Review Article - (2018) Volume 8, Issue 6
Advances and Challenges in Assessing 2-Hydroxyglutarate in Gliomas by Magnetic Resonance Spectroscopy: A Short Review
- *Corresponding Author:
- Yukihiko Fujii, MD, PhD
Professor, Department of Neurosurgery, Brain Research Institute, University of Niigata, Niigata, Japan
Tel: 025-227-0653
Abstract
The metabolite 2-hydroxyglutarate (2HG) accumulates in isocitrate dehydrogenase (IDH)- mutant gliomas and high-levels of 2HG can be non-invasively detected in living human brain by magnetic resonance spectroscopy (MRS). The concept of being able to detect a metabolite associated with an important gene mutation has generated considerable excitement in the fields of neurooncology and neuroradiology. However, challenges remain in reliably detecting 2HG before we can use it as a tool for making clinical decisions. In this review, we outline the advances and challenges in assessing 2HG by MRS.
Keywords
2-Hydroxyglutarate, Gliomas, Magnetic Resonance Spectroscopy
Introduction
A groundbreaking study showed isocitrate dehydrogenase (IDH) mutations in about 10% of glioblastomas [1], and subsequent studies showed IDH mutations to occur in 50-80% of astrocytomas, oligodendrogliomas, and secondary glioblastomas [2-5]. The current understanding is that IDH mutations occur frequently, early in the cascade of astrocytomas and oligodendrogliomas [3], and is deeply involved in gliomagenesis. IDH mutations are known to be a powerful positive prognostic factor in World Health Organization (WHO) grade 3 and 4 [6,7]. IDH mutations in gliomas give rise to the metabolite 2-hydroxyglutarate (2HG) [8], which can be detected by magnetic resonance spectroscopy (MRS).
▪ Functions of IDH and 2HG
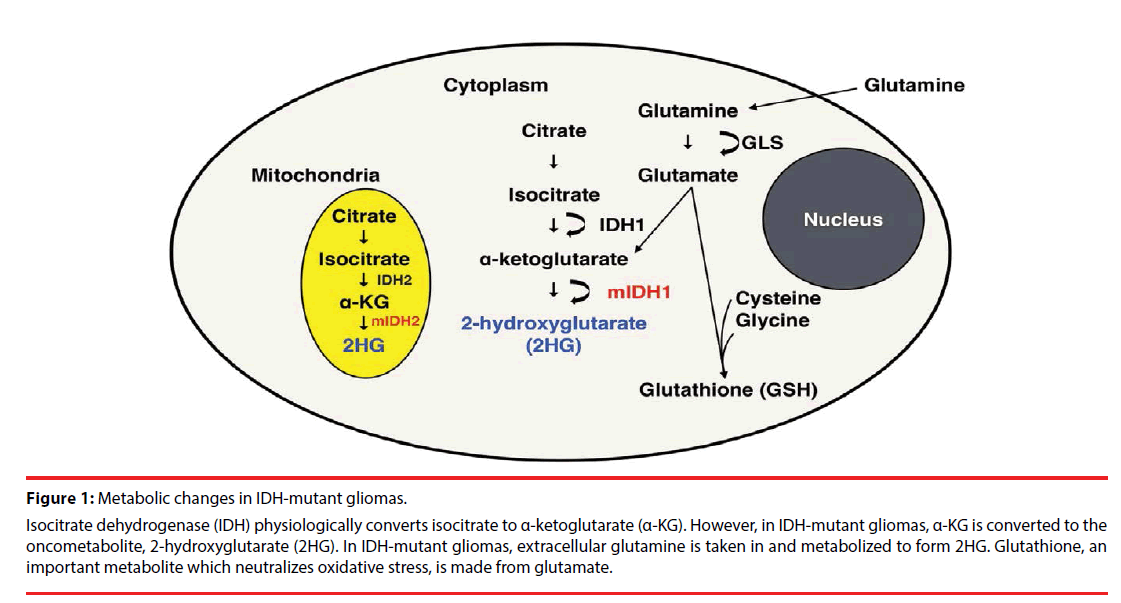
The family of IDH enzymes includes three isoforms: IDH1, which is located in the cytoplasm, and IDH2 and IDH3, which localize in mitochondria (Figure 1). IDH3, which uses the cofactor NAD+ (as opposed to NADP+ for IDH1 and IDH2) as the electron acceptor, converts isocitrate to α-ketoglutarate (α-KG) as part of the tricarboxylic acid (TCA) cycle.
Figure 1: Metabolic changes in IDH-mutant gliomas.
Isocitrate dehydrogenase (IDH) physiologically converts isocitrate to α-ketoglutarate (α-KG). However, in IDH-mutant gliomas, α-KG is converted to the oncometabolite, 2-hydroxyglutarate (2HG). In IDH-mutant gliomas, extracellular glutamine is taken in and metabolized to form 2HG. Glutathione, an important metabolite which neutralizes oxidative stress, is made from glutamate.
IDH mutation is a gain-of-function mutation, in which 2HG is produced from α-KG (Figure 1) [9]. At least 5 point mutations of IDH1 and 3 point mutations of IDH2 have been reported in gliomas; IDH3 mutation has not been reported in gliomas. The most commonly occurring point mutation in gliomas is IDH1 R132H with a frequency of 85-95% [7,10], causing a missense mutation from Arginine (R) to histidine (H). 2HG is structurally similar to α-KG and acts as a competitive antagonist, causing inhibition of α-KG-dependent dioxygenases. These include the JmjC domain-containing histone demethylases (KDMs), which cause histone demethylation [11-13], and the ten-eleven translocation (TET) family of DNA hydroxylases, which cause DNA demethylation [13,14]. As a result of this competitive inhibition, diffuse DNA hypermethylation is noted in IDH-mutant gliomas [15], the so-called glioma-CPG island methylator phenotype (G-CIMP).
▪ Assessment of 2-hydroxyglutarate
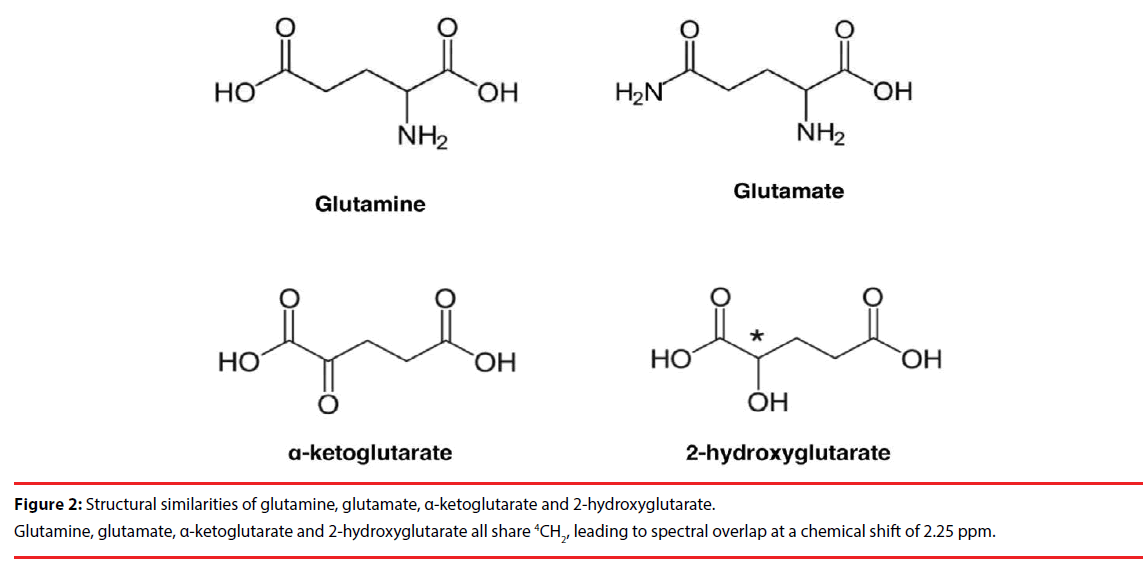
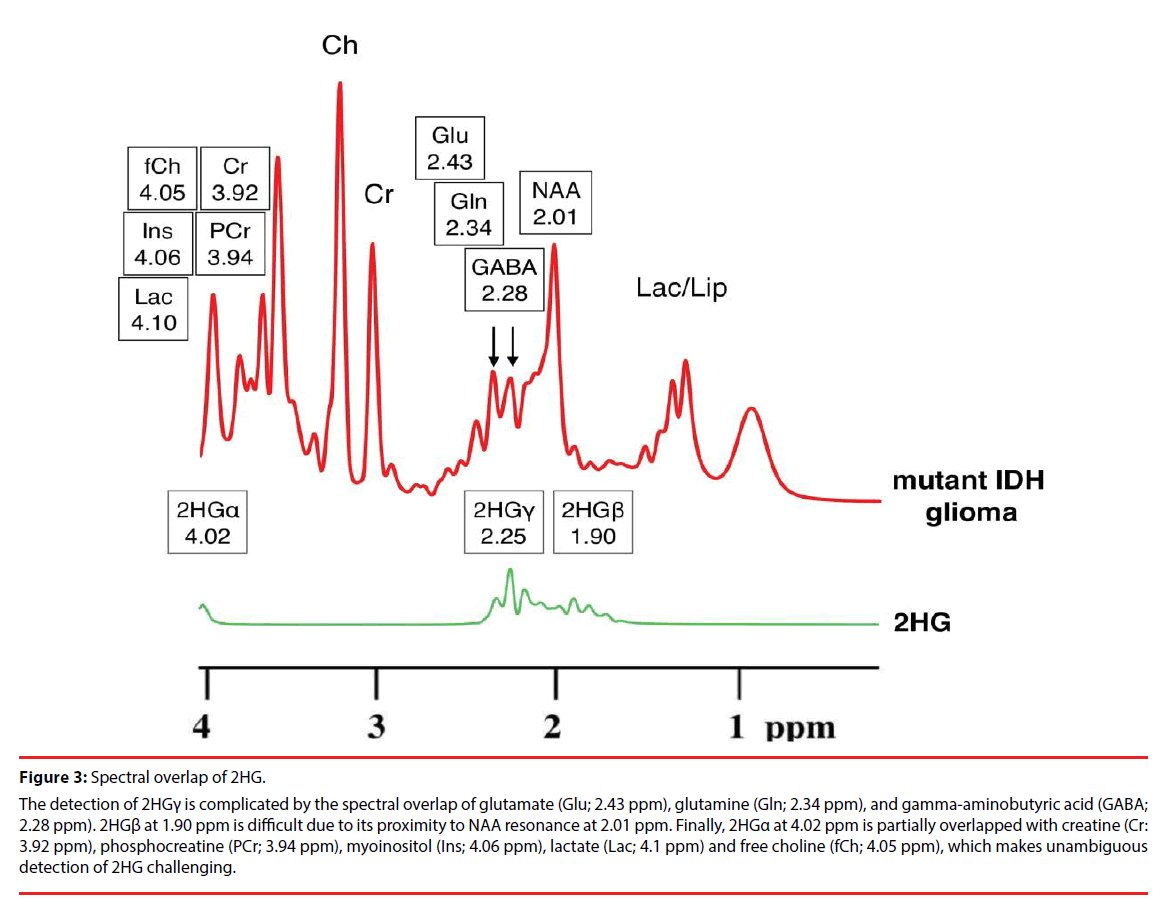
The 2HG molecule contains five nonexchangeable protons, giving rise to multiplets at three locations on MRS: approximately 4.02, 2.25, and 1.90 ppm [16]. The largest multiplet is located at 2.25 ppm. The detection of this multiplet is complicated by the spectral overlap of glutamate (Glu; 2.43 ppm), glutamine (Gln; 2.34 ppm), and gamma-aminobutyric acid (GABA; 2.28 ppm), which share the 4CH2 group [17]. This can be expected given the structural similarities of Glu, Gln and 2HG (Figure 2). Direct detection of the multiplet at 1.90 ppm is difficult due to its proximity to NAA resonance at 2.01 ppm, which shares 3CH2. Also, a CH3 peak of acetic acid at 1.90 ppm may further obscure the multiplet, especially in cases of pathologic state [18,19]. Finally, the multiplet at 4.02 ppm is partially overlapped with creatine (Cr: 3.92 ppm), phosphocreatine (PCr; 3.94 ppm), myoinositol (Ins; 4.06 ppm), lactate (Lac; 4.1 ppm) and free choline (fCh; 4.05 ppm), sharing 2CH2 [16], which makes unambiguous detection of 2HG challenging (Figure 3).
Figure 3: Spectral overlap of 2HG.
The detection of 2HGγ is complicated by the spectral overlap of glutamate (Glu; 2.43 ppm), glutamine (Gln; 2.34 ppm), and gamma-aminobutyric acid (GABA; 2.28 ppm). 2HGβ at 1.90 ppm is difficult due to its proximity to NAA resonance at 2.01 ppm. Finally, 2HGα at 4.02 ppm is partially overlapped with creatine (Cr: 3.92 ppm), phosphocreatine (PCr; 3.94 ppm), myoinositol (Ins; 4.06 ppm), lactate (Lac; 4.1 ppm) and free choline (fCh; 4.05 ppm), which makes unambiguous detection of 2HG challenging.
Furthermore, detection of 2HG is compounded by the fact that there are two enantiomers of 2HG, namely [D]-2HG and [L]-2HG, which cannot be distinguished by 1H MRS [20]. IDH mutations produce only the [D]- 2HG enantiomer, causing more than 100–fold increases [8,21]. In normal cells, both [D]-2HG and [L]-2HG are considered to be unwanted byproducts of cellular metabolism, and their intracellular levels are maintained at <0.1 mM [22]. However, recent studies have found that ischemia can cause up to 45-fold increases in [L]-2HG in cancer cells [23,24], so the presence of [L]-2HG must be taken into account when interpreting possible false-positive measurements of 2HG by MRS.
Also, there is a possibility that 2HG is produced in tumors with elevated glutamine metabolism. Teranuma et al. reported that 2 to 5 millimolar levels of 2HG, which is comparable to levels detected in IDH-mutant gliomas and acute myeloid leukemia (AML), was detected in IDHwildtype breast cancer tissues with MYC pathway activation and increased glutamine metabolism [25]. This phenomenon has not been reported to be common in gliomas.
Assessing 2HG in serum, urine, and cerebrospinal fluid is another hot topic of clinical research. Detection of 2HG from body fluids would enable less invasive diagnosis of IDH-mutant gliomas and longitudinal assessment of treatment response in these tumors. Recent studies have indicated that 2HG can be detected in cerebrospinal fluid [26,27] and urine [28], but it is still a challenge to assess 2HG in the serum of IDH-mutant gliomas [28,29]. It remains to see whether this can be overcome with more sensitive methods, or if 2HG accumulates in the serum of IDH-mutant glioma patients at all. The latter seems probable given that detection of 2HG in the serum of IDH-mutant AML patients has been well established [30-34]. An interesting study found that detection of circulating glioma cells is possible in 39% of glioblastoma patients using a cocktail of 5 antibodies, SOX2, tubulin beta-3, EGFR, A2B5, and c-MET. These circulating glioma cells were enriched for the mesenchymal signature [35]. IDH-mutant gliomas, which are known to be enriched with the proneural signature [36], may lack circulating tumor cells, thus contributing the absence of 2HG in the serum.
▪ Methods of assessing 2-hydroxyglutarate by magnetic resonance spectroscopy
Long-echo MRS with TE at 97 ms with the use of three-dimensional volume-localized basis (VLB) spectra has been shown to be optimal for detection of 2HG [16,37]. A comparative study of PRESS sequences at short- (35 ms) and long- TE (97 ms) found long- TE to be superior by minimizing the effect of macromolecule signals [16]. Unambiguous detection of 2HG in mutant IDH gliomas was achieved by 2D correlation spectroscopy (COSY) [38-41] and J-difference spectroscopy [38]. In 2D COSY, the overlapping signals are resolved along a second orthogonal chemical shift dimension, and the cross-peaks resulting from the scalar coupling of Hα-Hβ protons show up in a region that is free of the contribution of other metabolites in both normal cells and wildtype-IDH tumors. While 2D COSY retains all of the metabolites in the spectrum, J-difference spectroscopy focuses on the metabolite of interest, such as 2HG. In the case of 2HG, the editing pulses tuned at around 1.9 ppm indirectly influence the line shape of the multiplet at around 4.0 ppm due to J-coupling by the spins. By subtracting the two spectra acquired at the same TE with and without editing pulses, removal of the contribution of overlapping metabolites Lac and Ins, which do not have their J-couple counterpart at about 1.9 ppm is possible, and the signal 2HGα signal can be detected at 4.02 ppm [38,42,43]. J-difference spectroscopy has historically been used in gliomas to subtract the lactate peak from lipid peak. 2D COSY has the highest resolving power to separate overlapping metabolites, but has less sensitivity and involves more complex quantification; J-difference spectroscopy has increased sensitivity, and quantification is straightforward, but is susceptible to subtraction errors. These methods are less available clinically and involve longer acquisition time [42]. Jafari-Khouzani and colleagues have reported MRS imaging (MRSI) of 2HG using a 3-tesla machine [44]. MRS using ultra-high magnetic field machines such as 7-tesla and higher, with a high signal-to-noise ratio, is able to better separate the 2HG peak at 2.25 ppm from those of Glu, Gln and GABA [45]. Preclinical trials involving intravenous injection of hyperpolarized 13C-labeled α-KG to detect decreased glutamate production in IDH-mutant tumors [46], and hyperpolarized pyruvate to monitor the conversion of pyruvate to lactate, which is decreased in IDH-mutant tumors [47], presumably reflecting increase of metabolic substrate entering into TCA cycle. These techniques are outlined in three important review articles [42,43,48]. Previously reported methods to assess 2HG are also summarized in Table 1.
| Method | Strengths and limitations | References |
|---|---|---|
| Short-echo MRS (PRESS, TE 30 ms) |
Widely available for clinical use; high false positive rate |
[21,49,50] |
| Long-echo MRS (PRESS, TE 97 ms) |
Less false positives; relies on spectral editing to detect 2HG | [16,37] |
| 7T MRS (PRESS TE 78 ms) |
High signal-to-noise ratio; less available clinically | [45] |
| J-difference MRS | No false positives, increased sensitivity; less available clinically | [38] |
| 2D-COSY | High specificity; less available clinically, complex quantification, low sensitivity, long acquisition time | [38-41] |
| Hyperpolarized 13C, pyruvate | Assessment of metabolic flux; less available clinically |
[46,47] |
| MRSI | Assessment of localization of 2HG; semi-quantitative |
[44] |
Table 1: Summary of in vivo MRS methods used to detect 2HG.
▪ Data post-processing
We have achieved high sensitivity and comparable specificity in detecting 2HG in WHO 2/3 gliomas [49] and glioblastomas [50] with modulation of 2HG resonances by spectral fitting. Good spatial resolution, scanner stability, and absence of motion artifacts are required to reliably disentangle 2HG at 2.25 ppm. Currently available time- and frequency- domain analysis software includes Advanced Method for Accurate, Robust and Efficient Spectral fitting (AMARES) in jMRUI [51] and CFIT [52] respectively. Another popular frequency domain approach is LCModel, which has been widely used to fit in vivo 1H NMR spectra in humans and rodents. We used LCModel software (Stephen Provencher, Oakville, Ontario, Canada) [53] for spectral analysis. This software automatically adjusts the phase and chemical shift of the spectra, estimates the baseline, and performs eddy current corrections. Relative metabolite concentrations and their uncertainties were estimated by fitting the spectrum to a basis set of spectra acquired from individual metabolites in solution. To calculate the absolute metabolite concentrations, an unsuppressed water signal was used as a reference. The precision of spectral fitting is typically reported for individual metabolites in terms of Cramer-Rao lower bounds (CRLB) that sets a lower bound on the variance of the fitting estimate. Quantification is considered unreliable when the CRLB, returned as a percentage of standard deviation (%SD), is over 20% [54]. To achieve precise spectral fitting, shimming should be performed so that full width at half maximum (FWHM) was less than or equal to 10 Hz.
▪ Other metabolic changes detected in IDH mutant gliomas
Significant reduction of Glx (the sum of glutamate (Glu) and glutamine (Gln)) in WHO grade 2-4 IDH mutant gliomas [49,50,55] and reduced glutathione (GSH) in WHO grade 2-3 IDH mutant gliomas [49] compared to IDH wildtype gliomas. In vitro studies which placed isotope labeled glutamine into media growing IDH-mutant cells showed that the labeled carbon is ultimately used by 2HG [8], suggesting that 2HG is primarily derived from glutamine in IDH-mutant gliomas (Figure 1). Glutamine is hydrolyzed by glutaminase to produce glutamate, which is subsequently converted to α-KG [8,56]. Glutaminase inhibitors such as bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl) ethyl sulfide) (BPTES) [56] and 6-diazo-5-oxo- L-noleucine (DON) [57] have been shown to be effective in IDH1-R132H expressing cells. Furthermore, a study injecting hyperpolarized 13C α-KG into rats injected with IDH-mutant glioblastoma cells found that glutamine production is reduced in mutant-IDH gliomas, mainly due to the decrease of branched-chain amino acid transaminase 1 (BCAT1) enzyme, which catalyzes the transamination of branchedchain amino acids while converting α-KG to glutamate [46]. A report from Nagashima et al., suggested that elevated 2HG as well as decreased Glu is diagnostic for IDH-mutant gliomas [55]. GSH, an important antioxidant, is a tripeptide formed from Glu, as well as cysteine and glycine (Figure 1). This metabolite was also decreased in WHO grade 2/3 gliomas [49]. This is in agreement with preclinical studies showing that IDH1-mutation in gliomas depletes GSH production [58,59], rendering IDH-mutant gliomas sensitive to radiation [60,61]. Susceptibility to reactive oxygen species may be another reason for the relatively good survival in IDH mutant gliomas. Furthermore, a metabolomic study looking at IDH1 and IDH2 mutations showed reduction of N-acetylaspartyl- glutamate (NAAG) in cells expressing both IDH1 and IDH2 mutation [62].
▪ Clinical relevance and future implications
Quantitative measurement of 2HG by MRS has potential therapeutic implications, although an almost 100% sensitivity and specificity in detecting IDH mutations would be necessary to make therapeutic decisions based on this measurement. Reliable detection of 2HG by MRS would make non-invasive, preoperative analysis of IDH mutations in glioma patients possible. Second, determination of therapeutic response after surgery and chemoradiotherapy is possible by serial evaluation of 2HG [63,64]. Mutant IDH1 inhibitors, which are known to deplete 2HG, are being evaluated in clinical trials for mutant-IDH gliomas [65-69], so monitoring of therapeutic response by MRS would be rational. Third, since all IDH mutations are known to produce 2HG [70], detection of rare IDH1 and IDH2 mutations are possible. Fourth, for select cases of gliomas near eloquent areas, total removal of the tumor may be feasible after neoadjuvant chemotherapy [71] or radiation. Since no other brain tumor besides gliomas, including ependymomas and medulloblastomas, have IDH mutations [4], detection of 2HG is diagnostic for IDH-mutant gliomas, thus a biopsy for pathological diagnosis may be omitted. A clinical report indicates the sensitivity of secondary glioblastomas to temozolomide [72], and is a candidate for neoadjuvant chemotherapy in mutant-IDH gliomas. Also, preclinical studies IDH1 R132H mutations render cells to be more sensitive to radiation [60,61]. Furthermore, a retrospective analysis suggests a survival benefit for radical surgery, including FLAIR hyperintensity areas, in mutant-IDH but not wildtype-IDH gliomas [73]. Preoperative evaluation of 2HG by MRS may be helpful in determining indication for radical surgery.
IDH mutation triggers widespread effects, including histone and DNA hypermethylation and metabolic changes such as increased glutamine metabolism and decreased glutathione production. Recent evidence points to the production of 2HG as the main cause of these effects. Furthermore, new treatments targeting IDH mutations are currently being evaluated. Technical advances are being made to reliably detect 2HG by MRS in glioma patients which will lead to making clinical decisions with this data.
References
- Parsons DW, Jones C, Zhang X et al. An integrated genomic analysis of human glioblastoma multiforme. Science 321(5897), 1807-1812 (2008).
- Balss J, Meyer J, Mueller W et al. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta. Neuropathol 116(6), 597-602 (2008).
- Ohgaki H, Kleihues P. Genetic profile of astrocytic and oligodendroglial gliomas. Brain. Tumor. Pathol 28(3), 177-183 (2011).
- Yan H, Parsons DW, Jin G et al. IDH 1 and IDH 2 Mutations in Gliomas. N. Engl. J. Med 360(8), 765-773 (2009).
- Dunn GP, Andronesi OC, Cahill DP. From genomics to the clinic: biological and translational insights of mutant IDH1/2 in glioma. Neurosurg. Focus 34(2), 1-15 (2013).
- Ogura R, Tsukamoto Y, Natsumeda M et al. Immunohistochemical profiles of IDH1, MGMT and P53: practical significance for prognostication of patients with diffuse gliomas. Neuropathology 35(4), 324-335 (2015).
- Yan H, Parsons DW, Jin G et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med 360(8), 765-773 (2009).
- Dang L, White DW, Gross S et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462(7274), 739-744 (2009).
- Pietrak B, Zhao H, Qi H et al. A tale of two subunits: how the neomorphic R132H IDH1 mutation enhances production of alphaHG. Biochemistry 50(21), 4804-4812 (2011).
- Arita H, Yamasaki K, Matsushita Y et al. A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta. Neuropathol. Commun 4(1), 79 (2016).
- Lu C, Ward PS, Kapoor GS et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483(7390), 474-478 (2012).
- Chowdhury R, Yeoh KK, Tian YM et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO. Rep 12(5), 463-469 (2011).
- Xu W, Yang H, Liu Y et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer. Cell 19(1), 17-30 (2011).
- Figueroa ME, Abdel-Wahab O, Lu C et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer. cell 18(6), 553-567 (2010).
- Turcan S, Rohle D, Goenka A et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 483(7390), 479-483 (2012).
- Choi C, Ganji S, Hulsey K et al. A comparative study of short- and long-TE (1)H MRS at 3 T for in vivo detection of 2-hydroxyglutarate in brain tumors. NMR. Biomed 26(10), 1242-1250 (2013).
- Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR. Biomed 13129-153 (2000).
- Igarashi H, Suzuki Y, Huber VJ et al. N-acetylaspartate decrease in acute stage of ischemic stroke: a perspective from experimental and clinical studies. Magn. Reson. Med. Sci 14(1), 13-24 (2015).
- Dehghani M, Kunz N, Lanz B et al. Diffusion-weighted MRS of acetate in the rat brain. NMR. Biomed 30(10), (2017).
- Struys EA. 2-Hydroxyglutarate is not a metabolite; D-2-hydroxyglutarate and L-2-hydroxyglutarate are! Proc. Natl. Acad. Sci. U. S. A 110(51), E4939 (2013).
- Pope WB, Prins RM, Albert Thomas M et al. Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J. Neurooncol 107(1), 197-205 (2012).
- Losman JA, Kaelin WG Jr. What a difference a hydroxyl makes: mutant IDH, (R)-2-hydroxyglutarate, and cancer. Genes. Dev 27(8), 836-852 (2013).
- Intlekofer AM, Dematteo RG, Venneti S et al. Hypoxia Induces Production of L-2-Hydroxyglutarate. Cell. metabolism 22(2), 304-311 (2015).
- Oldham WM, Clish CB, Yang Y et al. Hypoxia-Mediated Increases in L-2-hydroxyglutarate Coordinate the Metabolic Response to Reductive Stress. Cell. metabolism 22(2), 291-303 (2015).
- Terunuma A, Putluri N, Mishra P et al. MYC-driven accumulation of 2-hydroxyglutarate is associated with breast cancer prognosis. J. Clin. Inv 124(1), 398-412 (2014).
- Kalinina J, Ahn J, Devi NS et al. Selective Detection of the D-enantiomer of 2-Hydroxyglutarate in the CSF of Glioma Patients with Mutated Isocitrate Dehydrogenase. Clin. Cancer. Res 22(24), 6256-6265 (2016).
- Locasale JW, Melman T, Song S et al. Metabolomics of human cerebrospinal fluid identifies signatures of malignant glioma. Mol. Cell. Proteomics 11(6), M111 014688 (2012).
- Fathi AT, Nahed BV, Wander SA et al. Elevation of Urinary 2-Hydroxyglutarate in IDH-Mutant Glioma. The. Oncologist 21214-219 (2016).
- Capper D, Simon M, Langhans C-D et al. 2-Hydroxyglutarate concentration in serum from patients with gliomas does not correlate with IDH1/2 mutation status or tumor size. Int. J. Canc 131(3), 766-768 (2012).
- Fathi AT, Sadrzadeh H, Borger DR et al. Prospective serial evaluation of 2-hydroxyglutarate, during treatment of newly diagnosed acute myeloid leukemia, to assess disease activity and therapeutic response. Blood 120(23), 4649-4652 (2012).
- DiNardo CD, Propert KJ, Loren AW et al. Serum 2-hydroxyglutarate levels predict isocitrate dehydrogenase mutations and clinical outcome in acute myeloid leukemia. Blood 121(24), 4917-4924 (2013).
- Wang JH, Chen WL, Li JM et al. Prognostic significance of 2-hydroxyglutarate levels in acute myeloid leukemia in China. Proc. Natl. Acad. Sci. U. S. A 110(42), 17017-17022 (2013).
- Janin M, Mylonas E, Saada V et al. Serum 2-hydroxyglutarate production in IDH1- and IDH2-mutated de novo acute myeloid leukemia: a study by the Acute Leukemia French Association group. J. Clin. Oncol 32(4), 297-305 (2014).
- Balss J, Thiede C, Bochtler T et al. Pretreatment d-2-hydroxyglutarate serum levels negatively impact on outcome in IDH1-mutated acute myeloid leukemia. Leukemia 30(4), 782-788 (2016).
- Sullivan JP, Nahed, BV, Madden MW et al. Brain tumor cells in circulation are enriched for mesenchymal gene expression. Cancer. Discov 4(11), 1299-1309 (2014).
- Verhaak RG, Hoadley KA, Purdom E et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer. Cell 17(1), 98-110 (2010).
- Choi C, Ganji SK, DeBerardinis RJ et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nature. Medicine 18(4), 624-629 (2012).
- Andronesi OC, Kim GS, Gerstner E et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci. Transl. Med 4(116), 116ra114 (2012).
- Elkhaled A, Jalbert LE, Phillips JJ et al. Magnetic resonance of 2-hydroxyglutarate in IDH1-mutated low-grade gliomas. Sci. Transl. Med 4(116), 116ra115 111-110 (2012).
- Esmaeili M, Vettukattil R, Bathen TF. 2-hydroxyglutarate as a magnetic resonance biomarker for glioma subtyping. Transl. Oncol 6(2), 92-98 (2013).
- Verma G, Mohan S, Nasrallah MP et al. Non-invasive detection of 2-hydroxyglutarate in IDH-mutated gliomas using two-dimensional localized correlation spectroscopy (2D L-COSY) at 7 Tesla. J. Transl. Med 14(1), 274 (2016).
- Andronesi OC, Rapalino O, Gerstner E et al. Detection of oncogenic IDH1 mutations using magnetic resonance spectroscopy of 2-hydroxyglutarate. J. Clin. Inv 123(9), 3659-3663 (2013).
- Kim H, Kim S, Lee HH et al. In-Vivo Proton Magnetic Resonance Spectroscopy of 2-Hydroxyglutarate in Isocitrate Dehydrogenase-Mutated Gliomas: A Technical Review for Neuroradiologists. Korean. J. Radiol 17(5), 620-632 (2016).
- Jafari-Khouzani K, Loebel F, Bogner W et al. Volumetric relationship between 2-hydroxyglutarate and FLAIR hyperintensity has potential implications for radiotherapy planning of mutant IDH glioma patients. Neuro. Oncol 18(11), 1569-1578 (2016).
- Ganji SK, An Z, Tiwari V et al. In vivo detection of 2-hydroxyglutarate in brain tumors by optimized point-resolved spectroscopy (PRESS) at 7T. Magn. Reson. Med 77(3), 936-944 (2016).
- Chaumeil MM, Larson PE, Woods SM et al. Hyperpolarized [1-13C] glutamate: a metabolic imaging biomarker of IDH1 mutational status in glioma. Cancer. Res 74(16), 4247-4257 (2014).
- Chaumeil MM, Radoul M, Najac C et al. Hyperpolarized (13)C MR imaging detects no lactate production in mutant IDH1 gliomas: Implications for diagnosis and response monitoring. Neuroimage. Clin 12180-189 (2016).
- Hu J, Salzillo TC, Sailasuta N et al. Interrogating IDH mutation in brain tumor: magnetic resonance and hyperpolarization. Top. Magn. Reson. Imaging 2627-32 (2017).
- Natsumeda M, Igarashi H, Nomura T et al. Accumulation of 2-hydroxyglutarate in gliomas correlates with survival: a study by 3.0-tesla magenetic resonance spectroscopy. Acta. Neuropathologica. Communications 2(158), (2014).
- Natsumeda M, Motohashi K, Igarashi H et al. Reliable diagnosis of IDH-mutant glioblastoma by 2-hydroxyglutarate detection: a study by 3-tesla magnetic resonance spectroscopy Neurosurg. Rev (2017).
- Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J. Magn. Reson 129(1), 35-43 (1997).
- Gabr RE, Ouwerkerk R, Bottomley PA. CFIT: A novel circle-fitting approach to spectral analysis. Proc. Intl. Soc. Mag. Reson. Med 142529 (2006).
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med 30(6), 672-679 (1993).
- Graveron-Demilly D. Quantification in magnetic resonance spectroscopy based on semi-parametric approaches. MAGMA 27(2), 113-130 (2014).
- Nagashima H, Tanaka K, Sasayama T et al. Diagnostic value of glutamate with 2-hydroxyglutarate in magnetic resonance spectroscopy for IDH1 mutant glioma. Neuro. Oncol 18(11), 1559-1568 (2016).
- Seltzer MJ, Bennett BD, Joshi AD et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Canc. Res 70(22), 8981-8987 (2010).
- Ohka F, Ito M, Ranjit M et al. Quantitative metabolome analysis profiles activation of glutaminolysis in glioma with IDH1 mutation. Tumour. Biol 35(6), 5911-5920 (2014).
- Shi J, Zuo H, Ni L et al. An IDH1 mutation inhibits growth of glioma cells via GSH depletion and ROS generation. Neurol. Sci Advance online publishing (2013).
- Shi J, Sun B, Shi W et al. Decreasing GSH and increasing ROS in chemosensitivity of gliomas with IDH1 mutation. Tumor. Biol 36(2), 655-662 (2014).
- Li S, Chou AP, Chen W et al. Overexpression of isocitrate dehydrogenase mutant proteins renders glioma cells more sensitive to radiation. Neuro. Oncol 15(1), 57-68 (2013).
- Kessler J, Guttler A, Wichimann H et al. IDH1(R132H) mutation causes a less aggressive phenotype and radiosensitizes human malignat glioma cells independent of the oxygenation status. Radiother. Oncol 116(3), 381-387 (2015).
- Reitman ZJ, Jin G, Karoly ED et al. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc. Natl. Acad. Sci. U. S. A 108(8), 3270-3275 (2011).
- Dunn GP, Andronesi OC, Cahill DP. From genomics to the clinic: biological and translational insights of mutant IDH1/2 in glioma. Neurosurg. Focus 34(2), E2 1-15 (2013).
- Andronesi OC, Loebel F, Bogner W et al. Treatment response assessment in IDH-mutant glioma patients by non-invasive 3D functional spetroscopic mapping of 2-hydroxyglutarate. Clin. Can. Res 22(7), 1632-1641 (2016).
- Rohle D, Popovici-Muller J, Palaskas N et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 340(6132), 626-630 (2013).
- Davis M PR, Popovici-Muller J, Gross S, Thorne N, Salituro F, Fantin V, Straley K, Su M, Dang L, Simeonov A, Shen M, Boxer MB. ML309: A potent inhibitor of R132H mutant IDH1 capable of reducing 2-hydroxyglutarate production in U87 MG glioblastoma cells. Probe Reports from the NIH Molecular Libraries Program (2012).
- Popovici-Muller J, Saunders JO, Salituro FG et al. Discovery of the First Potent Inhibitors of Mutant IDH1 That Lower Tumor 2-HGin Vivo. ACS. Med.Chem. Lett 3(10), 850-855 (2012).
- Zheng M, Sun M, Gao S et al. Structure based discovery of clomifene as a potent inhibitor of cancer-associated mutant IDH1 Oncotarget (2017).
- Kopinja J, Sevilla RS, Levitan D et al. A Brain Penetrant Mutant IDH1 Inhibitor Provides In Vivo Survival Benefit. Sci. Rep 7(1), 13853 (2017).
- Ward PS, Patel J, Wise DR et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Canc. Cell 17(3), 225-234 (2010).
- Sasaki H, Nishiyama Y, Yoshida K. Neoadjuvant therapy in the treatment of brain tumors. Jpn. J. Canc. Chemother 41709-712 (2014).
- SongTao Q, Lei Y, Si G et al. IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer. Sci 103(2), 269-273 (2012).
- Beiko J, Suki D, Hess KR et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro. Oncology 16(1), 81-91 (2014).