Research Article - (2018) Volume 8, Issue 3
Aging Disparity of Initial Dose in Patients with Schizophrenia
- Corresponding Author:
- For-Wey Lung, MD, ScD
Calo Psychiatric Center, No.12-200, Jinhua Road, Xinpi Township, Pingtung County, 925, Taiwan
Tel: +886-8-7982484
Fax: +886-8-7982487
Abstract
Abstract
Background/Aims: Data is limited on the relationship between drug dose and psych osoci al per formance inpatients with schizophrenia receiving antipsychotics. In this study, we used a latent growthcurve model (LGM) to explore the interactions between drug dose and psychosocial
performance.
Methods: We conducted a 12-week multisite, non-randomized, single-arm, open-label, flexibledose clinica l trial. All participants received a flexible dose of paliperidone extended-release(ER) of between 3 and 12 mg. Treatment efficacy was measured using Personal and SocialPerformance (PSP) scales at 0, 4, and 12 weeks. An LGM was used to analyze the associationbetween the dose of paliperidone ER and PSP scores. Three hundred thirty-one participantswere in cluded in the study by intention to treat, of whom 255 completed the 12-week studyperiod.
Results: The initial dose of paliperidone ER was estimated at 5.87-6.59 mg/day. Regarding theasso c iation between dose and psychosocial functioning, elderly patients and patients witha short duration of illness demonstrated a greater improvement in psychosocial functioning.Elderly patients also need less dose than the younger patients. A longer education year wasassociated with better initial psychosocial functioning.
Conclusion: Greater changes in dose were associated with le ss improvement in psychosocial fun ctioning astime went on. LGM represents a useful analytic method for clinical medical studies, includingpsychopharmacologic research, to predict related variables.
Keywords
Paliperidone extended-release, Personal and social performance score, Latent growth curve, Initial dose
Introduction
Schizophrenia is a serious mental illness that is challenging to the primary caregiver [1,2]. Therefore, antipsychotic drugs have become an important part of the treatment for and symptom control of schizophrenia. However, controversy exists about the efficacy and tolerability of first-generation (typical) and secondgeneration antipsychotics. The adverse effects of antipsychotics include extrapyramidal symptoms (EPS), tardive dyskinesia, sexual dysfunction, cardiovascular risk, increased prolactin levels, and the risk of metabolic syndrome [3-5]. Increased plasma glucose and cholesterol were also found in patients with schizophrenia taking antipsychotic drugs [6]. These adverse effects, and other characteristics such as sex, advanced age, or drug discontinuation, are important factors that may influence treatment outcomes in patients with schizophrenia [3,5,7,8]. Paliperidone extendedrelease (ER) has been studied for its efficacy and tolerability; its use results in a lower withdrawal rate for any reason and lower weight gain than other atypical antipsychotics [9]. Many studies have shown that flexible doses of 3–15 mg/day of paliperidone ER are suitable for the treatment of schizophrenia [10-13]. In addition, the pharmacokinetic characteristics of paliperidone ER are similar in different ethnic groups [14].
Generally, the initial dose of paliperidone ER is prescribed on the basis of pharmacokinetic studies. Many clinical trials studying the efficacy and dose of paliperidone ER have been analyzed using generalized linear models, such as analysis of variance (ANOVA) and analysis of covariance (ANCOVA) [12,15]. Linear models such as ANOVA and ANCOVA, when used in repeatedmeasures studies, traditionally need equal group sizes, consistent time intervals, and no missing data [16]. In contrast, latent growth curve models (LGM) have been used in the analysis of longitudinal data [17-19]. LGM methods have the advantage of investigating the initial status (intercept) and changes with time (slope) of dependent and independent variables [20,21]; additionally, they can also provide predictors of different levels , for example those that are fixed or flexible over time [22]. However, few clinical trials of drugs analyzed using LGM are available, and there has been no proper evaluation of the initial drug dose and change in dose throughout a whole clinical trial.
▪ Aims of the study
In this study, we aimed to evaluate the association between predictor variables related to the individual, changes in clinical psychosocial functioning, and the dose of paliperidone ER, using an LGM approach.
Materials and Methods
▪ Participants
A total of 255 participants were enrolled in the study. To be eligible for inclusion, patients had to:
• meet the Diagnostic and Statistical Manual of Mental Disorders IV criteria for schizophrenia;
• have the ability to read, understand, and sign the Institutional Review Board-approved informed consent form;
• if female, have been postmenopausal for at least 1 year, irreversibly surgically sterilized (via hysterectomy, oophorectomy, or bilateral tubal ligation), or practicing adequate and reliable contraception for the duration of the trial;
• be in good health, on the basis of a physical examination and vital signs at screening;
• Be over the age of 18.
Patients were excluded if they:
• we’re experiencing their first episode of a psychotic state;
• had a serious unstable medical condition, including recent and current clinically relevant laboratory abnormalities;
• had a diagnosis of schizoaffective disorder, mental retardation, or another cognitive disorder;
• if female, were pregnant or breast-feeding;
• if female, were of childbearing potential without adequate contraception (adequate contraception included: abstinence; oral contraceptives; intra-uterine devices; barrier methods [diaphragm or condom] plus spermicide; contraceptive implants).
• had participated in an investigational drug trial in the 30 days prior to selection;
• had a known hypersensitivity to or had experienced severe adverse events when using paliperidone ER or risperidone.
The enrolled participants were assigned an initial dose of paliperidone ER of between 3 and 15 mg, based on the physician’s evaluation.
▪ Study design
This was a 12-week, multisite, non-randomized, single-arm, open-label, flexible-dose study. Three hundred fifty-one patients were eligible to participate in the trial, 255 of whom completed the study and received paliperidone ER treatment for 12 weeks. Participants had to meet the inclusion/exclusion criteria and sign the informed consent form before entering the study. They were given paliperidone ER for 12 weeks after discontinuing their previous oral antipsychotics for at least 7 days and depot preparations for at least 4 weeks (the wash-out period). The initial dose of paliperidone ER was 3–12 mg once daily, based on the physician’s evaluation with symptoms of subjects, and the dose was adjusted according to the psychiatrist’s clinical judgment at follow-up visits. A senior psychiatrist in each site performed all assessments, to reduce inter-rater differences and increase reliability. With the exception of additional antipsychotic agents, concomitant medications were allowed throughout the study. The effect of treatment was assessed using Personal and Social Performance (PSP) scales [23,24] for the evaluation of psychosocial functioning at 0, 4, and 12 weeks, respectively. The baseline dose was assessed at the initial meeting (Week 0), and was followed up at 2, 4, 8, and 12 weeks.
▪ Statistical analysis
This study used SPSS 22.0 for Windows package software (SPSS Inc, Chicago, USA) for statistical analysis and organization of the data. Descriptive analysis of demographics was analyzed using SPSS, and structural equation analysis was performed using AMOS 22.0 for Windows (SPSS Inc, Chicago, USA).
In the first model, we estimated the unconditional growth models (without predictors) of the change over time in the dose of paliperidone ER. This model contained two latent variables: the intercept, which represented the level of the initial dose; and the linear slope, which represented the change over time in paliperidone ER dose. Second, we used the estimated conditional model of the parallel process of change over time in the mediator (paliperidone dose) and outcome (PSP score) to examine the significance of the predictor variables. The predictor variables, details of which were obtained from all study centers, comprising sex, hospital, occupation, in-/ outpatient treatment, illicit drug abuse, smoking status, alcohol consumption, age, age at onset, duration of illness, number of relapse, years of education, and number of previous periods of hospitalization, were examined in all the models. The chi-squared goodness-of-fit (GFI) test was used to investigate the model fit; non-significant chi-squared values (a p value greater than 0.05, and a GFI greater than 0.8) indicated that the model described the observed data adequately. Parsimonious models were presented, meaning that only statistically significant parameters were shown.
▪ Ethics considerations
This study was approved by the Institutional Review Boards of each site, and was performed in accordance with the Declaration of Helsinki. Informed consent was given before the study commenced, and participants were notified that they could withdraw at any time.
Results
▪ Demographics and disposition of participants
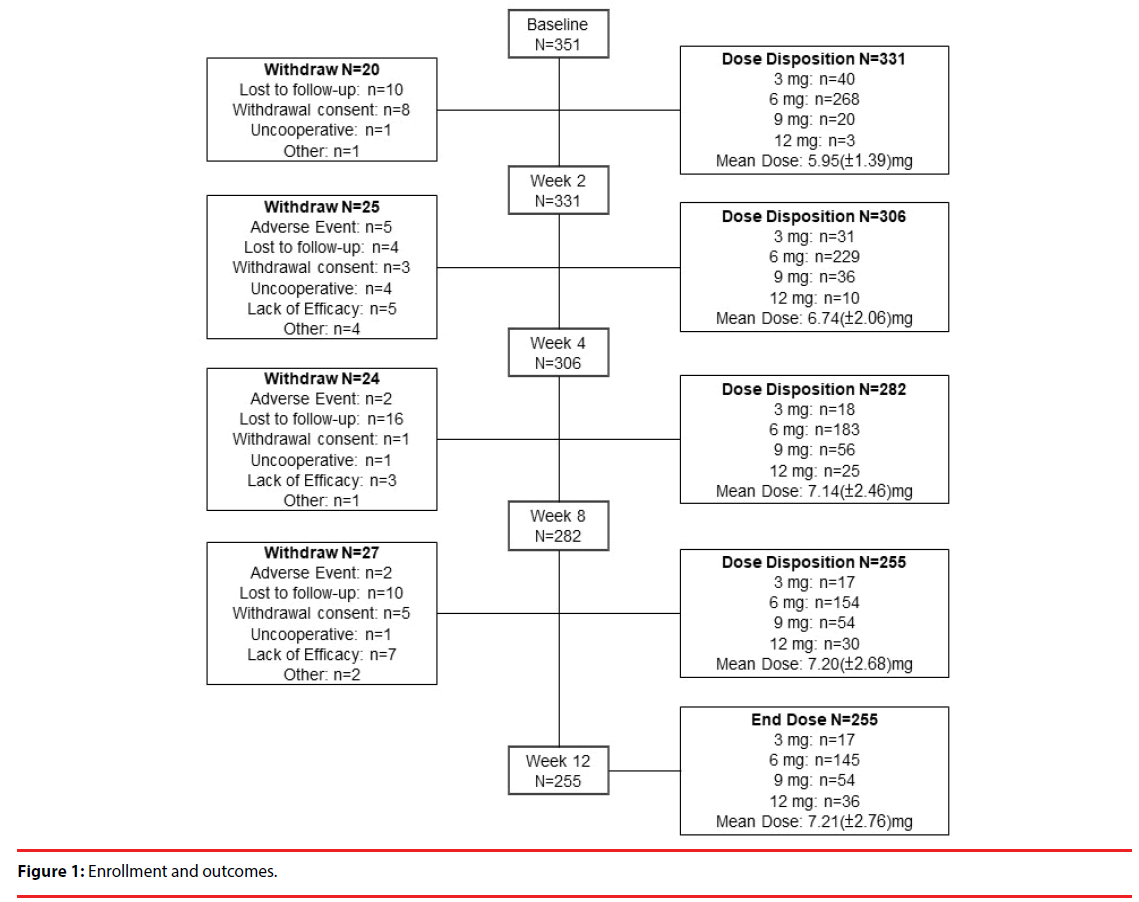
A total of 331 patients were included in the analysis on the basis of intention to treat [25] to investigate the potential effect of missing data. Table 1 shows the baseline demographics of the patients. We recorded each participant’s sex, psychiatric service model, occupation, smoking status, alcohol consumption, illicit drug abuse, in-/outpatient status, age, age at onset, duration of illness, educational level, number of relapse, and number of previous periods of hospitalization. Figure 1 delineates the enrollment and followup of the study participants: 351 patients enrolled in the study, of whom 255 completed the study. Ninety-six patients withdrew from the study, and the reasons for their withdrawal are summarized in Table 2. Loss to follow-up (40 patients; 11.4% of the participants enrolled) was the major reason for withdrawal from this study.
| Variables | n (%), or mean (SD) |
|---|---|
| Sex | |
| Male | 213 (64.4%) |
| Female | 118 (35.3%) |
| Hospital | |
| Redesigned network psychiatry service | 121 (36.6%) |
| Hospital-based psychiatry service | 210 (63.4%) |
| Occupation | |
| Yes | 21 (6.3%) |
| No | 310 (93.7%) |
| In-/Outpatient | |
| Inpatient | 230 (30.5%) |
| Outpatient | 101 (69.5%) |
| Drug abuse | |
| Yes | 22 (6.6%) |
| No | 309 (93.4%) |
| Smoking | |
| Yes | 192 (58%) |
| No | 139 (42%) |
| Alcohol | |
| Yes | 129 (39%) |
| No | 202 (61%) |
| Age, mean (SD), years | 41.17 (11.23) |
| Age at onset, mean (SD), years | 25.33 (7.88) |
| Duration of illness, mean (SD), years | 15.38 (10.25) |
| Relapse | 5.07 (4.20) |
| Number of previous periods of hospitalization | 6.08 (5.86) |
| Education | 10.46 (3.25) |
Table 1: Demographic characteristics of patients with schizophrenia (n = 331).
| Overall (n = 351) |
Initial Dose | |||||
|---|---|---|---|---|---|---|
| 0 mg/day (n = 5) |
3 mg/day (n = 47) |
6 mg/day (n = 276) |
9 mg/day (n = 20) |
12 mg/day (n = 3) |
||
| Adverse Effect | 9 (2.6%) | 0 | 3 | 6 | 0 | 0 |
| Lost to Follow-Up | 40 (11.4%) | 3 | 7 | 28 | 1 | 1 |
| ICF Withdrawal | 18 (5.1%) | 1 | 7 | 9 | 0 | 0 |
| Uncooperative | 7 (2%) | 1 | 0 | 6 | 0 | 0 |
| Lack of Efficacy | 15 (4.3%) | 0 | 3 | 10 | 2 | 0 |
| Other | 9 (2.6%) | 0 | 0 | 7 | 1 | 0 |
| Total | 98 (27.9%) | 5 | 20 | 66 | 4 | 1 |
Table 2: Summary of reasons for withdrawal.
▪ The estimated model of changes in dose over time
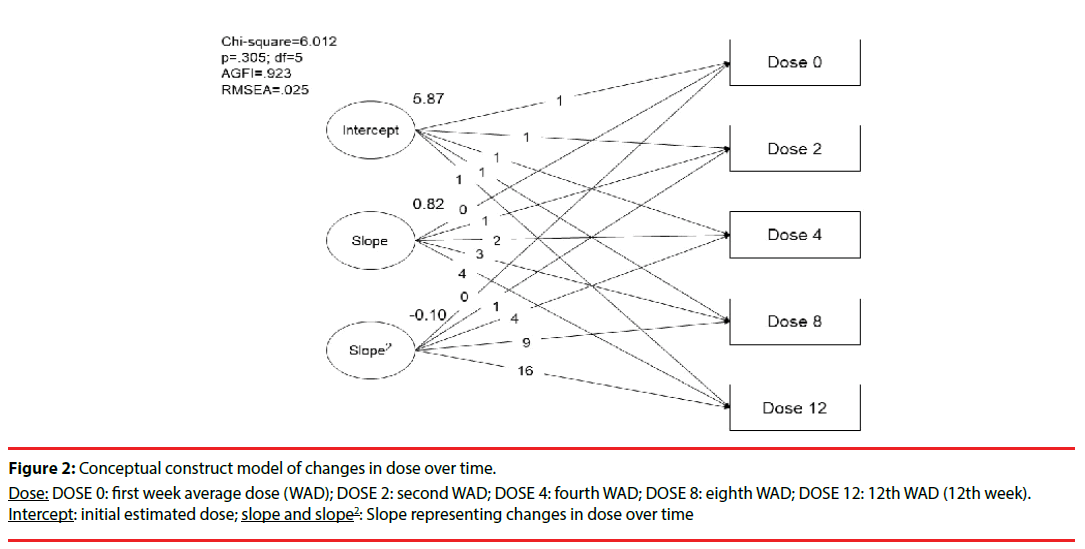
Repeated evaluations of the changes in dose and PSP scales over time were made in this study. Figure 2 shows that the initial dose of paliperidone ER was estimated as 5.87 mg/day. The results followed the description (chi-squared = 6.01; degree of freedom [df] = 5; adjusted GFI = 0.92; p = 0.305), and showed a linear growth trajectory consistent with the clinical practice of changes in dose over four time points. To estimate the fitness of this model, we tested the linear, polynominal regression (quadratic and cubic model of slope), and natural log transformation of the dose, and found that the quadratic correction model of the slope was most suitable for this study.
Figure 2: Conceptual construct model of changes in dose over time.
Dose: DOSE 0: first week average dose (WAD); DOSE 2: second WAD; DOSE 4: fourth WAD; DOSE 8: eighth WAD; DOSE 12: 12th WAD (12th week). Intercept: initial estimated dose; slope and slope2: Slope representing changes in dose over time
▪ The parsimonious model of dose and symptom changes, with adjustment for confounding variables
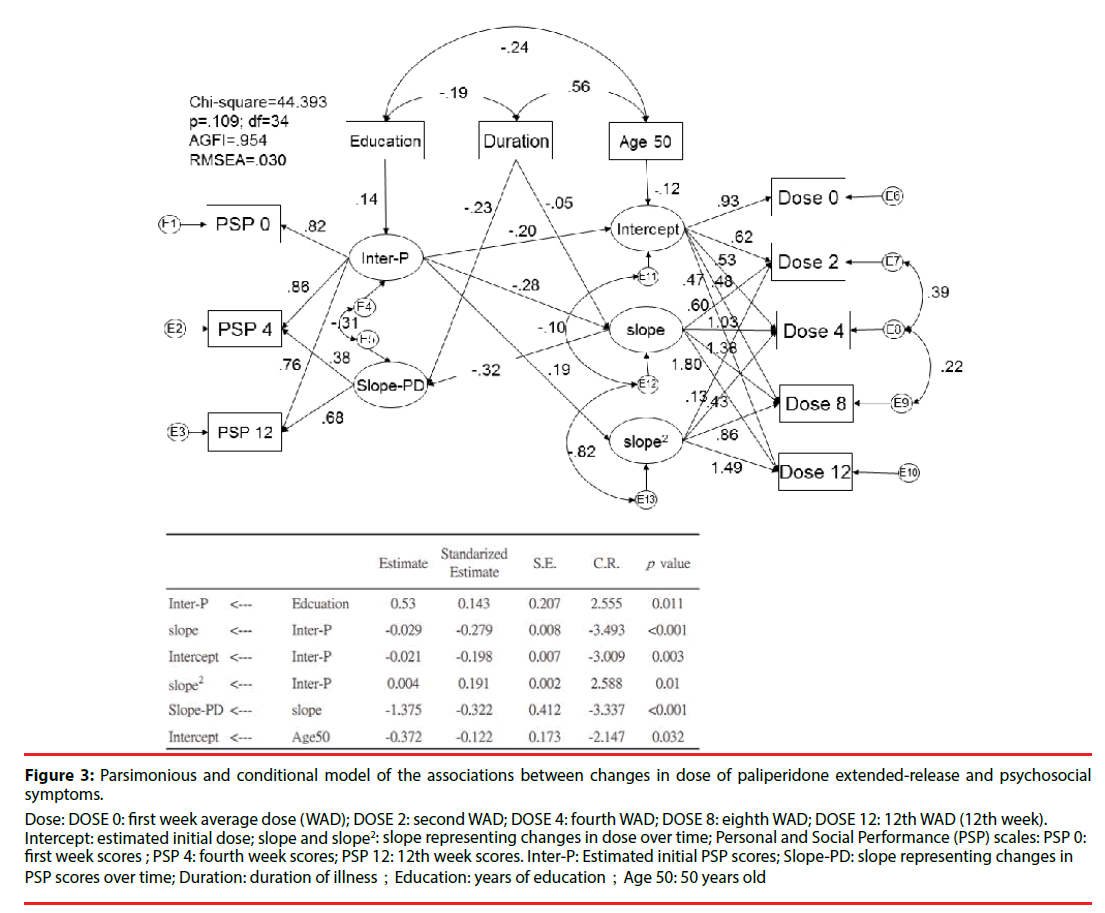
Figure 3 shows the parsimonious model and related significant predictors. This conditional parsimonious model exhibited a good fit, as measured by the following description: chisquared = 44.393; df = 34; adjusted GFI = 0.954; p = 0.109. The conditioned initial estimated dose of paliperidone ER was 6.59 mg/day. Those with a better initial PSP score had a lower initial dose (β = −0.20; p = 0.003) and demonstrated less improvement over time (β = −0.28; p < 0.001). Besides, elderly (cut off point of age above 50) is also associated lower initial dose of paliperidone (β = -0.12; p = 0.032). A shorter duration of illness was also associated with a greater improvement in PSP scores over time (β = −0.23; p < 0.001). A higher level of education was associated with better estimated initial PSP scores (β = 0.14; p = 0.011). More changes in dose over time (slope) were associated with worse PSP scores over time (β = −32; p < 0.001).
Figure 3:Parsimonious and conditional model of the associations between changes in dose of paliperidone extended-release and psychosocial symptoms.
Dose: DOSE 0: first week average dose (WAD); DOSE 2: second WAD; DOSE 4: fourth WAD; DOSE 8: eighth WAD; DOSE 12: 12th WAD (12th week).
Intercept: estimated initial dose; slope and slope2: slope representing changes in dose over time; Personal and Social Performance (PSP) scales: PSP 0: first week scores ; PSP 4: fourth week scores; PSP 12: 12th week scores. Inter-P: Estimated initial PSP scores; Slope-PD: slope representing changes in PSP scores over time; Duration: duration of illness;Education: years of education;Age 50: 50 years old
Discussion
We propose that the appropriate initial dose of the antipsychotic paliperidone ER should be 5.87–6.59 mg/day, based on PSP scores using a conditional and parsimonious model of change over time. A broad therapeutic dose range of 3–15 mg/day is suggested in many studies [10,25,26]; however, there is a lack of evidence to [24] support recommendations for the initial dose. Kane et al. [27] performed an international, multicenter, randomized study on acute exacerbations of schizophrenia, in which initial doses of 6, 9, and 12 mg had a significant therapeutic outcome as determined by Positive and Negative Syndrome scores, Clinical Global Impression scales-Symptoms, and PSP scales. Although other studies have suggested that an initial dose of 3-12 mg is suitable for patients with schizophrenia [11,26], little is known about the appropriate initial dose in relation to changes in PSP scales over time.
In our study, we found elderly subjects is associated with lower initial and last dose than younger ones. It is consistent with previous studies [28,29]. Then, we choose age of 50 as a cut-off point and divided into two groups (above the age of 50 and others). Advanced age of above 50 is associated with lower initial dose than others.
Our study was conducted as a multicenter, randomized, flexible-dose, open-label clinical trial. The initial dose was adjusted on the basis of the physician’s judgment, and was prescribed by referring to the PSP score of each individual. The dose was adjusted according to changes in psychosocial symptoms. Outcome measurement is important in clinical drug trials, in which, conventionally, a generalized linear model such as ANOVA and ANCOVA is used for the analysis of changes over time. The initial dose of paliperidone ER recommended by this study was 5.87 mg without considering adjustment for other variables, and 6.59 mg on the basis of a conditional and parsimonious measurement using LGM and considering individual changes over time [30,31]. In studies by Owen [32] and Nord and Farde [33], 6 mg of paliperidone ER (similar to our initial estimated paliperidone ER dose) showed 64% dopamine D2 receptor occupancy, and elicited a good treatment response with few EPS. However, those with greater than 80% D2 receptor occupancy were at an increased risk of EPS [33]. Considering the adverse effect of antipsychotics, the initial dose is important in encouraging adherence to long-term antipsychotic treatment [34]. In addition, a longitudinal trial is needed to study the interaction between changes over time and individual variables [16]. A quadratic individual change trajectory (slope) curve for paliperidone ER dose and outcome evaluation was proposed from our results, using LGM analysis [20,21].
In this study, we investigated predictor variables that influenced the initial dose of paliperidone ER and changes in treatment dose and psychosocial symptoms over time. To determine which curve fitted the data most appropriately across time, linear and nonlinear models were assessed. A hypothesized quadratic slope model had the best GFI characteristic in our trial. It seems reasonable that changes in paliperidone ER dose over time are best represented by a nonlinear model, rather than a linear model, in clinical trials. The estimated unconditional and conditional models showed that an initial dose of 5.87-6.59 mg is suitable in this group of patients, and that changes in dose over time are affected by the predictors age, duration of illness, and years of education.
Based on our observations, advanced age is associated with a greater improvement in PSP score (slope change) than youth. According to a pharmacokinetic study by Feng et al. [35], age is significantly related to the clearance of 9-hydroxy-risperidone, and this may explain, at least in part, why elderly patients exhibited a greater change in psychosocial symptoms than young patients. A study by Apiquian et al. [36] revealed that lower PSP scores in patients with schizophrenia were related to a shorter education, unemployed status, and a longer duration of illness. This is consistent with our results: we found that a longer education was related to high initial PSP scores, and indirectly related to a lower initial dose of paliperidone ER. Also, patients with a shorter duration of illness showed a greater improvement in PSP scores than those with a longer duration of illness. It is also possible that a longer duration of illness is related to worse cognitive function (which affects PSP scores) and poor adherence (which affects the efficacy of paliperidone ER). A greater change in dose was associated with a smaller change in PSP scores in our study. We believe that PSP scores may be influenced by adverse effects of the drug, such as EPS.
Our study had several limitations. First, other associated factors—such as adverse effects, concomitant anxiolytic therapy, prolactin levels, and gene interactions, all of which may affect symptoms and dose—were not recorded [37-39]. Second, we did not consider changes in cognition, which can improve social competence related to PSP scores [40]. Third, the trial period was only 3 months. Our results showed that the change in dose as related to PSP scores is described by a quadratic curve model, but the 3-month period was too short to assess this adequately. A longer observation period will be necessary in future studies [41]. Furthermore, use of another nonlinear model of dose change, such as a cubic curve model, may be more suitable in a longer study. Finally, symptom measurement, for example using PANSS or Brief Psychiatric Rating Scales, should be conducted in future studies to investigate the interaction between dose and symptoms.
In conclusion, in this multisite, nonrandomized, single-arm, open-label, flexibledose clinical trial, we aimed to investigate the relationship between drug dose and psychosocial performance in patients with schizophrenia receiving the antipsychotic paliperidone ER. The estimated initial dose and interactions between treatment dose and psychosocial symptoms over time were studied using an LGM. We identified age, duration of illness, years of education, and changes in dose as predictors of changes in symptoms over time. LGM represents a useful analytic method for clinical medical studies, and should be used in future studies, including clinical trials with a long-term follow-up, investigating the interaction between drug dose and symptoms.
Conflicts of Interest
This research was supported by Janssen-Cilag Taiwan, Johnson & Johnson. The funding body had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. No authors have other conflicts of interest to declare.
References
- Wheeler C. The diagnosis of schizophrenia and its impact on the primary caregiver. Nurs. Prax. N. Z. 9, 15-23 (1994).
- White RG, McCleery M, Gumley AI, et al. Hopelessness in schizophrenia: the impact of symptoms and beliefs about illness. J. Nerv. Ment. Dis 195, 968-975 (2007).
- Aichhorn W, Whitworth AB, Weiss EM, et al. Second-generation antipsychotics: is there evidence for sex differences in pharmacokinetic and adverse effect profiles? Drug. Saf 29(1), 587-598 (2006).
- Friedman JH. Atypical antipsychotics have very different adverse effect profiles and should not be lumped together. Arch. Intern. Med 166(1), 586 (2006).
- Maixner SM, Mellow AM, Tandon R. The efficacy, safety, and tolerability of antipsychotics in the elderly. J. Clin. Psychiatry 60 Suppl 8(1), 29-41 (1999).
- Lindenmayer JP, Czobor P, Volavka J, et al. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. Am. J. Psychiatry 160, 290-296 (2003).
- Jaffe AB, Levine J. Efficacy and effectiveness of first- and second-generation antipsychotics in schizophrenia. J. Clin. Psychiatry 64 Suppl 17(1), 3-6 (2003).
- Kapur S, Remington G. Atypical antipsychotics: new directions and new challenges in the treatment of schizophrenia. Annu. Rev. Med 52(1), 503-517 (2001).
- Jones MP, Nicholl D, Trakas K. Efficacy and tolerability of paliperidone ER and other oral atypical antipsychotics in schizophrenia. Int. J. Clin. Pharmacol. Ther 48(1), 383-399 (2010).
- Boom S, Talluri K, Janssens L, et al. Single- and multiple-dose pharmacokinetics and dose proportionality of the psychotropic agent paliperidone extended release. J. Clin. Pharmacol 49(1), 1318-1330 (2009).
- Davidson M, Emsley R, Kramer M, et al. Efficacy, safety and early response of paliperidone extended-release tablets (paliperidone ER): results of a 6-week, randomized, placebo-controlled study. Schizophr. Res 93(1), 117-130 (2007).
- Kane J, Canas F, Kramer M, et al. Treatment of schizophrenia with paliperidone extended-release tablets: a 6-week placebo-controlled trial. Schizophr. Res 90(1), 147-161 (2007).
- Marder SR, Kramer M, Ford L, et al. Efficacy and safety of paliperidone extended-release tablets: results of a 6-week, randomized, placebo-controlled study. Biol. Psychiatry 62(1), 1363-1370 (2007).
- Si T, Shu L, Liu Y, et al. Single-dose pharmacokinetics of paliperidone extended-release tablets in healthy Chinese subjects. Hum. Psychopharmacol 25(1), 404-409 (2010).
- Patrick DL, Burns T, Morosini P, et al. Measuring social functioning with the personal and social performance scale in patients with acute symptoms of schizophrenia: interpretation of results of a pooled analysis of three Phase III trials of paliperidone extended-release tablets. Clin. Ther 32(1), 275-292 (2010).
- Shek DT, Ma CM. Longitudinal data analyses using linear mixed models in SPSS: concepts, procedures and illustrations. Sci. World J 11(1), 42-76 (2011).
- Hamid JS, Roslin NM, Paterson AD, et al. Using a latent growth curve model for an integrative assessment of the effects of genetic and environmental factors on multiple phenotypes. BMC Proc 3 Suppl 7(1), S44 (2009).
- Stull DE. Analyzing growth and change: latent variable growth curve modeling with an application to clinical trials. Qual. Life. Res 17(1), 47-59 (2008).
- Roesch SC, Norman GJ, Adams MA, et al. Latent growth curve modeling of adolescent physical activity: testing parallel process and mediation models. J. Health. Psychol 14(1), 313-325 (2009).
- Miner JL, Clarke-Stewart KA. Trajectories of externalizing behavior from age 2 to age 9: relations with gender, temperament, ethnicity, parenting, and rater. Dev. Psychol 44(1), 771-786 (2008).
- Speer DC, Greenbaum PE. Five methods for computing significant individual client change and improvement rates: support for an individual growth curve approach. J. Consult. Clin. Psychol 63(1), 1044-1048 (1995).
- Lenzenweger MF, Johnson MD, Willett JB. Individual growth curve analysis illuminates stability and change in personality disorder features: the longitudinal study of personality disorders. Arch. Gen. Psychiatry 61(1), 1015-1024 (2004).
- Juckel G, Schaub D, Fuchs N, et al. Validation of the Personal and Social Performance (PSP) Scale in a German sample of acutely ill patients with schizophrenia. Schizophr. Res 104(1-3), 287-93 (2008).
- Nasrallah H, Morosini P, Gagnon DD. Reliability, validity and ability to detect change of the Personal and Social Performance scale in patients with stable schizophrenia. Psychiatry Res 161(2), 213-24 (2008).
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 319(1), 670-674 (1999).
- Fowler JA, Bettinger TL, Argo TR. Paliperidone extended-release tablets for the acute and maintenance treatment of schizophrenia. Clin. Ther 30(1), 231-248 (2008).
- Kane J, Canas F, Kramer M, et al. Treatment of schizophrenia with paliperidone extended-release tablets: a 6-week placebo-controlled trial. Schizophr. Res 90(1-3), 147-161 (2007).
- Uchida H, Mamo DC, Mulsant BH, et al. Increased antipsychotic sensitivity in elderly patients: evidence and mechanisms. J. Clin. Psychiatry 70(3), 397-405 (2009).
- Uchida H, Pollock BG, Bies RR, et al. Predicting age-specific dosing of antipsychotics. Clin. Pharmacol. Ther 86(4), 360-362 (2009).
- Cheong J, Mackinnon DP, Khoo ST. Investigation of Mediational Processes Using Parallel Process Latent Growth Curve Modeling. Struct. Equ. Modeling 10(1), 238 (2003).
- Willett JB, Ayoub CC, Robinson D. Using growth modeling to examine systematic differences in growth: an example of change in the functioning of families at risk of maladaptive parenting, child abuse, or neglect. J. Consult. Clin. Psychol 59(1), 38-47 (1991).
- Owen RT. Extended-release paliperidone: efficacy, safety and tolerability profile of a new atypical antipsychotic. Drugs. Today 43(1), 249-258 (2007).
- Nord M, Farde L. Antipsychotic Occupancy of Dopamine Receptors in Schizophrenia. CNS Neurosci. Ther 17(1), 97-103 (2011).
- Jeste DV, Rockwell E, Harris MJ, et al. Conventional vs. newer antipsychotics in elderly patients. Am. J. Geriatr. Psychiatry 7(1), 70-76 (1999).
- Feng Y, Pollock BG, Coley K, et al. Population pharmacokinetic analysis for risperidone using highly sparse sampling measurements from the CATIE study. Br. J. Clin. Pharmacol 66(1), 629-639 (2008).
- Apiquian R, Elena Ulloa R, Herrera-Estrella M, et al. Validity of the Spanish version of the Personal and Social Performance scale in schizophrenia. Schizophr. Res 112(1), 181-186 (2009).
- Hill M, Crumlish N, Whitty P, et al. Nonadherence to medication four years after a first episode of psychosis and associated risk factors. Psychiatr. Serv 61(1), 189-192 (2010).
- Lung FW, Tzeng DS, Shu BC. Ethnic heterogeneity in allele variation in the DRD4 gene in schizophrenia. Schizophr. Res 57(1), 239-245 (2002).
- Lung FW, Shu BC, Kao WT, et al. Association of DRD4 uVNTR and TP53 codon 72 polymorphisms with schizophrenia: a case-control study. BMC Med. Genet 10(1), 147 (2009).
- Mazza M, Tozzini C, Giosue P, et al. Social cognition and atypical antipsychotic agents in the treatment of persons with schizophrenia: preliminary data from a naturalistic study. Clin. Ter 154(1), 79-83 (2003).
- Chen YL, Cheng TS, Lung FW. Prolactin Levels in Olanzapine Treatment Correlate With Positive Symptoms of Schizophrenia: Results From an Open-Label, Flexible-Dose Study. Prim. Care Companion. J. Clin. Psychiatry 11(1), 16-20 (2009).