Research Article - Neuropsychiatry (2018) Volume 8, Issue 2
Abnormal Intrinsic Functional Hubs in Relapsing- Remitting Multiple Sclerosis: Evidence from a Voxel-Wise Degree Centrality Analysis
- *Corresponding Author:
- Jiyong Zheng
Department of Medical Imaging, Huai’an First People’s Hospital
Nanjing Medical University, 6 Beijing Road West
Huai’an, Jiangsu 223300, P. R. China
Abstract
Abstract
Objective: To explore the abnormal intrinsic functional hubs in relapsing-remitting multiple sclerosis (RRMS) using degree centrality approach and their relationships with clinical features.
Methods: Total of 26 RRMS (13 males, 13 females; mean age, 35.38 ± 11.33 years) and 27 statusmatched healthy groups (HGs; 12 males, 15 females; mean age, 35.37 ± 11.67 years) were recruited. RRMS underwent a physical examination using Expanded Disability Status Scale (EDSS) by an experienced neurologist. Degree centrality and functional connectivity approach was used to assess the abnormal intrinsic functional hubs features. Partial correlation analysis was conducted to investigate the relationships between clinical features and abnormal functional hubs. Receiver operating characteristic (ROC) curve was applied to calculate the sensitivities and specificities of those altered functional hubs to distinguish RRMS from HGs.
Results: Compared with HGs, RRMS exhibited significantly higher degree centrality values in multiple brain areas and lower degree centrality values in bilateral salience network and left cingulate gyrus. Decreased functional connectivity was found within left-right salience network, and between salience network and cingulate gyrus. Duration of disease and EDSS revealed negative correlations with degree centrality value in the salience network. ROC
analysis showed good performances of these abnormal hubs in distinguishing RRMS from HGs with high degree of sensitivities and specificities.
Conclusions: RRMS is associated with multiple specific abnormal functional hubs including relatively reduced and increased degree centrality value, which expands our understanding of the functional characteristics and may provide a new insight into understanding the pathophysiological mechanisms of RRMS.
Keywords
Multiple sclerosis, Degree centrality, Functional magnetic resonance imaging, Functional connectivity, Receiver operating characteristic, Resting-state
Introduction
Multiple sclerosis (MS), as an inflammatory and demyelinating disease in axonal degeneration of the central nervous system, is commonly diagnosed in the prime of life and in most cases. In addition to classic inflammatory white matter lesions, gray matter demyelination is common and extensive [1,2]. Abnormalities in the gray matter are also consistently found with in vivo magnetic resonance imaging (MRI) [3-5] and correlated with clinical deficits [6,7]. Extensive involvements of disease-related cortical gray matter organization may delay and perturb syntonic intrinsic signals across cortico-cortical and cortico-subcortical wirings, which lead to several clinical manifestations such as fatigue, sensorimotor deficits, cognitive dysfunction, and even chronic disability [2]. Considering the high prevalence and the known neurotoxic effects of MS, it’s essential to further expound its complex brain network impairments. However, the underlying mechanism of the neuropsychiatric alterations remains ambiguous.
The brain imaging has facilitated its non-invasive exploration. Multiple lines of neurobiological researches have revealed brain function alterations associated with MS. Previous functional magnetic resonance imaging (fMRI) studies have traditionally focus their attentions on neuroimaging effects on task-evoked neuronal activity. In this process, neuroimaging methods were widely used to demonstrate task-specific or cue-specific alterations in regional brain activity. Since the different performance and task patterns make it difficult to interpret the biological mechanisms of diseases and hinder comparisons between various populations, it is difficult to display a consistent story among various studies when we solely focus on regional functional brain activity alterations under task-specific or cue-specific conditions because of behavioral variability [8-11]. This may hamper us to insight into integrated global brain function alterations during resting-state or non-task condition.
It is proposed that resting-state fMRI (rs-fMRI) is a useful tool for the mechanism research of brain disease and is very hot in neuroimaging area [12]. Some approaches limitedto one or some specific regions of interest and are not designed to detect activation changes across the entire brain. Resting-state functional connectivity analysis has benefitted from the rsfMRI allowing to outline the functional network connectome between distant brain regions, but it only provides limited information of the relationships between the given seed point region and other brain regions. Recently, graph theory-based network analyses has been applied to explore brain connectivity within wholebrain networks [13-15]. Specifically, degree centrality is a class of graph-theoretic measures assessing the importance of each node in brain network, in terms of its connectivity strength to every voxel [16,17]. In contrast to traditional seed-based functional connectivity approach, degree centrality, based on voxel-based wholebrain correlation analysis, provides an unbiased opportunity to search abnormalities within the entire connectivity matrix of full-brain functional connectome without priori hypothesis, and don’t require a priori definition of regions of interest (ROIs). This approach is a criterion to measure the importance of the individual node and reflects the functional brain network “hub” properties, i.e., the communication ability of network information [18], and exhibits relatively high test-retest reliability [19]. Recently it has been successfully used to disclose the neurobiological mechanism for several diseases, such as obsessive compulsive disorder [20], Alzheimer’s disease [21], major depressive disorder [22] and alcohol dependence [23]. However, RRMS has not been studied. RRMS is associated with the changes in behavior, brain function, and brain structure. However, the nature of these changes has not been well understood. Studying the functional connectivity with whole-brain degree centrality may be of value. In this study, it’s the first to use whole-brain degree centrality approach to identify altered intrinsic functional connectivity hubs in patients with relapsing-remitting MS (RRMS) relative to healthy groups (HGs) from the entire connectivity matrix of full-brain functional connectome. Then, we conducted linear correlation analysis to evaluate the relationships between behavioral performances and degree centrality value of those significant alterations in intrinsic functional hubs in RRMS.
Materials and Methods
▪ Subjects
Total of twenty six RRMS (13 males, 13 females; mean age, 35.38 ± 11.33 years; mean education, 10.08 ± 2.81 years) and twenty seven age-, sex- , and education matched HGs (12 males, 15 females; mean age, 35.37 ± 11.67 years; mean education, 10.41 ± 3.87 years) were recruited in this study.
The RRMS met the pertinent inclusion criteria: (1) met the diagnostic criteria of RRMS as defined by the McDonald; (2) had mild to moderate RRMS as defined by the EDSS (EDSS≤5), in order to prevent confounding factors in the analysis; (3) had not received steroids-based therapy or experiencing a clinical relapse, or other concomitant therapy as antidepressant or therapy for fatigue for at least two months prior and during this study; (4) had not sleep disorders and other psychiatric disorders as defined by the DSM-IV; (5) had not brain tumor, brain injury, intracerebral hemorrhage according to the conventional MRI. The disease duration was recorded in months from the symptom onset date to the MRI scan date. The mean duration of RRMS is 20.71 ± 28.96 days (Table 1).
The HGs had not any history of brain tumor, brain injury, inborn or other acquired diseases, neurological or psychiatric disorders sleep disorders, alcohol, drug abuse, systemic illness, intracerebral hemorrhage and foreign implants in the body. All subjects were right-handed as defined by the Edinburgh Inventory.
▪ 1.2 Research design and process
Before MRI examination, all subjects underwent a thorough physical examination using the Expanded Disability Status Scale (EDSS) by an experienced neurologist. The EDSS score is from zero to ten, where zero score indicates normal, and higher EDSS score shows more disability. All volunteers participated voluntarily and were explained the purposes, methods, and potential risks. Before the MRI session, all subjects voluntarily signed their informed consent form. This study was approved by Human Research Ethics Committee.
▪ MRI
MRI scan was performed on 3-Tesla MR scanners (Trio, Siemens, Erlangen, Germany). High-resolution T1-weighted anatomical images were acquired with a three-dimensional spoiled gradient-recalled sequence in an sagittal orientation: 176 images (repetition time=1900 ms, echo time=2.26 ms, thickness=1.0 mm, gap=0.5 mm, acquisition matrix=256×256, field of view=250 mm×250 mm, flip angle=90) were obtained. Next, an 8-min rs-fMRI scan was obtained with eyes closed. Total of 240 functional images (repetition time = 2000 ms, echo time = 30 ms, thickness = 4.0 mm, gap = 1.2 mm, acquisition matrix = 64 × 64, flip angle = 90°, field of view = 220 mm × 220 mm, 32 axial slices with Gradient-Recalled Echo-Planar Imaging pulse sequence) covering the whole brain were obtained.
▪ Data analysis
MRIcro software (www.MRIcro.com) was used to ensure data quality. The first 10 time points of the functional images were discarded due to the possible instability of initial MRI signal and the participants’ adaptation to scanning environment [24]. On the basis of MATLAB2010a (Mathworks, Natick, MA, USA), the rest of the data pre-processing was performed by Data Processing & Analysis for Brain Imaging (DPABI 2.1, http://rfmri.org/DPABI) toolbox, including Digital Imaging and Communications in Medicine (DICOM) standards for form transformation, slice timing, head motion correction, spatial normalization, smooth with a Gaussian kernel of 6×6×6 mm3 full-width at half-maximum. The 3D T1-weighted images for each subject were co-registered to the mean of the realigned EPI template, and then segmented into gray matter, white matter, and cerebrospinal fluid (CSF) using the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) segmentation. The participants who had more than 1.5 mm maximum translation in x, y, or z and 1.5° degree of motion rotation were rejected. The Friston 24 head motion parameters model, including 6 head motion parameters, 6 head motion parameters, and 12 corresponding squared items, was used to regress out the head motion effects based on recent work showing that the higher-order models benefit from the removal of head motion effects [25,26]. In RRMS, T1-hypointense lesions were manually identified and were semiautomated painted as regions of interest (ROIs) using the T1 sagittal images converted to axial. Lesion masks for each patient were created (transforming the ROIs into independent images) and then binarized using ImCalc module. After this step, the binary lesion masks together with the T1 sagittal images were used to create new structural images. The created images were automatically transformed from the individual space to the Montreal Neurological Institute (MNI) space. Linear regression was applied to remove other sources of spurious covariates along with their temporal derivatives, including the global mean signal, white matter and cerebrospinal fluid signal. After the head-motion correction, the functional MRI images were spatially normalized to the Montreal Neurological Institute (MNI) space and re-sampled at a resolution of 3 mm × 3 mm × 3 mm. After preprocessing, the time series for each voxel were temporally bandpass filtered (0.01–0.1 Hz) and linearly detrended to reduce the low-frequency drift and physiological highfrequency respiratory and cardiac noise.
▪ Calculation of degree centrality maps
Degree centrality attributes a greater value to a voxel if it has strong connections with many other voxels in the brain. For the calculation of voxel-wise degree centrality, the processing data were used to compute the voxel-based wholebrain functional correlation analysis. Pearson’s correlation coefficient (r) between each pair of brain gray matter voxels was computed. As a result, we acquired a matrix of pearson correlation coefficients depicting whole-brain functional connectivity pattern. To obtain each subject’s graph, whole-brain functional network was then constructed by defining threshold of each correlation [16,17]. Degree centrality was calculated by counting the number of significant suprathresholded correlations (or the degree of the binarized adjacency matrix) for each subject based on the individual voxel-wise functional network. Finally, the voxel-wise degree centrality map for each individual was converted into a z-score map using the following equation:

where i is voxel index, degree centrality i is degree centrality value for the i -th voxel, std is standard deviation, and Zi is z-score for i-th voxel.
In this study, we repeated the network analysis using a range of correlation r thresholds (i.e., r=0.10, 0.15, 0.20, 0.25, 0.30, 0.35, 0.40 and 0.5) to determined that the betweengroup differences in degree centrality were not substantially affected by the selection of different r-value thresholds or nodes to construct the brain networks.
▪ Seed-based connectivity analyses
Those abnormal degree centrality in brain regions were saved as seed points, and the average time series from these seed points were extracted from the residual image. Seed-based connectivity analyses were conducted to investigate the functional connectivity of these seed points with other voxles in whole brain.
To make the data fit the normal distribution, we calculated the coefficient of pearson correlation between regions of interest and other voxels of whole brain, and the resulting coefficient was participated in Fisher’s Z transformation. To reduce the global effects of variability across the participants, the functional connectivity of each voxel was divided by the global mean value for each participant.
Statistical Analysis
▪ Behavioral data
The demographic factors (age, education, and years of education) and the questionnaire data were compared between groups using two sample t-test. Chi-square (χ2) test was used for categorical data. The statistical analysis was performed using IBM Statistical Package for the Social Sciences version 21.0 (SPSS 21.0). Data are presented as mean ± standard deviation. All results were quoted as two-tailed, and P<0.05 was considered as statistically significant.
▪ Voxel-wise degree centrality data
For voxel-wise degree centrality, two-sample t-tests were used to investigate the voxel-wise degree centrality and the functional connectivity differences in brain regions between RRMS and HGs with age, sex, and years of education as nuisance covariates of no interest. We analyzed group differences in two ways. First, we performed a threshold of two-tailed voxelwise p<0.05 and cluster-level p<0.05, corrected for multiple comparisons by false discovery rate (FDR) or Gaussian random field (GRF) theory. Second, once no between-group differences were found using the corrected threshold, then we used a loose uncorrected statistical threshold of p<0.01 with a minimum continuous cluster voxel volume of 810 mm3.
▪ Pearson linear correlation analysis
Partial correlation analysis was performed to evaluate the relationships between the behavioral performances and the degree centrality value of those different brain regions with age, sex, and years of education as nuisance covariates of no interest. p<0.05 was considered to be significant differences.
Results
▪ Sample characteristics
The demographic characteristics of the subjects are presented in Table 1. There were no significant differences in sex (χ2= 0.164, p=0.685), mean age (t= 0.005, p=0.996) and mean education (t= -0.352, p=0.726) between RRMS and HGs. The mean disease duration and mean EDSS score of RRMS was (21.9 ± 33.03) days and (3.6 ± 1.84), respectively.
| RRMS | HGs | t/χ2 value | p value | |
|---|---|---|---|---|
| Sex (Male, Female) | 13/13 | 12/15 | 0.164 | 0.685* |
| Mean age, year | 35.38 ± 11.33 | 35.37 ± 11.67 | 0.005 | 0.996 # |
| Mean education, year | 10.08 ± 2.87 | 10.41 ± 3.87 | -0.352 | 0.726 # |
| Mean disease duration, day | 21.9 ± 33.03 | N/A | N/A | N/A |
| Mean EDSS score | 3.6 ± 1.84 | N/A | N/A | N/A |
Notes: Data are mean ± standard deviation values; *, χ2 test was used; #, independent t-test. Abbreviations: RRMS, Relapsing-remitting multiple sclerosis; HGs, Healthy groups; N/A, Not applicable; EDSS, Expanded disability status scale.
Table 1: Demographics and clinical characteristics.
▪ Binarized degree centrality differences between groups
We analysed binary voxel-wise functional correlations and further investigated intra- and inter-group differences of binary voxel-wise functional brain centrality. We observed highly similar, not dependent on different thresholding schemes, intra-group differences of binary degree centrality in several threshold at r=0.10, 0.15, 0.20, 0.25, 0.30, 0.35, 0.40 and 0.50 (Figure 1A-H). For this reason, in this study we only reported the results in binary network with threshlod at r=0.3.
Figure 1: Binarized degree centrality maps with several threshlod between RRMS and HGs.
Note: Between t-group differences of binary network with different threshold at r=0.10 (A), 0.15 (B), 0.20 (C), 0.25 (D), 0.30 (E), 0.35 (F), 0.40 (G) and 0.50 (H).
Abbreviations: RRMS, Relapsing-remitting multiple sclerosis; HGs, Healthy controls; R, right; L, left.
First, we reported within-group statistic maps for degree centrality measurement for RRMS group (Figure 2A) and HGs group (Figure 2B) using one sample t-test (P<0.01, continuous cluster voxel volume ≥ 810 mm3, FDR corrected). We found that the two groups showed significantly similar different binarized degree centrality value in brain areas (Figure 2A-2B).
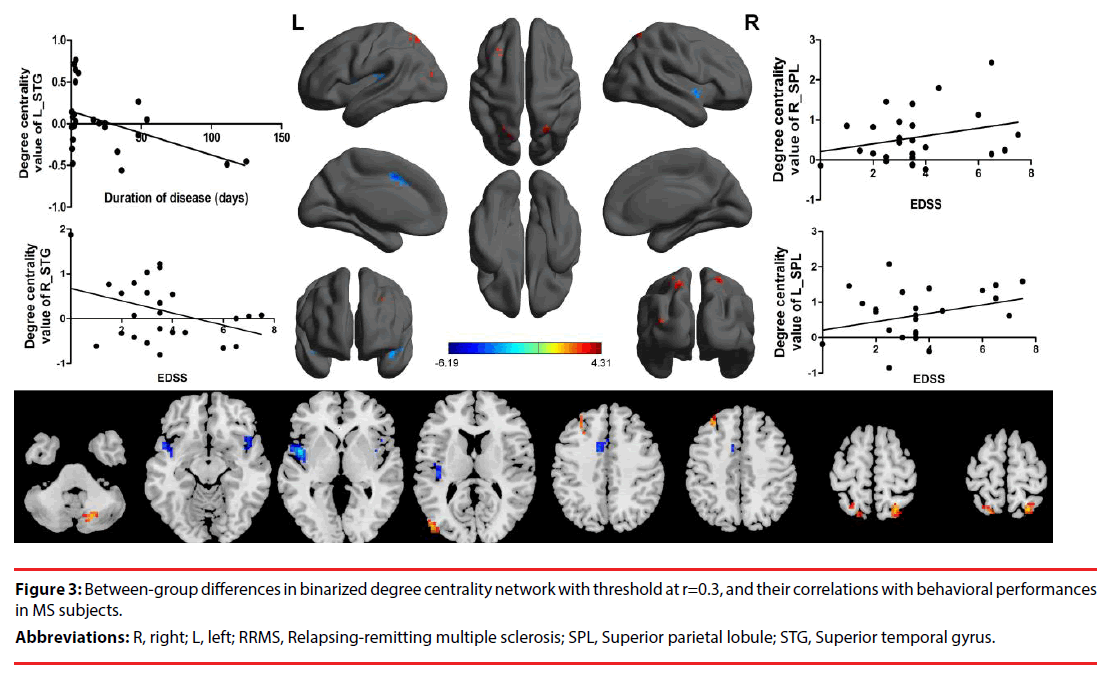
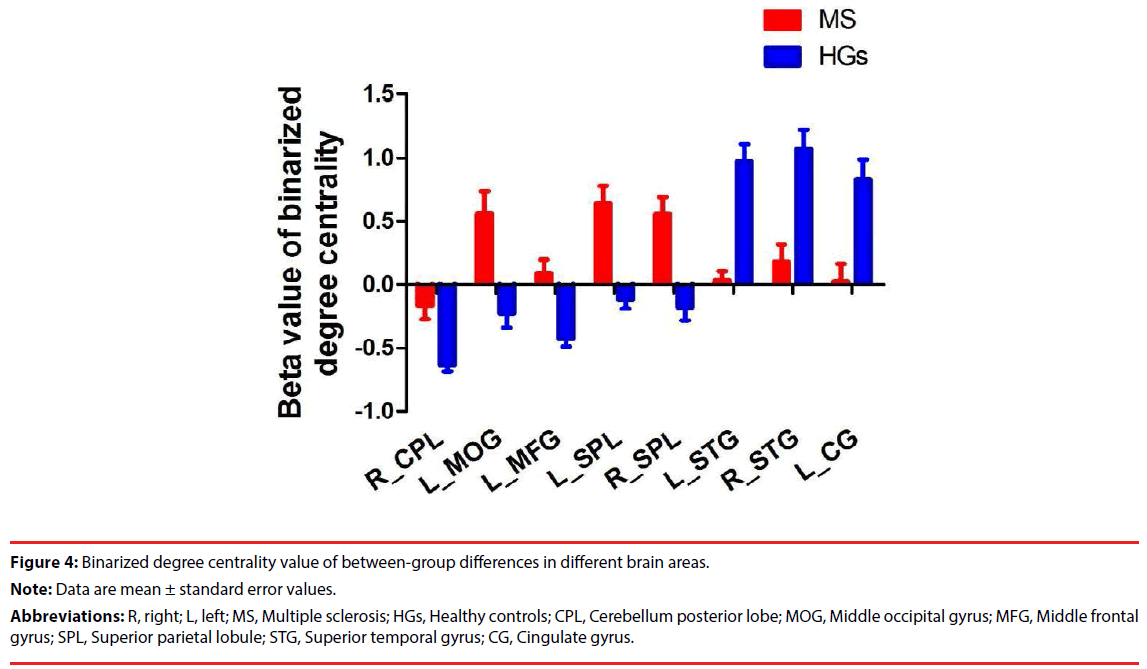
Next, we compared the binary degree centrality differences between RRMS group and HGs group (Figure 3, Table 2). Compared with HGs, RRMS subjects exhibited significantly higher degree centrality values in the right cerebellum posterior lobe, left middle occipital gyrus (BA19), left dorsolateral prefrontal cortex (BA9), bilateral superior parietal lobule (BA7), and lower degree centrality values in the bilateral temporal pole (BA38) extending to insula (BA13) in the salience network, and left cingulate gyrus (BA32) Figure 4.
Figure 3: Between-group differences in binarized degree centrality network with threshold at r=0.3, and their correlations with behavioral performances in MS subjects.
Abbreviations: R, right; L, left; RRMS, Relapsing-remitting multiple sclerosis; SPL, Superior parietal lobule; STG, Superior temporal gyrus.
| Conditions | Brain regions of peak coordinates | R/L | BA | Voxel volume (mm3) | t-score of peak voxel | MNI coordinates |
|---|---|---|---|---|---|---|
| X, Y, Z | ||||||
| RRMS>HGs | Cerebellum Posterior Lobe | R | N/A | 1377 | 3.9867 | 18 -69 -39 |
| RRMS>HGs | Middle Occipital Gyrus | L | 19 | 1107 | 4.0961 | -48 -81 12 |
| RRMS>HGs | Middle Frontal Gyrus | L | 9 | 918 | 3.8967 | -30 33 45 |
| RRMS>HGs | Superior Parietal Lobule | L | 7 | 1431 | 3.7095 | -18 -66 66 |
| RRMS>HGs | Superior Parietal Lobule | R | 7 | 1161 | 4.3143 | 24 -63 63 |
| RRMS<HGs | 颞极Superior Temporal Gyrus, Insula | L | 13, 38 | 5319 | -6.1879 | -45 0 0 |
| RRMS<HGs | 颞极Superior Temporal Gyrus, Insula | R | 13, 38 | 1107 | -3.6455 | 45 9 -12 |
| RRMS<HGs | Cingulate Gyrus | L | 32 | 999 | -4.1558 | -9 6 42 |
Notes: Between-group differences in binarized degree centrality thresholded at r=0.3. The statistical threshold was set at uncorrected voxel threshold of p<0.01 with a minimum voxel volume threshold of 540 mm3. Abbreviations: RRMS: Relapsing-Remitting Multiple Sclerosis; Hgs: Healthy Controls; R: Right; L: Left; BA: Brodmann’s Area; MNI: Montreal Neurological Institute; N/A: Not Applicable
Table 2: The binarized degree centrality differences between RRMS and HGs.
Figure 4: Binarized degree centrality value of between-group differences in different brain areas.
Note: Data are mean ± standard error values.
Abbreviations: R, right; L, left; MS, Multiple sclerosis; HGs, Healthy controls; CPL, Cerebellum posterior lobe; MOG, Middle occipital gyrus; MFG, Middle frontal gyrus; SPL, Superior parietal lobule; STG, Superior temporal gyrus; CG, Cingulate gyrus.
Interestingly, in the present study we found a strong unilateral lateralization between RRMS and HGs, showing that the higher, lower, and sum of higher and lower degree centrality value in brain areas consistently had more numbers of different degree centrality value in brain areas in left than that of right, and more total numbers of voxel volume of different degree centrality value in brain areas in left than that of right.
▪ Seed-based functional connectivity analysis
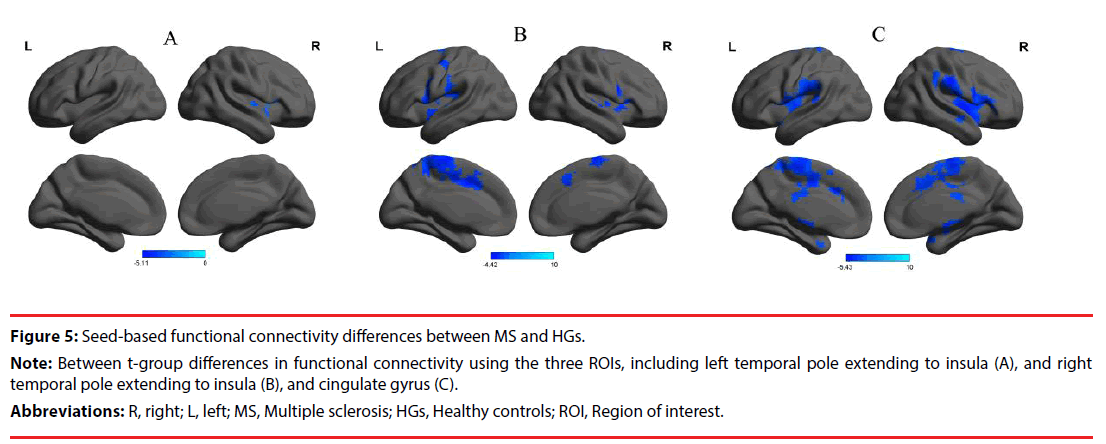
Those abnormal degree centrality in brain regions were selected as ROIs for further resting-state functional connectivity analyses. Two-sample t-tests revealed several resting-state functional connectivity differences between RRMS and HGs (Table 3, Figure 5). When the reduced degree centrality in brain regions were used as seed-points, several seed-based functional connectivity differences were found in intraand inter-network between RRMS and HGs (p<0.05, corrected by GRF). Compared with HGs, RRMS exhibited significantly decreased functional connectivity between the left and the right temporal pole extending to insula in the salience network (Table 3, Figure 5A). The right temporal pole extending to insula as an ROI showed decreased functional connectivity with three areas, including bilateral cluster of temporal pole, insula and inferior frontal gyrus in the salience network, and right cluster of cingulate gyrus and frontal lobe (Table 3, Figure 5B). The left cingulate gyrus as an ROI showed decreased functional connectivity with three areas, including bilateral cluster of temporal pole, insula and frontal lobe in the salience network, and bilateral cluster of cingulate gyrus and frontal lobe (Table 3, Figure 5C).
| Seed point | Brain regions of peak coordinates | R/L | BA | Voxel volume (mm3) | t-score of peak voxel | MNI coordinates |
|---|---|---|---|---|---|---|
| X, Y, Z | ||||||
| L_STG, Insula | STG, Insula | R | 13, 22, 38 | 23895 | -5.1139 | 51 15 -12 |
| R_STG, Insula | STG, Insula, Inferior Frontal Gyrus | L | 13, 22, 38, 44 | 23463 | -4.4191 | -33 -27 24 |
| STG, Insula, Inferior Frontal Gyrus | R | 13, 22, 38, 44 | 18819 | -4.0761 | 63 12 3 | |
| Cingulate Gyrus, Frontal lobe | L | 4, 6, 32 | 28296 | -4.3022 | -9 9 42 | |
| L_CG | STG, Insula | L | 13, 22, 38 | 34344 | -4.7806 | -39 -12 -3 |
| STG, Insula, Inferior Frontal Gyrus | R | 13, 22, 38, 44 | 51516 | -5.4255 | 36 -6 0 | |
| Cingulate Gyrus, Frontal lobe | L, R | 6, 24, 32 | 39717 | -4.6696 | -6 -6 48 | |
Notes: The statistical threshold was set at voxel threshold of p<0.05 and cluster threshold of p<0.05with GRF corrected. Abbreviations: MS: Multiple Sclerosis; Hgs: Healthy Controls; R: Right; L: Left; STG: Superior Temporal Gyrus; CG: Cingulate Gyrus, BA: Brodmann’s Area; MNI: Montreal Neurological Institute; GRF: Gaussian Random Field
Table 3: Seed-based functional connectivity differences between MS and HGs.
Figure 5: Seed-based functional connectivity differences between MS and HGs.
Note: Between t-group differences in functional connectivity using the three ROIs, including left temporal pole extending to insula (A), and right temporal pole extending to insula (B), and cingulate gyrus (C).
Abbreviations: R, right; L, left; MS, Multiple sclerosis; HGs, Healthy controls; ROI, Region of interest.
▪ Pearson linear correlation
As shown in Figure 3, duration of disease in RRMS subjects revealed a negative linear correlation with degree centrality value in the left temporal pole (r= -0.483, p=0.02). EDSS revealed a positive linear correlation with degree centrality value in the right superior parietal lobule (r=0.485, p=0.019), and a negative linear correlation with degree centrality value in the right temporal pole (r= -0.43, p=0.041). Furthermore, the EDSS also revealed a approximate positive linear correlation with degree centrality value in the left superior parietal lobule (r=0.374, p=0.079). No other significant linear correlations between degree centrality value in those different brain areas and the behavioural performances (p>0.05).
▪ High sensitivity and specificity
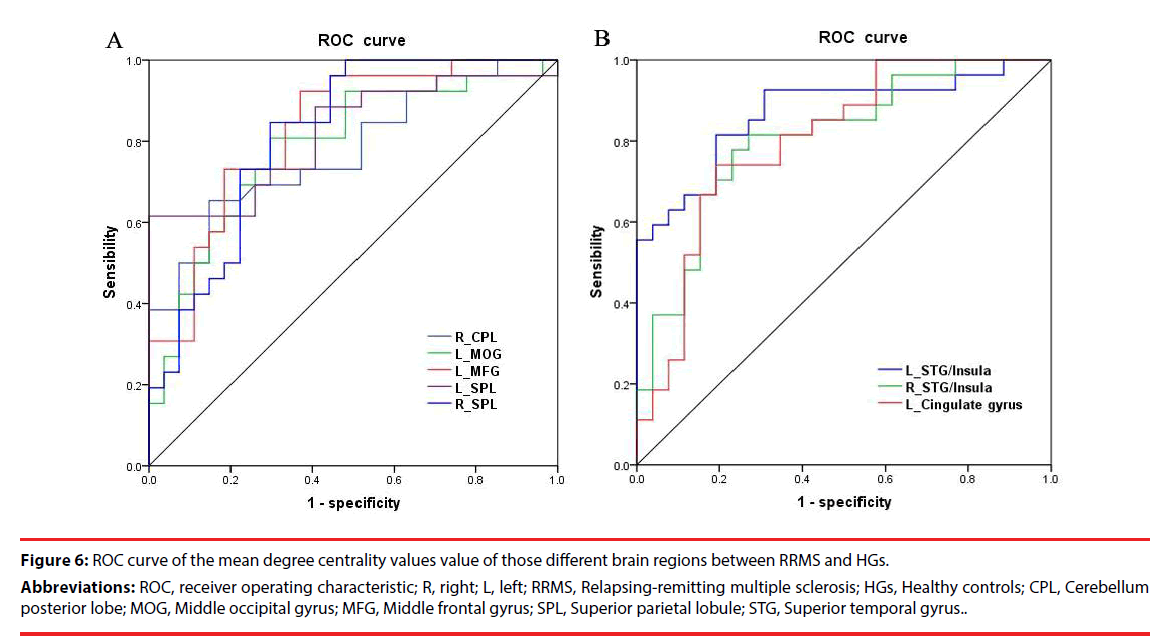
Since the different degree centrality areas might be utilized as markers to separate the RRMS from the HGs, the mean degree centrality values of these different areas were extracted and used for ROC curveto explore whether these specific areas have the ability to distinguish the RRMS from the HGs. In the present study, the ROC analysis revealed that the different degree centrality areas showed six good AUC values and two fair AUC values (Figure 6, Table 4). Further diagnostic analysis demonstrated that the mean beta values of these different brain areas demonstrated high degree of sensitivities (61.5%~92.3%) and specificities (63%~100%). This indicates that the ALFF index could serve as a good to excellent marker to distinguish the RRMS from the HGs.
Figure 6: ROC curve of the mean degree centrality values value of those different brain regions between RRMS and HGs.
Abbreviations: ROC, receiver operating characteristic; R, right; L, left; RRMS, Relapsing-remitting multiple sclerosis; HGs, Healthy controls; CPL, Cerebellum posterior lobe; MOG, Middle occipital gyrus; MFG, Middle frontal gyrus; SPL, Superior parietal lobule; STG, Superior temporal gyrus..
| Degree centrality index | ROC curve | |||
|---|---|---|---|---|
| AUC | Sensitivity | Specificity | Cutoff point of mean degree centrality value | |
| R_CPL | 0.777 | 65.4% | 85.2% | -0.4055 |
| L_MOG | 0.785 | 80.8% | 70.4% | -0.1264 |
| L_MFG | 0.828 | 92.3% | 63% | -0.4695 |
| L_SPL | 0.821 | 61.5% | 100% | 0.5147 |
| R_SPL | 0.815 | 84.6% | 70.4% | -0.0423 |
| L_STG | 0.869 | 81.5% | 80.8% | 0.317 |
| R_STG | 0.806 | 77.8% | 76.9% | 0.5946 |
| L_CG | 0.801 | 74.1% | 80.8% | 0.345 |
Abbreviations: ROC: Receiver Operating Characteristic; Area Under Curve: AUC; R: Right; L: Left; RRMS: Relapsing-Remitting Multiple Sclerosis; Hgs: Healthy Controls; CPL: Cerebellum Posterior Lobe; MOG: Middle Occipital Gyrus; MFG: Middle Frontal Gyrus; SPL: Superior Parietal Lobule; STG: Superior Temporal Gyrus; CG: Cingulate Gyrus
Table 4: ROC curve analysis for the different binarized degree centrality areas between RRMS and HGs.
Discussion
To the best of our knowledge, the current study is the first to apply resting-state degree centrality analysis approach to investigate the abnormal intrinsic functional hubs in RRMS relative to HGs, and their relationships with behavioral performances. RRMS was associated with a sequential pattern of widespread changes in resting-state degree centrality indices of intrinsic functional hubs, including significantly higher degree centrality values in the right cerebellum posterior lobe, left visual association cortex, left dorsolateral prefrontal cortex, bilateral superior parietal lobule, and lower degree centrality values in the bilateral temporal pole extending to insula, and left cingulate gyrus. Furthermore, these intrinsic functional hubs revealed linear correlation with EDSS and duration of disease, which may suggest that the critical network node could predict disease progression and disability status. Further functional connectivity demonstrated that the decreased degree centrality values in brain areas showing a decraesed functional connectivity with each other.
It was shown that the ROC curve has been widely accepted to be used for identifying and comparing the diseases from the HGs, and may be used as an early biological indicator for the detection of regional brain activity changes [12,27,28]. The identifying ability is considered to be excellent with the area under curve (AUC) value from 0.9 to 1, good from 0.8 to 0.9, fair from 0.7 to 0.8, poor from 0.6 to 0.7, and failed from 0.5 to 0.6 [29-30]. In the present study, the ROC analysis revealed that the different degree centrality areas showed high identifying ability with good AUC values and high degree of sensitivities and specificities. In this respect, our study provided the evidence that the resting-state degree centrality analysis approach is useful for characterizing the neural mechanisms underlying of RRMS and may be reliable predictive tool or an early biological indicator for the detection ofabnormal intrinsic functional hubs in RRMS.
Interestingly, in the present study we found a strong unilateral lateralization in RRMS, showing that higher, lower, and sum of higher and lower degree centrality value in brain areas between RRMS and HGs consistently showed more numbers of different degree centrality value in brain areas in left than that of right, and more total numbers of voxel volume of different degree centrality value in brain areas in left than that of right. The unilateral lateralization was also found in smoking dependence subjects [23,31], insomnia patients [24,32] and healthy subjects in auditory capability [33] and visual modality [34]. Thus the unilateral lateralization is not unique, it may happen in ill-condition even in normal condition. To improve the efficiency of brain function, the human brain represented strong unilateral lateralization to conduct specialization and cooperation. Thus the left lateralization may demonstrate that the left brain hemisphere displayed a facilitatory effect on production and transmission of brain activities in RRMS.
Compared to HGs, nonfatigued MS patients exhibited a higher degree of gray matter atrophy in the bilateral temporal and occipital lobes and whiter matter structural changes in the bilateral cingulate gyrus, bilateral temporal and occipital lobes, fatigued MS patients exhibited gray matter atrophy in the bilateral occipital lobe and cingulate gyrus, and whiter matter alterations in the temporal, occipital, cingulate gyrus, parietal lobes and cerebellum [35]. Some studies also demonstrated that RRMS showed altered regional brain activity in the bilateral cerebullum [36] and cingulate gyrus [37], and smaller gray matter volume in the left fronto-temporal cortex and bilateral cingulate gyrus [38]. These differences in brain areas were consistent with the differences of degree centrality indices in intrinsic functional hubs. In the present study we found significantly higher degree centrality values in the right cerebellum posterior lobe, left visual association cortex, left dorsolateral prefrontal cortex, bilateral superior parietal lobule between RRMS and HGs. One explanation of this finding could be that this is a brain compensation mechanism for when RRMS compromises the functionality of networks beyond self (within) compensation. Serial studies, including patients with variable level of cognitive performance, are required in order to determine whether subsequent loss of compensatory connectivity sub-serves cognitive decline. Therefore, higher degree centrality in these brain areas may utilize additional resources to help the individuals to achieve the same level of performance as before. RRMS is associated with widespread brain structure changes both in gray and white matter. Therefore, another explanation of the higher degree centrality values in those brain areas could be explained by that the hyperactivation in these areas may be interpreted as an enhanced neural effort to offset the brain structural damage.
Contrasted with HGs, RRMS showed greater task-related activation bilaterally in the cingulate gyrus and temporal pole during hand movement [39]. The cortical atrophy was also seen in the cingulate gyrus and temporal pole extending to insula consistently with past [40-42] literature. Consistently, in the present study we found RRMS was associated with lower degree centrality values in the bilateral temporal pole extending to insula in the salience network, and left cingulate gyrus, and these areas were associated with EDSS and duration of disease. Furthermore, we also found decreased functional connectivity within the bilateral salience network and between the cingulate gyrus and the bilateral salience network. The degree centrality provides information on the functional connectivity within the wholebrain network rather than with specific nodes or networks, and may serve as an important hub for information integration, superior information propagation, and critical way stations for information processing, and may thus leading to effective information flow [43]. Reduced degree centrality value in brain functional hubs in brain areas represent that these areas showed fewer correlated activity and that the role of these hubs in facilitating neural network communication is impaired [44]. Whether engaging in a task or during resting-state, processing inefficiency in RRMS could be caused by the disruption of one or multiple functional brain networks. Thus, the inefficient processing may arise from or reveal disorganization of one or multiple functional brain networks, leading to inefficiency in information transmission from one place to other places. In this framework, the decreased degree centrality and connectivity pattern of the cingulate gyrus and salience network observed in the present study may be an expression of inefficiency in information transmission and disorganization of these functional brain networks associated with RRMS, and may be associated with aspects of attention and motivation in RRMS that are not well controlled in the contrast with HGs who may – in general – perform the task with less effort [39,45].
Conclusions
Our study provides new insight of whole brain network analysis of RRMS using graph-theoretic measurement, unbiased opportunity to search abnormalities within the entire connectivity matrix of full-brain functional connectome without priori hypothesis and a priori definition of ROIs. The present study showed the pattern of negative functional deficits in the cingulate gyrus and salience network, appear to be disrupted by RRMS. These findings add evidence to the concept of altered functional connectivity and also highlight the role of functional connectivity in the pathophysiology of RRMS. The present study provided intriguing insights into the promising issue of the personalized medicine suggesting that these critical network node in RRMS could predict disease progression and disability status, and may guide tailored treatment decisions [46,47].
References
- Vercellino M, Masera S, Lorenzatti M, et al. Demyelination, inflammation, and neurodegeneration in multiple sclerosis deep gray matter. J. Neuropathol. Exp. Neurol 68(1), 489-502 (2009).
- Minagar A, Barnett MH, Benedict RH, et al. The thalamus and multiple sclerosis modern views on pathologic, imaging, and clinical aspects. Neurology 80(1), 210-219 (2013).
- Vrenken H , Geurts JJ , Knol DL , et al. Whole-brain T1 mapping in multiple sclerosis: global changes of normal appearing gray and white matter. Radiology 240(3), 811-820 (2006).
- Calabrese M , De Stefano N , Atzori M , et al. Detection of cortical infl ammatory lesions by double inversion recovery magnetic resonance imaging in patients with multiple sclerosis. Arch. Neurol 64(10), 1416-1422 (2007).
- Geurts JJ, Pouwels PJ , Uitdehaag BM, et al. Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging. Radiology 236 (1), 254 -260 (2005).
- Rovaris M , Filippi M , Minicucci L , et al.Cortical/subcortical disease burden and cognitive impairment in patients with multiple sclerosis. Am. J. Neuroradiol 21(2), 402-408 (2000).
- Vrenken H, Pouwels PJ, Geurts JJ, et al. Altered diffusion tensor in multiple sclerosis normal-appearing brain tissue: cortical diffusion changes seem related to clinical deterioration. J. Magn. Reson. Imaging 23(5), 628-636 (2006).
- Tintore M, Rovira A. MRI criteria distinguishing seropositive NMO spectrum disorder from MS. Neurology 80(1), 1336 (2013).
- Bakshi R, Thompson AJ, Rocca MA, et al. MRI in multiple sclerosis: current status and future prospects. Lancet. Neurol 7(1), 615-625 (2008).
- Filippi M, Rocca MA. Functional MR imaging in multiple sclerosis. Neuroimaging. Clin. N. Am 19(1), 59–70 (2009).
- Filippi M, Riccitelli G, Mattioli F, et al. Multiple sclerosis: effects of cognitive rehabilitation on structural and functional MR imaging measures-an explorative study. Radiology 262(1), 932-940 (2012).
- Dai XJ, Liu CL, Zhou RL, et al. Long-term sleep deprivation decreases the default spontaneous activity and connectivity pattern in healthy male subjects: a resting-state fMRI study. Neuropsychiatr. Dis. Treat 11(1), 761–772 (2015).
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci 10(1), 186–198 (2009).
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52(1), 1059–1069 (2010).
- Wang J, Zuo X, He Y. Graph-based network analysis of resting-state functional MRI. Front. Syst. Neurosci 4(1), 16 (2010).
- Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J. Neurosci 29(1),1860-1873 (2009).
- Zuo XN, Ehmke R, Mennes M, et al. Network centrality in the human functional connectome. Cereb. Cortex 22(1), 1862–1875 (2012).
- Di Martino A, Zuo X, Kelly C, et al. Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biological. Psychiatry 74(1), 623-632 (2013).
- Zuo X, Xing X. Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: a systems neuroscience perspective. Neuroscience & Biobehavioral Reviews 45(1), 100-118 (2014)
- Gottlich M, Kramer UM, Kordon A, et al. Resting-state connectivity of the amygdala predicts response to cognitive behavioral therapy in obsessive compulsive disorder. Biol. Psychol; 111(1),100–109 (2015).
- Guo Z, Liu X, Hou H, et al. Abnormal degree centrality in Alzheimer’s disease patients with
- depression: A resting-state functional magnetic resonance imaging study. Exp. Gerontol 79(1), 61-66 (2016).
- Shen Y, Yao J, Jiang X, et al. Sub-hubs of baseline functional brain networks are related to early improvement following two-week pharmacological therapy for major depressive disorder. Hum. Brain. Mapp 36(1), 2915–2927 (2015).
- Luo X, Guo L, Dai XJ, et al. Abnormal intrinsic functional hubs in alcohol dependence: evidence from a voxelwise degree centrality analysis. Neuropsychiatr. Dis. Treat 13(1), 2011-2020 (2017).
- Dai XJ, Gong HH, Wang YX, et al. Gender differences in brain regional homogeneity of healthy subjects after normal sleep and after sleep deprivation: a resting-state fMRI study. Sleep. Med 13(6), 720-727 (2012).
- Satterthwaite TD, Elliott MA, Gerraty RT, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 64(1), 240-256 (2013).
- Yan CG, Cheung B, Kelly C, et al. A Comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage 76(1), 183-201 (2013).
- Li HJ, Dai XJ, Gong HH, et al. Aberrant spontaneous low-frequency brain activity in male patients with severe obstructive sleep apnea revealed by resting-state functional MRI. Neuropsychiatr. Dis. Treat 11(1), 207-214 (2015).
- Dai XJ, Nie X, Liu X, et al. Gender differences in regional brain activity in patients with chronic primary insomnia: evidence from a resting-state fMRI study. J. Clin. Sleep. Med; 12(3), 363-374 (2016).
- Huang X, Zhong YL, Zeng XJ, et al. Disturbed spontaneous brain activity pattern in patients with primary angle-closure glaucoma using amplitude of low-frequency fluctuation: a fMRI study. Neuropsychiatr. Dis. Treat 11(1), 1877-1883 (2015).
- EI Khouli RH, Macura KJ, Barker PB, et al. Relationship of temporal resolution to diagnostic performance for dynamic contrast enhanced MRI of the breast. J. Magn. Reson. Imaging 30(1), 999-1004 (2009).
- Hahn C, Pogun S, Gunturkun O. Smoking modulates language lateralization in a sex-specific way. Neuropsychologia 48(1), 3993-4002 (2010).
- Li S, Tian J, Bauer A, Huang R, et al. Reduced Integrity of Right Lateralized White Matter in Patients with Primary Insomnia: A Diffusion-Tensor Imaging Study. Radiology 152038 (2016).
- Hugdahl K, Westerhausen R, Alho K, et al. Attention and cognitive control: unfolding the dichotic listening story. Scandinavian. Journal. Of. Psychology 50(1), 11-22 (2009).
- Nicholls ME, Wood AG, Hayes L. Cerebral asymmetries in the level of attention required for word recognition. Laterality 6(1), 97-110 (2001).
- Cruz Gomez, A.J., Ventura Campos, N., Belenguer, A., Avila, C., Forn, C., 2013. Regional brain atrophy and functional connectivity changes related to fatigue in multiple sclerosis. PLoS. One 8, e77914.
- Dogonowski AM, Andersen KW, Madsen KH, et al. Multiple sclerosis impairs regional functional connectivity in the cerebellum. Neuroimage. Clin 4(1), 130-138 (2013).
- Liu H, Chen H, Wu B, et al. Functional cortical changes in relapsing-remitting multiple sclerosis at amplitude configuration: a resting-state fMRI study. Neuropsychiatr. Dis. Treat 12(1), 3031-3039 (2016).
- Prinster A, Quarantelli M, Orefice G, et al. Grey matter loss in relapsing-remitting multiple sclerosis: a voxel-based morphometry study. Neuroimage 29(3), 859-867 (2006).
- Wegner C, Filippi M, Korteweg, T., et al. Relating functional changes during hand movement to clinical parameters in patients with multiple sclerosis in a multi-centre fMRI study. Eur. J. Neurol 15(1), 113-122 (2008).
- Calabrese M, Battaglini M, Giorgio A, et al. Imaging distribution and frequency of cortical lesions in patients with multiple sclerosis. Neurology 75(1), 1234-1240 (2010).
- Morgen K, Sammer G, Courtney SM, et al. Evidence for a direct association between cortical atrophy and cognitive impairment in relapsing-remitting MS. Neuroimage 30(1), 891-898 (2006).
- Calabrese M, Atzori M, Bernardi V, et al. Cortical atrophy is relevant in multiple sclerosis at clinical onset. J. Neurol 254(1), 1212-1220 (2007).
- Sato JR, Salum GA, Gadelha A, et al. Decreased centrality of subcortical regions during the transition to adolescence: a functional connectivity study. Neuroimage 104(1): 44-51 (2015).
- Beucke JC, Sepulcre J, Talukdar T, et al. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA. Psychiatry 70(1) 619-629 (2013).
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends. In. Cognitive. Sciences 4(1), 215–222 (2000).
- Lattanzi S, Danni M, Cerqua R, et al. Prediction of disability progression in fingolimod-treated patients. J. Neurol. Sci 358(1-2), 432-434 (2015).
- Lattanzi S, Danni M, Taffi R, et al. Persistence to oral disease-modifying therapies in multiple sclerosis patients. J. Neurol 22 2017.