinology and Metabolism at Taichung Veterans General Hospital.
▪ Statistical analysis
All the continuous data are presented as the mean ± standard deviation, and categorical data are presented as numbers (percentages). Statistical analyses were conducted using the independent sample t-test to detect statistical differences in the continuous variables between two groups. One-way analysis of variance was conducted to compare the differences in continuous variables among more than two groups, whereas chisquare tests were used to detect the differences in the categorical variables. Multivariate linear regression analysis was used to analyze the factors associated with the MMSE score. Statistical analysis was performed using SPSS version 22.0 software (IBM Corp., Armonk, NY, USA).
Results
A total of 621 subjects met the study criteria for analyses. The mean age was 75 ± 7 years, and 301 (48.5%) of them were male. Table 1 shows the clinical characteristics of the subjects with PAD (ABI ≤ 0.90) and without PAD (ABI>0.90 and ≤ 1.40). Patients with PAD were significantly older than those without PAD (77 ± 8 vs. 74 ± 7 years, P=0.005). Higher cardiovascular risk factors, including higher systolic brachial blood pressure (148 ± 24 vs. 139 ± 19 mmHg, P=0.007), higher HbA1c value (7.7 ± 1.6 vs. 7.3 ± 1.3%, P=0.037), lower HDL cholesterol level (1.3 ± 0.4 vs. 1.4 ± 0.4 mmol/L, P=0.024), lower eGFR (57 ± 30 vs. 71 ± 24 mL/min/1.73 m2, P<0.001), and higher UACR (568 ± 1169 vs. 202 ± 609 mg/g, P<0.001) were noted in patients with PAD than in those without PAD. The proportion of patients using insulin therapy was significantly higher in the patients with PAD than in those without PAD (48.8% vs. 24.9%, P<0.001). The MMSE score was significantly lower in the patients with PAD than in those without PAD (23.4 ± 5.2 vs. 25.9 ± 3.7, P<0.001).
| With PAD†(n=43) | Without PAD(n=578) | P | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (year) | 77 ± 8 | 74 ± 7 | 0.005 |
| Male, n (%) | 23(53.5%) | 278(48.1%) | 0.530 |
| Current smoker, n (%) | 4(9.3%) | 46(8.0%) | 0.769 |
| Education, n (%) | 0.679 | ||
| <6 years | 13(30.2%) | 143(24.7%) | |
| 6‒9 years | 18(41.9%) | 245(42.4%) | |
| >9 years | 12(27.9%) | 190(32.9%) | |
| Hypoglycemia in the last 1 year, n (%) | 7 (16.3%) | 40 (6.9%) | 0.052 |
| CAD history, n (%) | 9(20.9%) | 52(9.0%) | 0.028 |
| Anthropometric data | |||
| BMI (kg/m2) | 25.6 ± 3.8 | 25.2 ± 3.7 | 0.434 |
| Systolic BP (mmHg) | 148 ± 24 | 139 ± 19 | 0.007 |
| Diastolic BP (mmHg) | 75 ± 12 | 76 ± 11 | 0.621 |
| Laboratory data | |||
| Fasting glucose (mmol/L) | 7.9 ± 2.1 | 7.9 ± 2.3 | 0.897 |
| HbA1c (%) | 7.7 ± 1.6 | 7.3 ± 1.3 | 0.037 |
| Total cholesterol (mmol/L) | 3.9 ± 0.7 | 4.0 ± 0.7 | 0.338 |
| HDL cholesterol (mmol/L) | 1.3 ± 0.4 | 1.4 ±0.4 | 0.024 |
| Triglyceride (mmol/L) | 1.6 ± 0.9 | 1.3 ± 0.9 | 0.057 |
| ALT | 22 ± 28 | 24 ±16 | 0.401 |
| eGFR (mL/min/1.73 m2) | 57 ± 30 | 71 ± 24 | <0.001 |
| UACR (mg/g)‡ | 568 ± 1169 | 202 ± 609 | <0.001 |
| Ankle-brachial profiles | |||
| ABI | 0.78 ± 0.10 | 1.11 ± 0.08 | <0.001 |
| baPWV (cm/sec) | 2341 ± 1335 | 2046 ±439 | <0.001 |
| MMSE Score | 23.4 ± 5.2 | 25.9 ± 3.7 | <0.001 |
| Current medications | |||
| Statins, n (%) | 33(76.7%) | 435(75.3%) | 0.999 |
| Antihypertensive agents, n (%) | |||
| ACE inhibitor or ARB | 23(53.5%) | 274(47.4%) | 0.527 |
| α-Blocker | 8(18.6%) | 50(8.7%) | 0.050 |
| β-Blocker | 10 (23.3%) |
127 (22.0%) |
0.849 |
| Calcium channel blocker | 4(9.3%) | 27(4.7%) | 0.262 |
| Diuretics | 1(2.3%) | 13(2.2%) | 0.999 |
| Antidiabetic drugs, n (%) | |||
| Insulin therapy | 21(48.8%) | 144(24.9%) | <0.001 |
| Insulin secretagogues | 17(39.5%) | 277(47.9%) | 0.343 |
| Metformin | 10(23.3%) | 175(30.3%) | 0.390 |
| DPP4 inhibitors | 26(60.5%) | 375(64.9%) | 0.621 |
| SGLT2 inhibitors | 0(0.0%) | 37(6.4%) | 0.100 |
| Thiazolidinediones | 7(16.3%) | 136(23.5%) | 0.349 |
| α-Glucosidase inhibitor | 5(11.6%) | 74(12.8%) | 0.999 |
| †An ABI ≤ 0.90 indicates PAD. ‡UACR was logarithm-transformed (log) in analyses due to skewed distributions ABB: ABI: Ankle-Brachial Index; ACE: Angiotensin-Converting Enzyme; ALT: Alanine Aminotransferase; ARB: Angiotensin II Receptor Antagonists; BMI: Body Mass Index; BP: Blood Pressure; CAD: Coronary Artery Disease; DPP4: Dipeptidyl Peptidase-4; eGFR: Estimated Glomerular Filtration Rate; HbA1C: Glycated Hemoglobin; HDL: High-Density Lipoprotein; MMSE: Mini-Mental State Examination; PAD: peripheral artery disease; baPWV: Brachial-Ankle Pulse Wave Velocity; SGLT2: Sodium-Glucose Co-Transporter 2; UACR: Urinary Albumin-To-Creatinine Ratio |
|||
Table 1: Clinical characteristics of the subjects with and without peripheral artery disease (PAD).
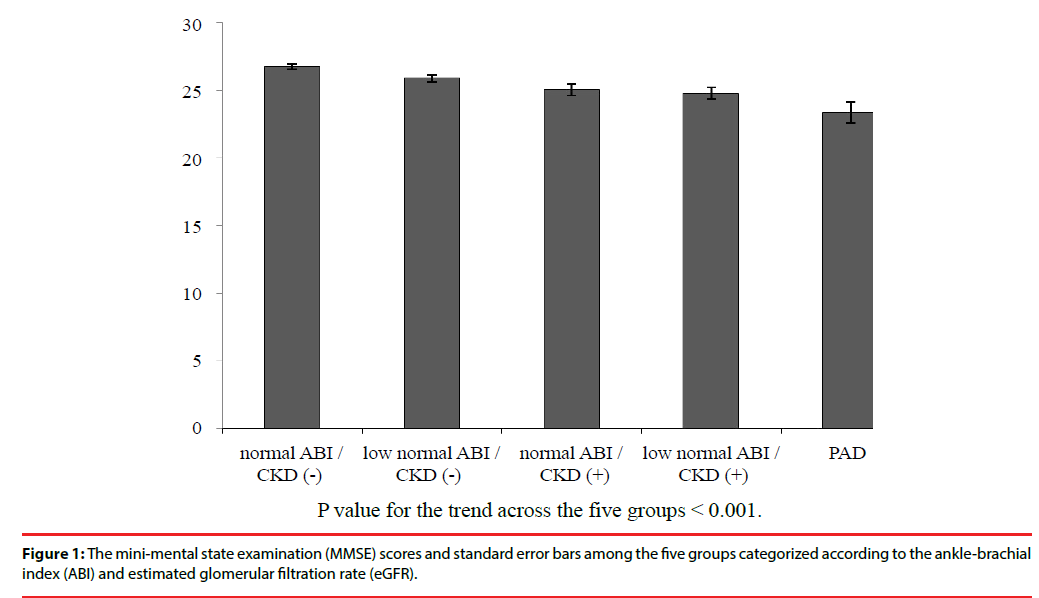
In addition to patients with PAD, the patients without PAD were further categorized into four groups based on the presence of CKD and their ABI values, including the low normal ABI/CKD (+), normal ABI/CKD (+), low normal ABI/ CKD (‒), and normal ABI/CKD (‒) groups. The mean MMSE score was significantly different among these five groups with a positive trend towards higher ABI and higher eGFR (Figure 1). Furthermore, there were significant differences in age, gender, body mass index (BMI), brachial systolic blood pressure, HDL cholesterol level, eGFR, UACR, ABI value, baPWV, and use of medications including α-blocker, insulin therapy, metformin, SGLT2 inhibitor, and ACE inhibitor or ARB among these five groups (Table 2).
| PAD (n=43) | Low normal ABI/ CKD (+) (n=88) | Normal ABI/ CKD (+)(n=94) | Low normal ABI/ CKD (‒)(n=183) | Normal ABI/CKD (‒) (n=213) | P | |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age (year) | 77 ± 8 | 77 ± 7 | 77 ± 7 | 74 ± 7 | 73 ± 6 | <0.001 |
| Male, n (%) | 23 (53.5%) | 40 (45.5%) | 59 (62.8%) | 76 (41.5%) | 103(48.4%) | 0.018 |
| Current smoker, n (%) | 4 (9.3%) | 4 (4.5%) | 8 (8.5%) | 11 (6.0%) | 23 (10.8%) | 0.311 |
| Education, n (%) | 0.189 | |||||
| <6 years | 13 (30.2%) | 30 (34.1%) | 26 (27.7%) | 42 (23.0%) | 45 (21.1%) | |
| 6‒9 years | 18 (41.9%) | 35 (39.8%) | 44 (46.8%) | 80 (43.7%) | 86 (40.4%) | |
| >9 years | 12 (27.9%) | 23 (26.1%) | 24 (25.5%) | 61 (33.3%) | 82 (38.5%) | |
| Hypoglycemia in the last one year, n (%) | 7 (16.3%) | 8 (9.1%) | 8 (8.5%) | 12 (6.6%) | 12 (5.6%) | 0.166 |
| CAD history, n (%) | 9 (20.9%) | 10 (11.4%) | 9 (9.6%) | 14 (7.7%) | 19 (8.9%) | 0.116 |
| Anthropometric data | ||||||
| BMI (kg/m2) | 25.6 ± 3.8 | 26.3 ± 3.6 | 26.0 ± 4.3 | 25.0 ± 3.5 | 24.5 ± 3.4 | <0.001 |
| Systolic BP (mmHg) | 148 ± 24 | 145 ± 19 | 142 ± 20 | 140 ± 20 | 136 ± 17 | <0.001 |
| Diastolic BP (mmHg) | 75 ± 12 | 77 ± 11 | 75 ± 12 | 76 ± 11 | 75 ± 11 | 0.477 |
| Laboratory data | ||||||
| Fasting glucose (mmol/L) | 7.9 ± 2.1 | 7.7 ± 2.6 | 7.7 ± 2.1 | 8.1 ± 2.3 | 7.9 ± 2.4 | 0.563 |
| HbA1c (%) | 7.7 ± 1.6 | 7.3 ± 1.3 | 7.3 ± 1.2 | 7.4 ± 1.3 | 7.3 ± 1.2 | 0.317 |
| Total cholesterol (mmol/L) | 3.9 (0.7) | 4.0 (0.8) | 3.8 (0.7) | 4.0 (0.7) | 4.0 (0.7) | 0.196 |
| HDL cholesterol (mmol/L) | 1.3 ± 0.4 | 1.4 ± 0.4 | 1.3 ± 0.3 | 1.4 ± 0.4 | 1.4 ± 0.4 | 0.003 |
| Triglyceride (mmol/L) | 1.6 ± 0.9 | 1.5 ± 1.0 | 1.4 ± 0.7 | 1.3 ± 1.2 | 1.2 ± 0.7 | 0.107 |
| ALT (U/L) | 22 ± 28 | 24 ± 21 | 25 ± 20 | 23 ± 12 | 24 ± 16 | 0.870 |
| eGFR (mL/min/1.73 m2) | 57 ± 30 | 41 ± 12 | 45 ± 12 | 84 ± 16 | 85 ± 16 | <0.001 |
| UACR¶ | 568 ± 1169 | 514 ± 1075 | 420 ± 828 | 68 ± 178 | 93 ± 345 | <0.001 |
| Ankle-brachial profiles | ||||||
| ABI | 0.78 ± 0.10 | 1.03 ± 0.06 | 1.17 ± 0.05 | 1.04 ± 0.05 | 1.17 ± 0.04 | <0.001 |
| baPWV (cm/sec) | 2341 ± 1335 | 2156 ± 448 | 2131 ± 526 | 2023 ± 431 | 1981 ± 387 | <0.001 |
| MMSE Score | 23.4 ± 5.2 | 24.8 ± 4.2 | 25.1 ± 4.2 | 25.9 ± 3.6 | 26.8 ± 3.0 | <0.001 |
| Current medications | ||||||
| Statins, n(%) | 33 (76.7%) | 57 (64.8%) | 76 (80.9%) | 139 (76.0%) | 163 (76.5%) | 0.132 |
| Antihypertensive agents, n (%) | ||||||
| ACE inhibitor or ARB | 23 (53.5%) | 62 (70.5%) | 54 (57.4%) | 72 (39.3%) | 86 (40.4%) | <0.001 |
| α-Blocker | 8 (18.6%) | 10 (11.4%) | 22 (23.4%) | 8 (4.4%) | 10 (4.70%) | <0.001 |
| β-Blocker | 10 (23.3%) | 26 (29.5%) | 25 (26.6%) | 35 (19.1%) | 41 (19.2%) | 0.205 |
| Calcium channel blocker | 4 (9.3%) | 1 (1.1%) | 5 (5.3%) | 12 (6.6%) | 9 (4.2%) | 0.225 |
| Diuretics | 1 (2.3%) | 2 (2.3%) | 4 (4.3%) | 3 (1.6%) | 4 (1.9%) | 0.706 |
| Antidiabetic drugs, n (%) | ||||||
| Insulin therapy | 21 (48.8%) | 31 (35.2%) | 27 (28.7%) | 37 (20.2%) | 49 (23.0%) | <0.001 |
| Insulin secretagogues | 17 (39.5%) | 37 (42.0%) | 49 (52.1%) | 95 (51.9%) | 96 (45.1%) | 0.300 |
| Metformin | 10 (23.3%) | 12 (13.6%) | 22 (23.4%) | 69 (37.7%) | 72 (33.8%) | <0.001 |
| DPP4 inhibitors | 26 (60.5%) | 63 (71.6%) | 67 (71.3%) | 107 (58.5%) | 138 (64.8%) | 0.134 |
| SGLT2 inhibitors | 0 (0.0%) | 2 (2.30%) | 3 (3.20%) | 13 (7.10%) | 19 (8.90%) | 0.042 |
| Thiazolidinediones | 7 (16.3%) | 21 (23.9%) | 21 (22.3%) | 38 (20.8%) | 56 (26.3%) | 0.562 |
| α-Glucosidase inhibitor | 5 (11.6%) | 13 (14.8%) | 16 (17.0%) | 20 (10.9%) | 25 (11.7%) | 0.616 |
| †ABI ≤ 0.90 indicates PAD, ABI>0.90 and<1.10 indicates low normal ABI, and ABI ≥ 1.10 indicates normal ABI. ‡eGFR<60 mL/min/1.73 m2 indicates CKD (+) ¶UACR was logarithm-transformed (log) in analyses due to skewed distributions ABB: ABI: Ankle-Brachial Index; ACE: Angiotensin-Converting Enzyme; ALT: Alanine Aminotransferase; ARB: Angiotensin II Receptor Antagonists; BMI: Body Mass Index; BP : Blood Pressure; CAD: Coronary Artery Disease; DPP4: Dipeptidyl Peptidase-4; eGFR: Estimated Glomerular Filtration Rate; HbA1C: Glycated Hemoglobin; HDL: High-Density Lipoprotein; MMSE: Mini-Mental State Examination; baPWV: Brachial-Ankle Pulse Wave Velocity; SGLT2: Sodium-Glucose Co-Transporter 2; UACR: Urinary Albumin-to-Creatinine Ratio |
||||||
Table 2: Characteristics of subjects categorized according to ankle-brachial index (ABI)† and chronic kidney disease (CKD)‡.
Table 3 shows the MMSE scores between the different groups categorized based on each associated factor in the patients without PAD. Age was significantly associated with a low MMSE score (24.3 ± 4.2 in ≥ 75 years, 26.3 ± 2.9 in 70‒74 years, and 27.4 ± 2.8 in<70 years; P<0.001). Women had a lower MMSE score than men (25.4 ± 4.0 vs. 26.5 ± 3.2; P<0.001). Shorter education duration was significantly associated with a low MMSE score (23.3 ± 4.5 in<6 years, 26 ± 3 in 6‒9 years, and 27.8 ± 2.4 in>9 years; P<0.001). Patients using ACE inhibitor or ARB had a significantly lower MMSE score, as compared to those not receiving these medications (25.5 ± 4 vs. 26.3 ± 3.4; P=0.018). Patients with CKD had a low MMSE score than those without CKD (24.9 ± 4.2 vs. 26.4 ± 3.3; P<0.001). Patients with increased UACR had a low MMSE score than those with UACR<30 mg/g (25.3 ± 4.0 vs. 26.4 ± 3.4; P<0.001). Patients with low normal ABI had a low MMSE score than those with normal ABI (25.5 ± 3.9 vs. 26.2 ± 3.5; P<0.001), and patients with high PWV had a low MMSE score than those with low PWV (25.0 ± 4.2 vs. 26.5 ± 3.3; P<0.001).
| N | Mean ± SD | P | |
| Demographic characteristics | |||
| Age | |||
| <70 years | 203 | 27.4 ± 2.8 | <0.001 |
| 70‒74 years | 142 | 26.3 ± 2.9 | |
| ≥ 75 years | 233 | 24.3 ± 4.2 | |
| Gender | |||
| Female | 300 | 25.4 ± 4.0 | <0.001 |
| Male | 278 | 26.5 ± 3.2 | |
| Current smoker | |||
| No | 532 | 25.8 ± 3.7 | 0.112 |
| Yes | 46 | 26.7 ± 3.1 | |
| Education | |||
| <6 years | 143 | 23.3 ± 4.5 | <0.001 |
| 6‒9 years | 245 | 26.0 ± 3.0 | |
| >9 years | 190 | 27.8 ± 2.4 | |
| Hypoglycemia in the last one year | |||
| No | 538 | 25.9 ± 3.7 | 0.408 |
| Yes | 40 | 26.4 ± 3.8 | |
| CAD history | |||
| No | 526 | 26.0 ± 3.7 | 0.361 |
| Yes | 52 | 25.5 ± 3.9 | |
| Anthropometric data | |||
| BMI† | |||
| <24.7 kg/m2 | 290 | 26.1 ± 3.7 | 0.361 |
| ≥ 24.7 kg/m2 | 288 | 25.8 ± 3.7 | |
| Systolic BP† | |||
| <138.0 mmHg | 286 | 26.2 ± 3.3 | 0.119 |
| ≥ 138.0 mmHg | 292 | 25.7 ± 4.1 | |
| Diastolic BP† | |||
| <75.5 mmHg | 289 | 25.8 ± 3.5 | 0.438 |
| ≥ 75.5 mmHg | 289 | 26.0 ± 3.8 | |
| Laboratory data | |||
| Fasting glucose (mmol/L) | |||
| <7.3 mmol/L | 276 | 26.1 ± 3.8 | 0.364 |
| ≥ 7.3 mmol/L | 302 | 25.8 ± 3.6 | |
| HbA1c (%) | |||
| <7.0% | 270 | 26.0 ± 3.7 | 0.673 |
| ≥ 7.0% | 308 | 25.9 ±3.7 | |
| Total cholesterol | |||
| ≥ 4.1 mmol/L | 230 | 25.8 ± 3.8 | 0.269 |
| <4.1 mmol/L | 348 | 26.1 ± 3.6 | |
| Low HDL cholesterol‡ | |||
| No | 426 | 26.0 ± 3.6 | 0.192 |
| Yes | 152 | 25.6 ± 3.9 | |
| Triglycerides | |||
| <1.13 mmol/L | 285 | 26.2 ± 3.5 | 0.075 |
| ≥ 1.13 mmol/L | 293 | 25.6 ± 3.9 | |
| eGFR | |||
| ≥ 60 mL/min/1.73m2 | 396 | 26.4 ± 3.3 | <0.001 |
| <60 mL/min/1.73m2 | 182 | 24.9 ± 4.2 | |
| UACR | |||
| <30 mg/g | 317 | 26.4 ± 3.4 | <0.001 |
| ≥ 30 mg/g | 261 | 25.3 ± 4.0 | |
| Ankle-brachial profiles | |||
| ABI | |||
| 1.10‒1.40 | 307 | 26.2 ± 3.5 | 0.021 |
| >0.90 and<1.10 | 271 | 25.5 ± 3.9 | |
| baPWV† | |
||
| <1970 cm/sec | 289 | 26.5 ± 3.3 | <0.001 |
| ≥ 1970 cm/sec | 289 | 25.0 ± 4.2 | |
| Current medications | |||
| Statins | |||
| No | 143 | 25.8 ± 3.8 | 0.737 |
| Yes | 435 | 25.9 ± 3.7 | |
| ACE inhibitor or ARB | |||
| No | 304 | 26.3 ± 3.4 | 0.018 |
| Yes | 274 | 25.5 ± 4.0 | |
| Calcium channel blocker, n (%) | |||
| No | 551 | 25.9 ± 3.7 | 0.298 |
| Yes | 27 | 25.2 ± 4.5 | |
| α-Blocker, n (%) | |||
| No | 528 | 25.9 ± 3.7 | 0.981 |
| Yes | 50 | 25.9 ± 3.7 | |
| β-Blocker, n (%) | |||
| No | 451 | 26.0 ± 3.7 | 0.365 |
| Yes | 127 | 25.7 ± 3.7 | |
| Diuretics, n (%) | |||
| No | 607 | 25.7 ± 3.9 | 0.794 |
| Yes | 14 | 26.0 ± 4.1 | |
| Insulin therapy, n (%) | |||
| No | 434 | 26.2 ± 3.4 | 0.003 |
| Yes | 144 | 25.1 ± 4.3 | |
| Insulin secretagogues, n (%) | |||
| No | 301 | 26.0 ± 3.7 | 0.535 |
| Yes | 277 | 25.8 ± 3.7 | |
| Metformin, n (%) | |||
| No | 403 | 25.7 ± 3.9 | 0.066 |
| Yes | 175 | 26.3 ± 3.1 | |
| DPP4 inhibitors, n (%) | |||
| No | 203 | 25.9 ± 3.5 | 0.843 |
| Yes | 375 | 25.9 ± 3.8 | |
| Thiazolidinediones, n (%) | |||
| No | 442 | 25.8 ± 3.7 | 0.190 |
| Yes | 136 | 26.3 ± 3.7 | |
| α-Glucosidase inhibitor, n (%) | |||
| No | 504 | 25.9 ± 3.7 | 0.834 |
| Yes | 74 | 25.8 ± 3.6 | |
| †Cutoff based on the median value ‡Low HDL cholesterol was defined as serum HDL cholesterol<40 mg/dL (1.0 mmol/L) in men or<50 mg/dL (1.3 mmol/L) in women. ABB: ABI: Ankle-Brachial Index; ACE: Angiotensin-Converting Enzyme; ARB: Angiotensin II Receptor Antagonists; BMI: Body Mass Index; BP: Blood Pressure; CAD: Coronary Artery Disease; DPP4: Dipeptidyl Peptidase-4; eGFR: Estimated Glomerular Filtration Rate; HbA1C: Glycated Hemoglobin; HDL : High-Density Lipoprotein; MMSE: Mini-Mental State Examination; baPWV: Brachial-Ankle Pulse Wave Velocity; SGLT2: Sodium-Glucose Co-Transporter 2; UACR: Urinary Albumin-To-Creatinine Ratio |
|||
Table 3: MMSE scores in the subjects without peripheral artery disease (n=578).
In addition to PAD, Table 4 showed either low normal ABI (95%CI: -1.604 to -0.116, P=0.024) or CKD (95%CI: -2.595 to -0.768, P<0.001) was significantly associated with a low MMSE score, as compared with the normal ABI/ CKD (‒) group in a crude analysis. There were synergistic effects of lower normal ABI and CKD on the decrease in MMSE score. Multivariate linear regression analysis showed that low normal ABI accompanied with CKD were independent risk factors for low MMSE scores (95%CI: -1.778 to -0.051, P=0.038) after adjusting for HbA1c value, experience of hypoglycemia, and MMSE-associated factors selected from Table 3.
| Crude | Model 1 | Model 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | 95%CI | P value | B | 95%CI | P value | B | 95%CI | P value | ||
| Normal ABI / CKD (‒) | reference | 1.000 | 1.000 | |||||||
| low normal ABI / CKD (‒) | -0.860 | (-1.604,-0.116) | 0.024 | -0.529 | (-1.175,0.118) | 0.109 | -0.533 | (-1.179,0.114) | 0.106 | |
| normal ABI / CKD (+) | -1.681 | (-2.595,-0.768) | <0.001 | -0.808 | (-1.622,0.005) | 0.051 | -0.809 | (-1.638,0.019) | 0.056 | |
| low normal ABI / CKD (+) | -1.983 | (-2.918,-1.048) | <0.001 | -0.923 | (-1.752,-0.095) | 0.029 | -0.914 | (-1.778,-0.051) | 0.038 | |
| PAD | -3.384 | (-4.618,-2.150) | <0.001 | -2.434 | (-3.524,-1.345) | <0.001 | -2.292 | (-3.401,-1.182) | <0.001 | |
| Age (year) | -0.132 | (-0.171,-0.093) | <0.001 | -0.137 | (-0.178,-0.097) | <0.001 | ||||
| Male | 0.495 | (-0.060,1.050) | 0.081 | 0.499 | (-0.058, 1.057) | 0.079 | ||||
| Education | 1.885 | (1.511,2.258) | <0.001 | 1.855 | (1.477, 2.232) | <0.001 | ||||
| Hypoglycemia in the last one year | 0.435 | (-0.540,1.409) | 0.381 | 0.439 | (-0.547,1.425) | 0.382 | ||||
| Use of ACE inhibitor or ARB | -0.048 | (-0.582,0.487) | 0.861 | |||||||
| Use of Insulin therapy | -0.451 | (-1.093,0.190) | 0.168 | |||||||
| HbA1c (%) | -0.149 | (-0.366,0.068) | 0.177 | |||||||
| UACR>30 | 0.150 | (-0.432,0.732) | 0.613 | |||||||
| baPWV (m/sec) | 0.007 | (-0.043,0.056) | 0.797 | |||||||
| ABB: ABI: Ankle-Brachial Index; ACE: Angiotensin-Converting Enzyme; ARB: Angiotensin II Receptor Antagonists; CI: Lconfidence Interval; CKD: Chronic Kidney Disease, eGFR: Estimated Glomerular Filtration Rate; HbA1C: Glycated Hemoglobin; baPWV: Brachial-Ankle Pulse Wave Velocity; SGLT2: Sodium-Glucose Co-Transporter 2; UACR: Urinary Albumin-To-Creatinine Ratio | ||||||||||
Table 4: Results of regression analysis for the effects of risk factors on the mini-mental state examination score.
Discussion
The main finding of the present study is that PAD is significantly associated with low MMSE scores, and that low normal ABI and CKD have synergistic effects on low MMSE scores as compared to normal ABI without CKD in older patients with type 2 diabetes. These results suggest that a low MMSE score was associated with both micro- and macro-vascular diseases, as indicated by a positive trend in the MMSE score to eGFR and ABI.
Studies on CKD and cognitive impairment have yielded inconsistent results in patients with type 2 diabetes. In the Diabetes Heart Study MIND study, CKD was associated with cerebral white-matter lesions which were consistent with the poor performance of speed of processing and working memory scored via the digit symbol coding in African American patients [26]; however, this significant finding was not observed in European American patients [27]. Umemura reported that CKD was associated with frontal lobe dysfunction, demonstrated by a low Digit Symbol Substitution score, in older Japanese patients [28]. In that study, the MMSE score was lower in patients with CKD than in those without CKD, although the difference was not statistically significant, probably due to the limited number of cases [28]. In the present study, the mean MMSE score was significantly lower in older Chinese patients with CKD than in those without CKD, and the results appeared similar to those in Asian, but not European American patients [27].
ABI<0.9 is known to be associated with cognitive impairment and dementia [29]. However, the relationship between cognitive function and ABI is rarely reported in subjects with normal ABI. In the present study, our findings provided evidence that a low normal ABI value is associated with a lower MMSE, as compared to those with normal ABI (between 1.10 and 1.40). To our knowledge, this is the first report to show the synergistic effects of CKD and low normal ABI on low MMSE scores in older patients with type 2 diabetes. Recently, an ABI value between 0.91 and 1.00 has been considered as a borderline ABI value, based on the clinical practice guidelines of the American College of Cardiology and American Heart Association Task Force [20]. However, Resnick et al. [30] reported that the lowest risk of all-cause mortality was observed in subjects with ABI between 1.1 and 1.3, and that the mortality risk showed an inverse trend with ABI in subjects with ABI<1.1 over 8.3 years of follow-up [30]. Due to the limited number of cases with ABI between 0.90 and 1.00, the present study used a cutoff value of 1.10 instead of 1.00 for low normal ABI in the subjects with ABI between 0.90 and 1.40.
Moreover, arterial stiffness has been found to be associated with cognitive impairment in older subjects [31,32]. In the present study, a lower MMSE score was also observed in the subjects with baPWV ≥1970 cm/sec than in those with baPWV<1970 cm/sec. However, the association between baPWV and MMSE became non-significant after adjusting for other factors. In addition, albuminuria has been reportedly associated with cognitive impairment [33]. Consistent with the current findings, do Carmo et al [34]. Reported either ACR>30 mg/g or eGFR<60 mL/min/1.73 m2 was associated with dementia. On multivariate regression analysis in the present study, CKD showed a more powerful association with MMSE, as compared to ACR, in older patients with type 2 diabetes.
Hypoglycemia is an important predictor of dementia [35-37]. In the present study, no significant association was observed between a history of hypoglycemia and the MMSE score, which might be due to several reasons. In particular, the hypoglycemia events were retrospectively recorded during an interview based on the patients’ memory; it is possible that patients with low MMSE scores might not remember the hypoglycemic event. A previous report has shown that hypoglycemia is a predictor of dementia over a follow-up period of 7 years [37]. However, only hypoglycemia in the last one year was recorded in the present study, although the effect of hypoglycemia might persist for longer in older patients with type 2 diabetes [38].
There were some possible mechanisms underlying the dementia involved in micro- and macro-vascular diseases. Nakagawa et al [39]. Reported that angiotensin II activation might be associated with blood-brain barrier disruption and induce cognitive decline in an animal study. Angiotensin II receptor blockers, with protective benefits on cardiovascular disease and diabetic nephropathy, could ameliorate cognitive decline [40]. Furthermore, a low circulating level of brain-derived neurotrophic factor (BDNF) was a risk factor of dementia in older patients with diabetes [41]. BDNF was reported to be inversely correlated with vascular cell adhesion molecule and renalase, which might involve in cardiovascular disease and chronic kidney disease [42,43]. However, further studies are warranted to understand the real mechanisms of dementia in older patients with a low ABI value and CKD.
The present study has certain limitations. First, in the PAD group, the MMSE scores were not significantly different (24.4 ± 4.9 vs. 22.2 ± 5.5, P=0.173) between the patients with CKD (n=23) and without CKD (n=20). The effect of CKD on the MMSE score in patients with PAD was not relevant due to the limited number of cases in the PAD group. Second, the causal relationship of low MMSE scores with CKD and low ABI value could not be assessed in this cross-sectional study. Finally, the mechanism between low MMSE scores and CKD or low ABI value was not evaluated.
Conclusion
PAD defined as ABI<0.9 was an independent risk factor for a low MMSE score in older patients with type 2 diabetes. Furthermore, CKD and low normal ABI were also associated with a low MMSE score, and there were synergistic effects of CKD and low normal ABI on low MMSE scores. Nevertheless, further investigations into the causal effects and mechanisms of a low MMSE score with CKD and PAD are needed.
Acknowledgements
Statistical analysis was performed by the Biostatistics Task Force of Taichung Veterans General Hospital, Taichung, Taiwan. We thank the Center for Geriatrics and Gerontology of Taichung Veterans General Hospital for their support.
Funding
This work was supported by a grant from Taichung Veterans General Hospital, Taichung, Taiwan [grant number TCVGH-1073504C, TCVGH-1063504D]. The funders had no role in the decision to submit the manuscript for publication.
Conflicts of Interest
There are no conflicts of interest to disclose.
References
- Koroukian SM, Schiltz NK, Warner DF, et al. Increasing burden of complex multimorbidity across gradients of cognitive impairment. Am. J. Alzheimers. Dis. Other. Demen 32(7), 408-417 (2017).
- Alexander M, Perera G, Ford L, et al. Age-stratified prevalence of mild cognitive impairment and dementia in European populations: A systematic review. J. Alzheimers. Dis 48(2), 355-359 (2015).
- Wu YT, Lee HY, Norton S, et al. Prevalence studies of dementia in mainland china, Hong Kong and taiwan: a systematic review and meta-analysis. PLoSOne 8(6), e66252 (2013).
- Sun Y, Lee HJ, Yang SC, et al. A nationwide survey of mild cognitive impairment and dementia, including very mild dementia, in Taiwan. PLoSOne 9(6), e100303 (2014).
- Chien IC, Lin YC, Chou YJ, et al. Treated prevalence and incidence of dementia among National Health Insurance enrollees in Taiwan, 1996-2003. J. Geriatr. Psychiatry. Neurol 21(2), 142-148 (2008).
- Umegaki H. Type 2 diabetes as a risk factor for cognitive impairment: current insights. Clin. Interv. Aging 9(1), 1011-1019 (2014).
- Ott A, Stolk RP, van Harskamp F, et al. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 53(9), 1937-1942 (1999).
- Cheng G1, Huang C, Deng H, et al. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern. Med. J 42(5), 484-491 (2012).
- Caspard H, Jabbour S, Hammar N, et al. Recent trends in the prevalence of type 2 diabetes and the association with abdominal obesity lead to growing health disparities in the USA: An analysis of the NHANES surveys from 1999 to 2014. Diabetes. Obes. Metab 20(3), 667-671 (2018).
- Li HY, Jiang YD, Chang CH, et al. Mortality trends in patients with diabetes in Taiwan: a nationwide survey in 2000-2009. J. Formos. Med. Assoc 111(11), 645-650 (2012).
- Chang HY, Hsu CC, Pan WH, et al. Gender differences in trends in diabetes prevalence from 1993 to 2008 in Taiwan. Diabetes. Res. Clin. Pract 90(3), 358-364 (2010).
- Simo R, Ciudin A, Simo-Servat O, et al. Cognitive impairment and dementia: a new emerging complication of type 2 diabetes-The diabetologist's perspective. Acta. Diabetol 54(5), 417-424 (2017).
- Berger I, Wu S, Masson P, et al. Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC. Med 14(1), 206 (2016).
- Seliger SL, Wendell CR, Waldstein SR, et al. Renal function and long-term decline in cognitive function: the Baltimore Longitudinal Study of Aging. Am. J. Nephrol 41(4-5), 305-312 (2015).
- Wang A, Jiang R, Su Z, et al. A low ankle-brachial index is associated with cognitive impairment: The APAC study. Atherosclerosis 255(1), 90-95 (2016).
- Sugawara N, Yasui-Furukori N, Umeda T, et al. Comparison of ankle-brachial pressure index and pulse wave velocity as markers of cognitive function in a community-dwelling population. BMC. Psychiatry 10(1), 46 (2010).
- Diehm C, Lange S, Darius H, et al. Association of low ankle brachial index with high mortality in primary care. Eur. Heart. J 27(14), 1743-1749 (2006).
- Foster MC, Ghuman N, Hwang SJ, et al. Low ankle-brachial index and the development of rapid estimated GFR decline and CKD. Am. J. Kidney. Dis 61(2), 204-210 (2013).
- Chen YC, Lee CT, Lin BJ, et al. Impact of pay-for-performance on mortality in diabetes patients in Taiwan: A population-based study. Medicine. (Baltimore) 95(27), e4197 (2016).
- Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the management of patients with lower extremity peripheral artery disease: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol 69(11), 1465-1508 (2017).
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am. J. Kidney. Dis 39(2S1), S1-266 (2002).
- American Diabetes Association. 10. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2018. Diabetes. Care 41(S1), S105-S118 (2018).
- Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N. Engl. J. Med 344(21), 1608-1621 (2001).
- Li YH, Lin SY, Sheu WH, et al. Relationship between percentage of mean arterial pressure at the ankle and mortality in participants with normal ankle-brachial index: an observational study. BMJ 6(3), e010540 (2016).
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res 12(3), 189-198 (1975).
- Sink KM, Divers J, Whitlow CT, et al. Cerebral structural changes in diabetic kidney disease: African American-Diabetes Heart Study MIND. Diabetes. Care 38(2), 206-212 (2015).
- Murea M, Hsu FC, Cox AJ, et al. Structural and functional assessment of the brain in European Americans with mild-to-moderate kidney disease: Diabetes Heart Study-MIND. Nephrol. Dial. Transplant 30(8), 1322-1329 (2015).
- Umemura T, Kawamura T, Umegaki H, et al. Association of chronic kidney disease and cerebral small vessel disease with cognitive impairment in elderly patients with type 2 diabetes. Dement. Geriatr. Cogn. Dis. Extra 3(1), 212-222 (2013)..
- Guerchet M, Aboyans V, Nubukpo P, et al. Ankle-brachial index as a marker of cognitive impairment and dementia in general population. A systematic review. Atherosclerosis 216(2), 251-257 (2011).
- Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: The strong heart study. Circulation 109(6), 733-739 (2004).
- Zeki Al Hazzouri A, Newman AB, Simonsick E, et al. Pulse wave velocity and cognitive decline in elders: the Health, Aging, and Body Composition study. Stroke 44(2), 388-393 (2013).
- Rabkin SW. Arterial stiffness: detection and consequences in cognitive impairment and dementia of the elderly. J. Alzheimers. Dis 32(3), 541-549 (2012).
- Zammit AR, Katz MJ, Bitzer M, et al. Cognitive Impairment and Dementia in Older Adults With Chronic Kidney Disease: A Review. Alzheimer. Dis. Assoc. Disord 30(4), 357-366 (2016).
- do Carmo FS, Pires SL, Gorzoni ML, et al. Cognitive and renal dysfunction in the elderly. Dement. Neuropsychol 7(4), 397-402 (2013).
- Sheen YJ, Sheu WH. Association between hypoglycemia and dementia in patients with type 2 diabetes. Diabetes. Res. Clin. Pract 116(1), 279-287 (2016).
- Mehta HB, Mehta V, Goodwin JS. Association of hypoglycemia with subsequent Dementia in older patients with type 2 diabetes mellitus. J. Gerontol. A. Biol. Sci. Med. Sci 72(8), 1110-1116 (2017).
- Lin CH, Sheu WH. Hypoglycaemic episodes and risk of dementia in diabetes mellitus: 7-year follow-up study. J. Intern. Med 273(1), 102-110 (2013).
- Whitmer RA, Karter AJ, Yaffe K, et al. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 301(15), 1565-1572 (2009).
- Nakagawa T, Hasegawa Y, Uekawa K, et al. Chronic kidney disease accelerates cognitive impairment in a mouse model of Alzheimer's disease, through angiotensin II. Exp. Gerontol 87(PtA), 108-112 (2017).
- Saavedra JM. Evidence to consider angiotensin ii receptor blockers for the treatment of early Alzheimer's disease. Cell. Mol. Neurobiol 36(2), 259-279 (2016).
- Passaro A, Dalla Nora E, Morieri ML, et al. Brain-derived neurotrophic factor plasma levels: relationship with dementia and diabetes in the elderly population. J. Gerontol. A. Biol. Sci. Med. Sci 70(3), 294-302 (2015).
- Lee IT, Wang JS, Lee WJ, et al. The synergistic effect of vascular cell adhesion molecule-1 and coronary artery disease on brain-derived neurotrophic factor. Clin. Chim. Acta 466(1), 194-200 (2017).
- Lee IT, Sheu WH. Serum renalase levels are predicted by brain-derived neurotrophic factor and associated with cardiovascular events and mortality after percutaneous coronary intervention. J. Clin. Med 7(11), E437 (2018).