Research Article - Neuropsychiatry (2018) Volume 8, Issue 2
Addenbrooke’s Cognitive Examination in Nondemented Patients after Stroke
- *Corresponding Author:
- Hana Zakopcanova Srovnalova, MD, Ph.D
Department of Neurology, University Hospital in Ostrava, 17. listopadu 1790, Ostrava-Poruba 70800, Czech Republic
Tel: +420597373083
Abstract
Abstract
Introduction: Cognitive deficit after stroke is common, and beginning cognitive rehabilitation as soon as possible is important to minimize the consequences of the impairment. The aim of this study was to use Addenbrooke’s Cognitive Examination to compare cognitive function in nondemented and nondepressed stroke patients, 3–6 months after the stroke, with sex- and age-matched controls.
Materials and Methods: A total of 156 participants were included (72 controls: 19 men, mean age 64.5 ± 12.4 years; 84 patients after stroke: 54 men, mean age 62.2 ± 9.0 years).
Keywords
Stroke, Cognitive functions, Cognitive deficit, Addenbrooke’s cognitive examination
Introduction
A stroke can involve both physical and cognitive impairments, although few studies have focused on cognitive deficits. The effect on cognitive abilities can be observed in every area: memory, attention, executive functions (i.e., decision making, organization, problem solving), visuospatial abilities, speech, thought, and symbolic functions [1,2]. Impairment of cognitive functions following stroke is very common but depends on stroke extent and region [2-5]. Some authors report the development of cognitive deficit within 3 months following a stroke, but it may develop even after this period. In patients evaluated 3 months after a stroke, impairment of executive function and attention have been confirmed, and those with problems in orientation, memory, and expressive language have a higher risk of progressing to dementia [6].
To describe mild cognitive deficit, some authors use the term ‘vascular mild cognitive impairment’, which may be understood as equivalent to the term ‘mild cognitive impairment’ (MCI) used in patients with neurodegenerative conditions such as Alzheimer’s disease (AD) [7]. The group of cognitive deficits with a vascular basis includes all types caused by cerebrovascular impairment, with variable severity of the deficit from mild trouble with cognitive functions (MCI) to dementia. Thus, vascular MCI is a syndrome associated with a vascular impairment of the brain that affects at least one cognitive domain [7].
The Addenbrooke’s Cognitive Examination (ACE)–Revised (ACE-R) is a screening tool used for detection of cognitive deficit in acute and chronic cerebral lesions [4]. The ACE-R allows for scoring in the domains of visuospatial skills, memory, attention, and executive function [8]. ACE-R is less time consuming than neuropsychological investigation and less burdensome for patients. Its application can speed up the diagnostic process of mild cognitive deficit and consequent treatment (pharmacological and non-pharmacological) intervention, such as cognitive rehabilitation [9].
Many studies have focused on cognitive deficit/ dementia in neurodegenerative disease, such a progressive supranuclear palsy (PSP), multiple system atrophy (MSA), AD, and Parkinson’s disease (PD), confirming the cognitive decline in these diseases. Krishnan, Mathuranath, Sarma, and Kishore [10] compared cognitive functions in patients with PD, MSA, and PSP and found the worst declines in PD patients, followed by those with PSP, and the least decline among those with MSA. The ACE-R Verbal Fluency domain distinguishes between PSP and PD with high sensitivity (0.92) and high specificity (0.87), and the total ACE-R score have specificity 0.87 and sensitivity 0.7 and visuospatial subscore have a specificity 0.84 and sensitivity 0.73 [11]. The sensitivity of ACE-R for detection of cognitive decline in PD was confirmed in another study that showed a performance difference on the ACE-R total score between PD and healthy controls [12]. The ability of ACE-R to detect cognitive decline in AD was confirmed in further studies [13].
A newly developed version of the ACE, known as ACE-III, is ready for use [14], but unfortunately a Czech version is not available. The maximum score for the ACE-III is 100 points, and items related to the Mini Mental State Exam (MMSE) have been excluded. Some remaining items have been modified, as well. In the domain Attention and Orientation, for example, it is not possible to substitute subtracting 7 sequentially from 100 with spelling words backwards. In the Language domain, for the item “Writing the sentence,” the participant is asked to write two or more sentences about a single topic. Two sayings now replace the phrase repetition items. For item naming, “pencil” and “watch” have been replaced by more familiar objects: “spoon” and “book.” In the Visuospatial Functions domain, overlapping infinity loops have replaced the intersecting pentagons. This new version of ACEIII is available for free at the website, (http:// dementiaroadmap.info/southgloucestershire/ resources/addenbrookes-cognitive-examinationiii/#. WFJbnPnhBPb) [14].
The aim of this study was to use ACE-R as a screening test to monitor and compare the level of cognitive function between a group of nondemented and nondepressed patients at 3-6 months following a stroke, and a group of age and sex-matched controls. Furthermore, we also compared subgroups of stroke patients (internal carotid artery right/left lesion with mild neurological deficit) and controls to see if stroke features such as region influence cognitive deficit. We hypothesized that the ACE-R can be a useful screening test for the rapid inclusion of patients 3–6 months after a stroke in cognitive rehabilitation [15].
Materials and Methods
▪ Patients
Patients were selected at the University Hospital in Ostrava from 2012–2015. Sex- and agematched participants without a history of brain injury who were hospitalized in the Department of Neurology were selected as the control group.
Inclusion criteria were as follows: MMSE score ≥ 25 points; self-sufficiency (modified Rankin score (mRS) ≤ 2 points); no problem in selfsufficiency in activities based on the Activities of Daily Living (ADL) and Instrumental ADL (iADL) scales; and signed, informed consent. Exclusion criteria were Beck Depression Inventory score suggesting depression; seriously impaired vision or hearing; known central nervous system disease, including brain injury, neuroinfection, dementia, and unconsciousness longer than 2 hours; known psychiatric disorder including depression; substance abuse (alcohol or psychoactive substances); aphasia; and terminal stage of any disease. Stroke patients were divided into two subgroups according to the side of the ischemic lesion.
The study was conducted in accordance with the Helsinki Declaration of 1964 and was approved by the local Ethics Committee of University Hospital in Ostrava. All participants signed informed consent.
▪ Tests
The Czech version of ACE-R [16], ADL, iADL, and Beck Depression Inventory II were administered to all participants. The test battery included MMSE, a global score for ACE-R (100 points), and scores for the individual cognitive domains (Attention and Orientation, total score 18 points; Memory, 26 points; Verbal Fluency, 14 points; Language, 26 points; and Visuospatial Abilities, 16 points) [9].
Within the domain Attention and Orientation, the participant was asked for information about day of the week, age, date, month, year, season, town, state, region, where they live, the name of the hospital, and the floor for the outpatient department. The domain Memory covers tasks related to short-term memory and the ability to recall new information. First, the patient has to repeat after the examiner three words that they are to recall for the moment. This test is followed by deducting 7 from the number 100 as an overlay for the newly stored memory traces (the deduction is repeated 5 times until the result is 65). After this task, the patient is asked to repeat the previously presented three words.
Following a memory task of learning an address, which is presented three times, after each reading, the patient is prompted to repeat the addresses, observing whether their performance improves with each new attempt (each attempt should demonstrate better patient performance). Patients also choose this address from three options at the end of the test to determine whether and to what extent the new information was stored in the short-term memory. This part is followed by tasks for long-term memory, in which the participant is asked about information such as the name of the prime minister, the first president of their country, the current president of their country, etc.
In the domain Verbal Fluency, the participant has to say as many words as possible with the letter “p” in one minute, which cannot include capitalized words or phrases with the same root (paint–painter, etc.) (semantic Verbal Fluency). The second part of the domain Verbal Fluency is to say as many animals as possible (categorical Verbal Fluency). In the domain Language, the participant names the pictures, repeats the words and sentences, and performs simple tasks to establish understanding and speech expression. Within the domain Visuospatial Functions, the participant draws pictures according to the draft (a cube, two overlapping pentagons), completes the Clock Test, counts dots without pointing a finger, and reads “imperfectly” printed letters.
▪ Statistics
For comparison of monitored parameters (MMSE, ACE-R and its individual domains) in both groups (controls vs patients), parametric and non-parametric methods were used, and normal distributions were tested. The cutoff scores for sensitivity and specificity in the statistically significant domain score were established using receiver operating characteristic analysis. Sample size was determined by power analysis: to achieve adequate statistical power (0.8) with a 5% type I error rate and expected effect size d=0.5 required a minimum sample size of 63.
The Kolmogorov–Smirnov test showed that all variables were non-normally distributed. Therefore, the Kruskal–Wallis tests, followed by a series of Mann–Whitney U tests, were conducted for all comparisons. For statistical analysis, descriptive statistics were used (mean, standard deviation, frequency; Table 1), and significance was assumed at 0.05 All analyses were completed using the Statistical Package for the Social Sciences (IMB SPSS 23.0).
| All | Male | Female | Age (mean) | Education (y) | |
|---|---|---|---|---|---|
| Stroke patients | 84 | 54 | 30 | 62.1 ± 12.4 | 13.2 ± 2.3 |
| Right-sided lesion | 44 | 26 | 18 | 62.4 ± 11.6 | 13.1 ± 2.1 |
| Left-sided lesion | 40 | 28 | 12 | 61.2 ± 13.6 | 13.5 ± 2.5 |
| Controls | 72 | 19 | 53 | 64.5 ± 8.9 | 13.3 ± 2.5 |
Table 1: Demographic characteristics.
Results
A total of 156 patients without dementia were enrolled in our study, of whom 84 were patients who had experienced a stroke 3–6 months previously (54 men, 30 women; mean age 62.2 ± 12.4 years; 44 patients with right lesions and 40 patients with left lesions) and 72 were previously healthy controls (19 men, 54 women; mean age 64.5 ± 9.0 years) (Table 1). Risk factors for stroke patients were arterial hypertension (63%), diabetes mellitus (18%), hyperlipidemia (48%), arrhythmia (13%), thrombophilia (5%), smoking (48%), and alcohol use (31%). Control participants with these risk factors for stroke were excluded from the study. Table 2 lists the clinical neurological condition of the participants, and Table 3 provides an overall clinical assessment as the mRS. All patients experienced only one episode of stroke. Localization of brain lesions was performed using computed tomography. All patients were right-handed, and they suffered from visual impairment, severe motor impairment, or aphasia.
| Upper limb paresis | Lower extremity paresis | Hypoesthesia of the upper limb | Hypoesthesia of the lower extremity | Central paresis of n. Facialis | |
|---|---|---|---|---|---|
| Left | 32 | 27 | 15 | 16 | 26 |
| Right | 24 | 20 | 9 | 7 | 13 |
| Speech disorders | |||||
| Type of speech disorder | Dysarthria | Expressive phatic disorder | Perception phatic disorder | Mixed phatic disorder | |
| Number of patients with speech disorder | 22 | 9 | 1 | 5 | |
Table 2: Clinical neurological condition of stroke patients.
| Modified Rankin score | % |
|---|---|
| 0 | 80 |
| I | 9 |
| II | 5 |
| III | 2 |
| IV | 3 |
| V | 0 |
Table 3: Self-sufficiency in stroke patients.
We used parametric and non-parametric statistical methods to cross-check results (Table 4). Based on the Kolmogorov–Smirnov test and inspection of histograms, almost all variables exhibited abnormal distribution. T-tests for independent samples demonstrated a statistically significant difference between the groups in the total score of ACE-R (patient score, 86.2 points vs controls, 91.2 points; p<0.01), Verbal Production domain (patients, 9.8 points vs controls, 11.5 points; p<0.01), and Memory domain (patients, 19.5 points vs controls, 21.7 points; p<0.01). A difference between stroke patient subgroups for left lesion (SPL) and right lesion (SPR) was not statistically significant. A statistically significant difference was identified, however, between the SPR and control groups in total ACE-R score (SPR, 88.3 vs controls, 91.3 points; p<0.05) and Verbal Production (SPR, 9.9 points vs controls, 11.5 points; p<0.01) domains. The SPL and control groups also differed in ACE-R global score (83.9 vs. 91.3 points, respectively; p<0.01) and for the Memory (18.6 vs 21.7 points, respectively; p<0.01) and Verbal Fluency (9.6 vs 11.5 points, respectively; p<0.01) domains (Table 4). The SPR group was not affected in the Memory domain; only in the Verbal Production domain.
| Kruskal–Wallis | T tests Mann–Whitney test | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients (n=84) | Controls (n=72) | p | Right-sided stroke (n=44) | Left-sided stroke (n=40) | p | Right-sided stroke (n=44) | Controls (n=72) | p | Left-sided stroke (n=40) | Controls (n=72) | p | Patients | Controls | P | |||||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | Median | Median | ||||||||
| MMSE | ≤0.242 | 28.54 | 1.46 | 28.82 | 1.25 | ≤0.272 | 28.75 | 1.31 | 28.30 | 1.59 | ≤0.215 | 28.75 | 1.31 | 28.82 | 1.25 | ≤0.820 | 28.30 | 1.59 | 28.82 | 1.25 | ≤0.114 | 29 | 29 | ≤0.141 | |
| ACE-R | ≤0.001 | 86.19 | 9.88 | 91.26 | 5.28 | ≤0.001 | 88.27 | 7.36 | 83.90 | 11.74 | ≤0.080 | 88.27 | 7.36 | 91.26 | 5.28 | ≤0.046 | 83.90 | 11.74 | 91.26 | 5.28 | ≤0.001 | 88 | 92 | ≤0.001 | |
| AO | ≤0.504 | 17.40 | 1.03 | 17.43 | 0.85 | ≤0.610 | 17.52 | 0.90 | 17.28 | 1.15 | ≤0.306 | 17.52 | 0.90 | 17.43 | 0.85 | ≤0.305 | 17.28 | 1.15 | 17.43 | 0.85 | ≤0.853 | 18 | 18 | ≤0.31 | |
| M | ≤0.004 | 19.48 | 4.59 | 21.68 | 3.12 | ≤0.003 | 20.30 | 4.14 | 18.58 | 4.93 | ≤0.149 | 20.30 | 4.14 | 21.68 | 3.12 | ≤0.100 | 18.58 | 4.93 | 21.68 | 3.12 | ≤0.002 | 20 | 23 | ≤0.001 | |
| VF | ≤0.001 | 9.75 | 2.85 | 11.53 | 1.84 | ≤0.001 | 9.93 | 2.76 | 9.55 | 2.97 | ≤0.576 | 9.93 | 2.76 | 11.53 | 1.84 | ≤0.001 | 9.55 | 2.97 | 11.53 | 1.84 | ≤0.001 | 10 | 12 | ≤0.001 | |
| L | ≤0.469 | 25.32 | 1.21 | 25.21 | 1.34 | ≤0.495 | 25.45 | 1.04 | 25.18 | 1.38 | ≤0.307 | 25.45 | 1.04 | 25.21 | 1.34 | ≤0.247 | 25.18 | 1.38 | 25.21 | 1.34 | ≤0.974 | 26 | 26 | ≤0.249 | |
| VA | ≤0.566 | 14.88 | 1.79 | 15.40 | 0.85 | ≤0.400 | 15.02 | 1.64 | 14.73 | 1.95 | ≤0.544 | 15.02 | 1.64 | 15.40 | 0.85 | ≤0.738 | 14.73 | 1.95 | 15.40 | 0.85 | ≤0.312 | 29 | 29 | ≤0.22 | |
MMSE: Mini Mental State Examination; ACE-R: Addenbrooke’s Cognitive Examination–Revised; AO: Domain Attention and Orientation in ACE-R; M: Domain Memory in ACE-R; VF: Domain Verbal production in ACE-R; L: Domain Language in ACE-R; VA: Domain Visuospatial Abilities in ACE-R
Table 4: Differences between controls and stroke patients in ACE-R global and domain scores.
Subsequent pairwise comparison of the three groups (two stroke patient subgroups and one control group) using the Mann–Whitney U test (with Bonferroni correction) revealed no significant differences among those with right or left ischemic stroke. Stroke patients and controls differed based on Mann–Whitney U test results. As Table 4 shows, patients with ischemic stroke had significantly lower overall ACE-R global, Memory, and Verbal Fluency scores. In a comparison of the three groups using the Kruskal–Wallis test, they differed significantly in terms of ACE-R overall score and Memory and Verbal Production domain scores (Table 4).
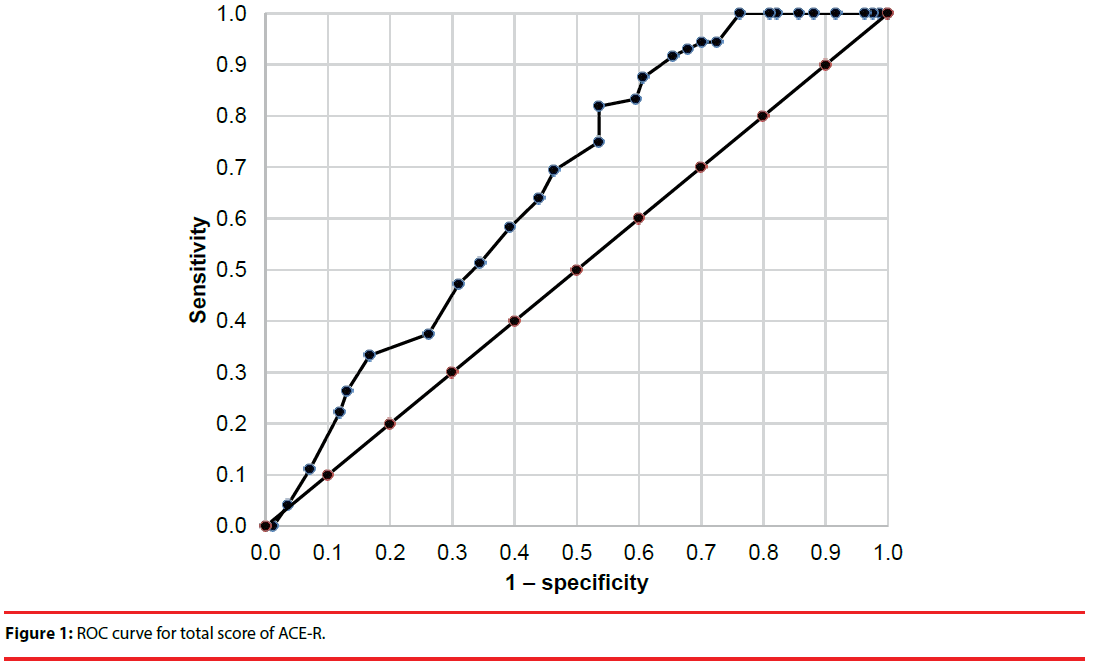
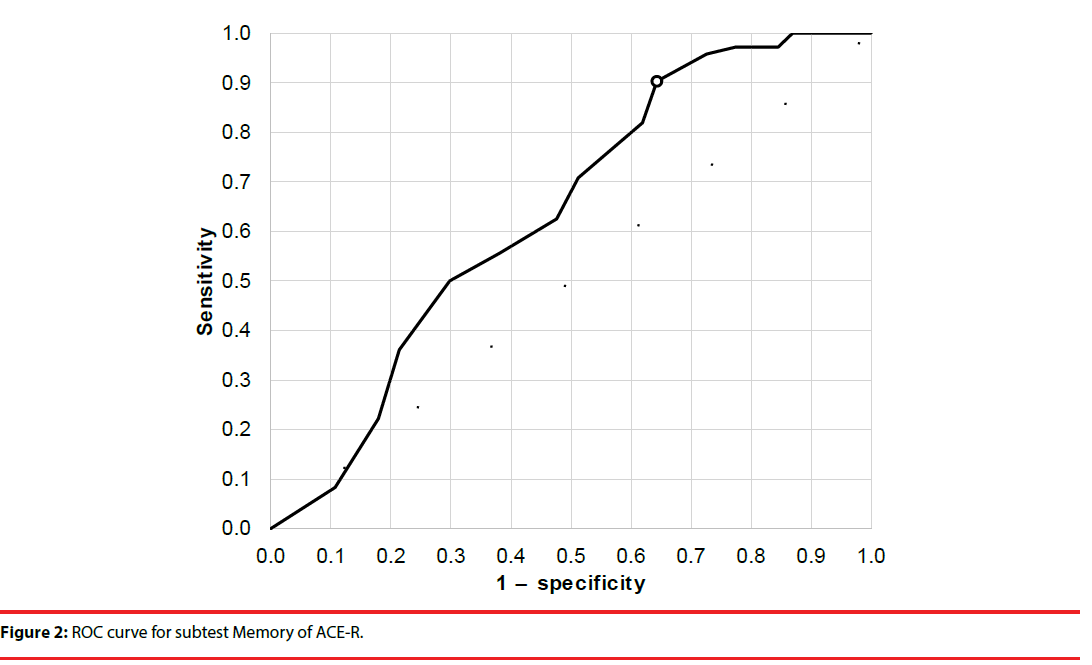
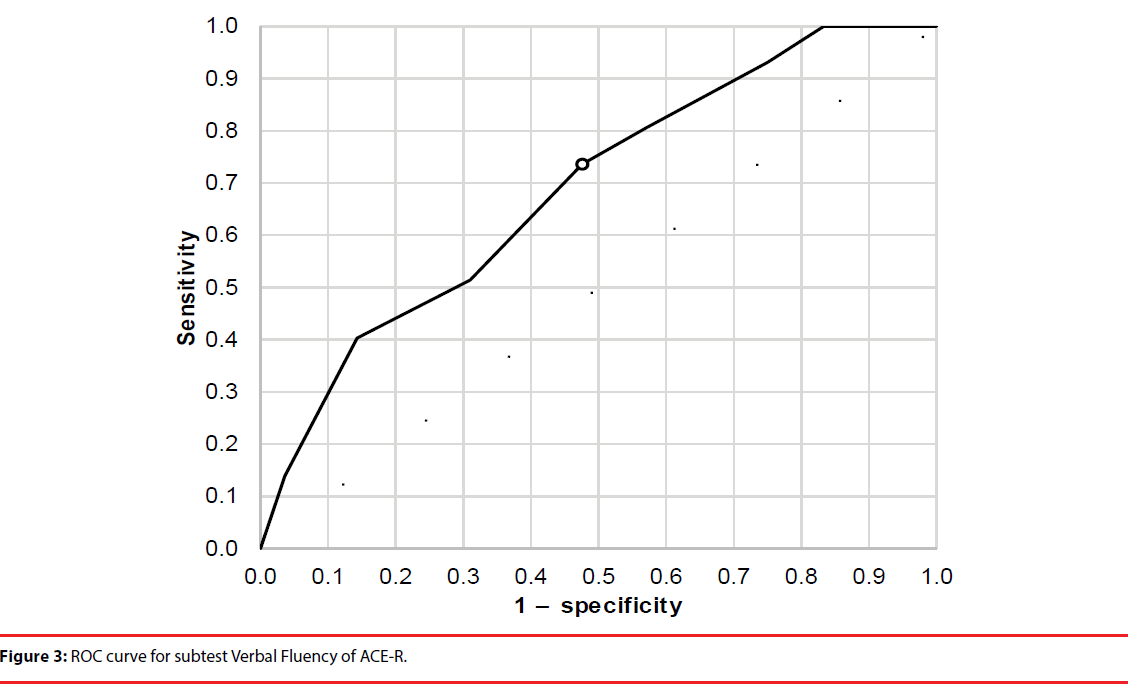
The cutoff scores and sensitivity and specificity for total ACE-R score and the Verbal Fluency and Memory domains were established using receiver operating characteristic analysis (Figures 1-3). Cutoffs for total ACE-R score and subtests to distinguish controls from stroke patients were set where the cumulated sensitivity and specificity reached their maximum. For the total ACE-R score, this value was 86.5 points (sensitivity 0.82, specificity 0.46; area under the curve, 0.66). For the Memory domain, the cutoff was 17.5 points (sensitivity 0.90, specificity 0.36; area under the curve, 0.64); for the Verbal Fluency subtest, it was 10.5 points (sensitivity 0.74, specificity 0.52; area under the curve, 0.69).
In comparing ACE-R and MMSE (Table 5), it was clear that ACE-R gave far more detailed information on cognitive decline than MMSE.
| Subtest/domain in ACE-R | ACE-R score; maximum number of points in individual domain | MMSE score; maximum number of points (evaluating ACE-R) compared to MMSE |
|---|---|---|
| Attention and orientation | 18 | Yes; 18 of 18 |
| Memory | 26 | Yes; 3 of 26 |
| Verbal fluency | 14 | No |
| Language | 26 | Yes; 8 of 26 |
| Visuospatial abilities | 16 | Yes; 1 of 14 |
| Total score | 100 | 30 |
Table 5: Comparison of ACE-R and MMSE in subtests/domain and their representation.
Discussion
Increasingly younger patients are affected by stroke [17], and long-term outcomes are a considerable concern. Death and permanent disability risks persist in the 4 years following the stroke, which also are associated with higher institutionalization rates [1]. Some of these patients suffer post-stroke dementia, but others without dementia still experience cognitive deficit. Because neuropsychological testing is time consuming and can lead to delays in accessing rehabilitation, a less intensive way to evaluate cognition is needed. With timely application, patients could be enrolled in cognitive rehabilitation programs within a reasonable period after a stroke to eliminate the progression of a cognitive deficit into dementia. ACE-R is a fast test of cognitive profile and deficits that takes approximately 15 minutes [18] and is less burdensome than a detailed neuropsychological assessment [19]. It has previously been shown to have good sensitivity and specificity for diagnosing MCI in vascular cognitive impairment [20] and can detect cognitive deficit that will manifest in the domain of cognitive functions, attention, and impairment of visuospatial abilities [8] in patients following a stroke.
In our study, we identified a statistically significant difference between controls and stroke patients in total ACE-R score and Verbal Production and Memory domain scores. A difference based on affected region was not found, but statistically significant differences in in total ACE-R test scores were observed between the SPL group and healthy controls. A statistically significant difference was also demonstrated between SPL and control groups, with higher scores among controls for global ACE-R and the Memory and Verbal Fluency domains.
Similar to our study, the statistically significant difference between controls and stroke patients in global ACE-R score may explain the lower scores for the Verbal Fluency and Memory domains, both of which are associated with impairment in executive function [21,22]. These impairments occur after a stroke in up to 75% of cases [23].
Morris conducted a study with a design similar to ours, but tested patients with acute stroke and found impairment in visuospatial, attention, and executive functions [8]. In contrast, our study tested patients at 3–6 months after ischemic stroke, and we used different tests.
The brain exhibits functional plasticity [24], and patients after a stroke enrolled in neuroimaging studies show changes in brain synapses, which points towards some functional reorganization following the insult. This reorganization may be the basis for restoration of damaged functions after a stroke, but we do not know the limits of these changes and what areas they may influence [25]. The time of 3–6 months after a stroke seems to be the best for evaluation of cognitive functions in patients and simultaneously the time to start cognitive rehabilitation (after spontaneous healing) [26]. For this reason, we studied patients at 3–6 months post stroke, when the functional reorganization process could be stabilized [27].
The global score differences in stroke patients and controls in our study suggest MCI in the former. A cutoff score of 88 points on the global ACE-R has been reported for the Czech population, with 100% sensitivity, or a cutoff of 83 points for 96.6% sensitivity [16]. A statistically significant difference associated with left- versus right-sided stroke was not demonstrated, likely because ACE-R is not sensitive enough to detect any small discrepancies between cognitive functions compared to detailed neuropsychological investigation [8,25].
ACE-R could help, however, for easier and faster inclusion of patients in cognitive rehabilitation programs because it allows for fast selection of patients and acquisition of a profile of cognitive deficit. It is not possible to compare ACE-R with the more detailed neuropsychological investigation, but ACE-R is sufficiently sensitive to screen for cognitive deficit 3–6 months after a stroke.
Conclusion
Mild cognitive deficit is frequently observed at 3–6 months after a stroke, and ACE-R can be used to detect MCI. Although ACE-R is not suitable for localizing lesions in stroke patients, it provides useful information about cognitive functions and cognitive deficits in patients without dementia after a stroke. Using ACE-R scores as a basis, we can begin cognitive rehabilitation for impaired functions as soon as possible and promote a higher quality of life for the patient.
It would be enriching to correlate ACE-R and its domains with specific neuropsychological tests to identify the sensitivity and specificity of ACE-R for different cognitive deficits in patients after stroke. Validation of the Czech version of ACE-III is an important step and is currently in the planning stages.
Acknowledgements
This work was supported by the Institutional support, University Hospital Ostrava. NO. 1 RVO-FNOs/2012 1.7.2012 - 1.7.2015.
Conflict of Interests and Sources of Funding
The authors report no conflict of interests or sources of funding or personal relationships with other people or organizations that could inappropriately influence or bias their work.
References
- Haring HP. Cognitive impairment after stroke. Curr. Opin. Neurol 15(1), 79-84 (2002).
- Gottesman RF, Hillis AE. Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol 9(9), 895-905 (2010).
- Eskes GA, Barrett AM. Neuropsychological rehabilitation. Neurovascular Neuropsychol, 281-305(2009).
- Tatemichi TK, Desmond DW, Stern Y, et al. Cognitive impairment after stroke: frequency, patterns and relationship to functional abilities. J Neurol. Neurosurg. Psychiatry 57, 202-207 (1994).
- Draper K, Ponsford J. Cognitive functioning ten years following traumatic brain injury and rehabilitation. Neuropsychol 22, 618–625 (2008).
- Stephens S, Kenny RA, Rowan E, et al. Neuropsychological characteristic of mild vascular cognitive impairment and dementia after stroke. Int J Geriatr Psychiatry 11, 1053-1057 (2004).
- Correia S, Lee SY, Voom T, et al. Quantitative tractography metrics of white matter integrity in diffusion-tensor MRI. Neuroimage 42, 568-81(2008).
- Morris KJ. The validity of the Addenbrooke’s Cognitive Examination-Revised (ACE-R) in acute stroke. University of Lincoln School of Health, Life and Social Sciences. Submitted in part fulfillment of the requirements for the Doctorate in Clinical Psychology (2004).
- Amaral-Carvalho V, Caramelli P. Normative Data for Healthy Middle-Aged and Elderly Performance on the Addenbrooke’s Cognitive Examination-Revised. Cogn. Behav. Neurol 25(2), 72-76 (2012).
- Kirshman S, Mathuranath PS, Sarma S, et al. Neuropsychological functions in progressive supranuclear palsy, multiple system atrophy and Parkinson’s disease. Neurol India 54(3), 268-272 (2006).
- Ritman T, Ghosh, BC, McColgan P, et al. The Addenbrooke’s Cognitive Examination for the differential diagnosis and longitudinal assessment of patients with parkinsonian disorders. J Neurol. Neurosurg. Psychiatry 84, 544-551(2013).
- Chade A, Roca M, Torralva T, et al. Detecting cognitive impairment in patients with Parkinson’s disease using a brief cognitive screening tool: Addenbrooke’s Cognitive Examination (ACE). Dementia Neuropsychol 2(3), 197-200 (2008).
- Davies RR, Dawson K, Mioshi E, et al. Differentioation of semantic dementia and Alzheimer’s disease using the Addenbrooke’s Cognitive Examination (ACE). International J Geriatr Psychiatry 23(4), 370-375 (2008).
- Hsieh S, Schubert S, Hoon C, et al. Validation of the Addenbrooke’s Cognitive Examination III in Frontotemporal Dementia and Alzheimer’s Disease. Dementia Geriatr Cogn 36(3-4), 242-250 (2013).
- Terpening Z, Cordato ZJ, Hepner IJ. Utility of the Addenbrookes’ Cognitive Examination-Revised for the diagnosis of dementia syndromes. Aust J Ageing 30(3), 113-118 (2010).
- Hummelová-Fanfrdlová Z, Rektorová I, Sheardová K, et al. Czech adaptation of the Addenbrook Cognitive Test. Czechoslovak psychol 3(4), 376 (2010).
- Bar M, Chmelová I. Caring for a patient after a stroke. Postgrad. Med 1(1), 12 (2011).
- Bak TH, Mioshi E. A cognitive bedside assessment beyond the MMSE: the Addenbrooke’s Cognitive Examination. Pact. Neurol 7(4), 245-249 (2007).
- Beránková D, Krulová P, Mračková M, et al. Addenbrook Cognitive Test - Indicative Standards for the Czech Population. Cesk. Slov. Neurol. N 111(3), 300-305 (2015).
- Pendlebury ST, Mariz J, Bull L, et al. MoCA, ACE-R, and MMSE Versus the National Institute of Neurological Disorders and Stroke–Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards Neuropsychological Battery After TIA and Stroke. Stroke 43(1), 464-469 (2012).
- Habib R, Nyberg L, Tulving E. Hemispheric asymmetries of memory: the HERA model revisited. Trends. Cogn. Sci 7(6), 241-245 (2003).
- Corballis M. Human laterality. New York, Academic Press (1983).
- Chung CSY, Pollock A, Campbell T, et al. Cognitive rehabilitation for executive dysfunction in adults with stroke or other adult non-progressive acquired brain damage. Cochrane. Database. Syst. Rev 4(1), 77-78 (2013).
- Pulsifer MB, Brandt J, Salorio, CF, et al. The Cognitive Outcome of Hemispherectomy in 71 Children. Epilepsy 45(3), 243-254 (2004).
- Johnson SC, Saykin AJ, Flashman LA, et al. Brain activation on fMRI and verbal memory ability: Functional neuroanatomic correlates of CVLT performance. J. Int. Neuropsychol. Soc 7(1), 55–62 (2001).
- Cappa SF, Benke T, Clarke S. EFNS guidelines 12(1), 665-680 (2005).
- Hallett M. Plasticity of the Human motor cortex and recovery from stroke. Brain. Res. Rev 36(2-3), 169-174 (2001).